Vector-borne diseases account for more than 17% of all infectious diseases. In the Americas, dengue, chikungunya and Zika constitute a potential epidemiological risk due to the recent increase in cases, complications, and severity. The co-circulation of the three diseases is a matter of public health interest due to the fact that they are transmitted by the same vector as well as the increase in the number of cases of microcephaly related to Zika virus, post-chikungunya chronic joint disease, and severe dengue. Therefore, it is important for clinicians to be familiar with the various clinical presentations and laboratory methods to make the differential diagnosis, start appropriate treatment, and prevent the associated complications.
Las enfermedades transmitidas por vector condicionan alrededor del 17% de la carga mundial estimada de enfermedades infecciosas. En América, el dengue, chikunguña y zika constituyen un potencial riesgo epidemiológico debido al reciente incremento de casos, complicaciones y gravedad. Es de interés en salud pública la co-circulación de las tres enfermedades debido a la transmisión por el mismo vector así como el aumento en el número de casos de microcefalia relacionado al virus del Zika, artropatías crónicas post chikunguña y dengue grave. Por ello, es de importancia para los clínicos conocer las diversas presentaciones clínicas y los métodos de laboratorio para realizar el diagnóstico diferencial, instituir el tratamiento oportuno y prevenir las complicaciones asociadas.
The dengue (DENV), chikungunya (CHIKV) and Zika (ZIKV) viruses, currently prevalent and co-circulating in several countries around the world, are arboviruses responsible for significant epidemics in recent years. In the Americas, they are mainly transmitted by the Aedes aegypti mosquito, a haematophagic arthropod of African origin very well adapted to living in both urban and rural areas and predominantly distributed in the tropical and subtropical areas of the Americas.1
Dengue is the most significant human viral disease transmitted by arthropods. It is estimated that 2.5 billion people live in areas where there is a risk of contracting dengue.2 Currently, a global alert has been issued for Zika given the increase in congenital abnormalities, Guillain–Barré syndrome, and other autoimmune manifestations, and the increase in chronic joint diseases due to chikungunya.3
DENGUE: Dengue is an exanthematic febrile disease with variable clinical progression and presentation. It is responsible for high rates of morbidity and mortality in various endemic regions around the world. Approximately 390 million people are infected per year; 96 million of them have clinical manifestations of any level of severity and up to 20,000 of them die.4
DENV belongs to the family Flaviviridae, genus Flavivirus. There are four serotypes of the virus: DENV-1, DENV-2, DENV-3 and DENV-4. The serotypes are antigenically similar, though different enough not to confer long-term cross-protection. Infection with one dengue serotype confers lifetime immunity against that serotype.2 The genome of the virus consists of a single RNA chain whose nucleocapsid is embedded with M and E structural proteins, and seven non-structural proteins. Of these non-structural proteins, the NS1 protein interacts with the host immune system and evokes T cell responses. This response has been used as a diagnostic marker of infection.2,5
The latest update, published by the Pan American Health Organization (PAHO) and the World Health Organisation (WHO) on 17 June 2016, reported a total of 1,679,537 probable cases of dengue and 309,465 cases of dengue confirmed through laboratory tests in the Americas.6
In Mexico, dengue is the most common vector-borne disease and the one with the greatest morbidity and mortality of all. In 2015, 61,710 cases of dengue fever corresponding to non-severe dengue were reported; 30,042 cases occurred in August, September and October. The number of reported cases was greater in women than in men: 36,383 and 25,327, respectively. The 25–44 year old age group was the most affected age group, with 20,986 cases. Regarding dengue haemorrhagic fever, equivalent to dengue with warning signs and severe dengue, in that same year, a total of 5626 cases were reported. It is estimated that for week 36 of 2016 there have been a total of 8975 cases of dengue, 2081 of which correspond to severe dengue, with 11 deaths. The most affected state in Mexico is Guerrero, with 1863 cases, corresponding to a rate of 51.92 cases per 100,000 inhabitants, followed by Veracruz, with 1120 cases, and Chiapas, with 688 cases.7,8
CHIKUNGUNYA: Chikungunya (CHIK) fever is a viral disease characterised by a sudden fever accompanied by skin rashes and joint pain, followed by persistent rheumatic symptoms.
It is caused by CHIKV, an RNA virus belonging to the genus Alphavirus of the family Togaviridae.9 Three lineages of CHIKV, with different antigenic characteristics and genotypes, have been identified: Western Africa (WAF), East/Central/Southern Africa (ECSA) and finally Asian.10
In 2015, the Pan American Health Organization (PAHO) reported 693,489 suspected cases and 37,480 confirmed cases. In 2016, a decreasing frequency has been observed. Up to 18 March, 31,000 cases had been reported to the PAHO. This figure is five times lower than in the same period in 2015.11
In Mexico in 2015, a total of 12,588 cases were reported with 4 deaths. The months in which the largest number of people affected were July, August and September, with 7297 cases. There were 8186 cases in women as opposed to 4402 cases in men. The 25–44 year old age group had the highest number of cases. This is similar to what happened with dengue. Up to September 2016, only 436 cases have been confirmed, with the greatest incidence in the states of Veracruz with 138 cases, Tamaulipas with 57 cases and Nayarit with 39 cases.7,12
ZIKA: Zika fever, like dengue and chikungunya, is a viral disease characterised by fever, exanthema and non-purulent conjunctivitis. Symptoms are self-limiting, although the majority of patients are asymptomatic. Despite its low morbidity, its association with cases of microcephaly and Guillain–Barré syndrome is worrisome to the health authorities in the countries with active transmission.
ZIKV is an RNA virus with 10,794 nucleotides that encode 3419 amino acids. It is a member of the family Flaviviridae of the genus Flavivirus. The virus has also been isolated in, Ae. apicoargenteus, Ae. luteocephalus, Ae. aegypti, Ae. vitattus and Ae. furcifer. Currently, 39 countries in the Americas have active transmission of the virus.13 To date, Brazil is the country with the largest number of reported cases of Zika; this number is estimated to be between 500,000 and 1,500,000.14
In Mexico in 2015, a total of 19 cases were reported, mainly during the months of November (8 cases) and December (10 cases). Regarding gender, the proportion is similar: 9 cases in women and 10 cases in men. The most affected age group was the 25–44 year old age group.7 Up to September 2016 there has been a constant increase, amounting to a total of 2782 confirmed cases. The state with most reported cases is Veracruz with 680 cases, followed by Guerrero with 666 cases and Chiapas with 547 cases. Of the 2782 confirmed cases, 1247 involve pregnant women. Chiapas is the most affected state with 339 pregnant women infected with the virus.15
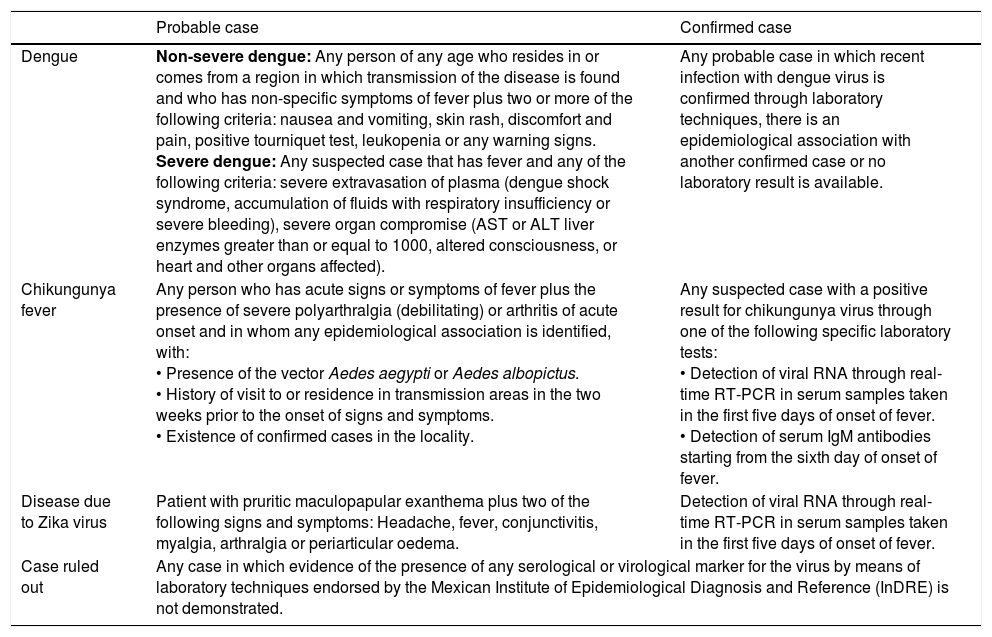
It is useful to take into consideration the current operational case definitions in Mexico for these three diseases, which have been prepared to unify the criteria for detection, reporting and classification of cases for epidemiological monitoring. Table 1 shows a summary adapted from various current guidelines.16–18
Operational case definitions for dengue, chikungunya and Zika.
| Probable case | Confirmed case | |
|---|---|---|
| Dengue | Non-severe dengue: Any person of any age who resides in or comes from a region in which transmission of the disease is found and who has non-specific symptoms of fever plus two or more of the following criteria: nausea and vomiting, skin rash, discomfort and pain, positive tourniquet test, leukopenia or any warning signs. Severe dengue: Any suspected case that has fever and any of the following criteria: severe extravasation of plasma (dengue shock syndrome, accumulation of fluids with respiratory insufficiency or severe bleeding), severe organ compromise (AST or ALT liver enzymes greater than or equal to 1000, altered consciousness, or heart and other organs affected). | Any probable case in which recent infection with dengue virus is confirmed through laboratory techniques, there is an epidemiological association with another confirmed case or no laboratory result is available. |
| Chikungunya fever | Any person who has acute signs or symptoms of fever plus the presence of severe polyarthralgia (debilitating) or arthritis of acute onset and in whom any epidemiological association is identified, with: • Presence of the vector Aedes aegypti or Aedes albopictus. • History of visit to or residence in transmission areas in the two weeks prior to the onset of signs and symptoms. • Existence of confirmed cases in the locality. | Any suspected case with a positive result for chikungunya virus through one of the following specific laboratory tests: • Detection of viral RNA through real-time RT-PCR in serum samples taken in the first five days of onset of fever. • Detection of serum IgM antibodies starting from the sixth day of onset of fever. |
| Disease due to Zika virus | Patient with pruritic maculopapular exanthema plus two of the following signs and symptoms: Headache, fever, conjunctivitis, myalgia, arthralgia or periarticular oedema. | Detection of viral RNA through real-time RT-PCR in serum samples taken in the first five days of onset of fever. |
| Case ruled out | Any case in which evidence of the presence of any serological or virological marker for the virus by means of laboratory techniques endorsed by the Mexican Institute of Epidemiological Diagnosis and Reference (InDRE) is not demonstrated. | |
Adapted from: Various guidelines.2,16,17,18
The global distribution of diseases transmitted by arboviruses represents a challenge for clinicians due to the similarities between their manifestations and sometimes overlapping symptoms.
In dengue, the disease has three phases: febrile, critical, and convalescence or recovery. Its incubation period lasts 4–10 days and is followed by the febrile phase, which lasts 2–7 days and causes non-specific signs and symptoms such as fever (38.8–40.5°C), frontal headache, retro-ocular pain, general malaise, myalgia, arthralgia, nausea, vomiting, anorexia and exanthema. In this phase, it may be difficult to clinically distinguish this disease from other febrile diseases and to determine whether it is in its severe or non-severe form. The tourniquet test may be performed in this phase. This consists in inflating a blood pressure cuff up to a midpoint between the diastolic pressure and systolic pressure of the patient for five minutes. It is considered to be positive when 10 or more petechiae are found in a 2.5cm2 area of skin on the forearm. If it is positive in this phase, the likelihood of making a diagnosis of dengue increases. The earliest abnormality in the complete blood count in the febrile phase is a progressive reduction in the total white blood cell count. This should increase the suspicion that the patient has signs and symptoms of dengue. The critical phase lasts 24–48h and occurs when the fever subsides. There may be bleeding and/or an increase in capillary permeability. The latter results in a leakage of plasma clinically manifested as pleural effusion and ascites. At the same time, there is an increase in haematocrit levels, a decrease in platelet count, leukopenia with neutropenia and lymphocytosis with 15–20% atypical shapes. These correspond to severe dengue. When the loss of plasma volume is critical, the risk of having shock increases; if the state of shock is prolonged, it may lead to progressive organ deterioration resulting in death.2,19
More encouragingly, if the critical phase is exceeded, there is a gradual reabsorption of fluids, general well-being improves and haemodynamic status stabilises in the following 48–72h. The total white blood cell count increases with the increase in neutrophils and decrease in lymphocytes. The increase in platelet count tends to be subsequent to the increase in white blood cells. This corresponds to the recovery phase.2,19
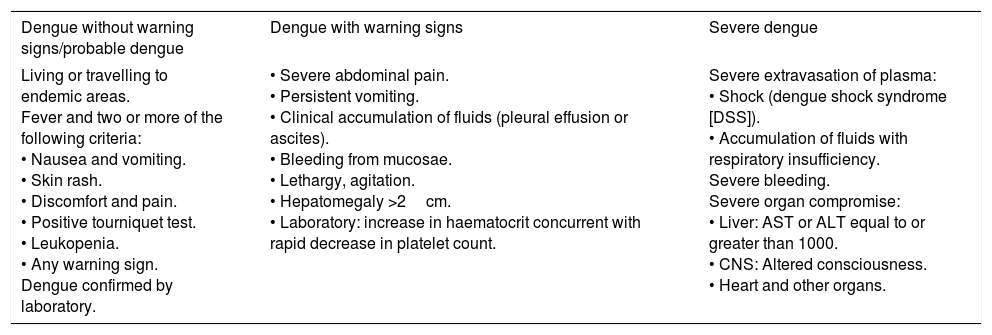
Depending on its clinical course, the disease may be classified as non-severe dengue with or without warning signs or severe dengue. In severe dengue, hepatomegaly, a rare sign that occurs in fewer than 2% of cases in the non-severe form of the disease, becomes a sign of severity.20Table 2 shows the current classification for dengue prepared by the World Health Organization.
Classification of dengue and levels of severity.
| Dengue without warning signs/probable dengue | Dengue with warning signs | Severe dengue |
|---|---|---|
| Living or travelling to endemic areas. Fever and two or more of the following criteria: • Nausea and vomiting. • Skin rash. • Discomfort and pain. • Positive tourniquet test. • Leukopenia. • Any warning sign. Dengue confirmed by laboratory. | • Severe abdominal pain. • Persistent vomiting. • Clinical accumulation of fluids (pleural effusion or ascites). • Bleeding from mucosae. • Lethargy, agitation. • Hepatomegaly >2cm. • Laboratory: increase in haematocrit concurrent with rapid decrease in platelet count. | Severe extravasation of plasma: • Shock (dengue shock syndrome [DSS]). • Accumulation of fluids with respiratory insufficiency. Severe bleeding. Severe organ compromise: • Liver: AST or ALT equal to or greater than 1000. • CNS: Altered consciousness. • Heart and other organs. |
Adapted from: World Health Organization.2
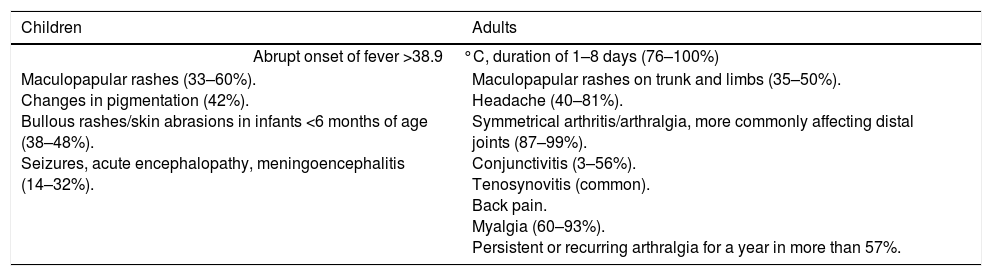
It is known that, in infection with CHIKV, 16–27% of adults and 35–40% of neonates and infants are asymptomatic.21 The incubation period is between 2 and 12 days.11 The clinical manifestations include fever of sudden onset, in 76–100% of cases, associated with polyarthralgia, headache, myalgia/arthralgia of greater severity than in other diseases and skin rashes.22 The most common manifestations are grouped in Table 3.
Most common clinical manifestations of CHIK in children and adults.
| Children | Adults |
|---|---|
| Abrupt onset of fever >38.9°C, duration of 1–8 days (76–100%) | |
| Maculopapular rashes (33–60%). Changes in pigmentation (42%). Bullous rashes/skin abrasions in infants <6 months of age (38–48%). Seizures, acute encephalopathy, meningoencephalitis (14–32%). | Maculopapular rashes on trunk and limbs (35–50%). Headache (40–81%). Symmetrical arthritis/arthralgia, more commonly affecting distal joints (87–99%). Conjunctivitis (3–56%). Tenosynovitis (common). Back pain. Myalgia (60–93%). Persistent or recurring arthralgia for a year in more than 57%. |
Adapted from: Various studies.9,21
Atypical presentations and complications of the disease predominate in patients with cardiovascular comorbidities, neurological comorbidities, respiratory comorbidities, systemic arterial hypertension and prior administration of non-steroidal anti-inflammatory drugs (NSAIDs).23 In addition, the pre-existing signs of chronic joint disease and other causes of chronic rheumatism predispose patients to chronicity of CHIK symptoms.24 Individuals at the extremes of life, that is to say, neonates and elderly people, develop severe forms of the disease.23
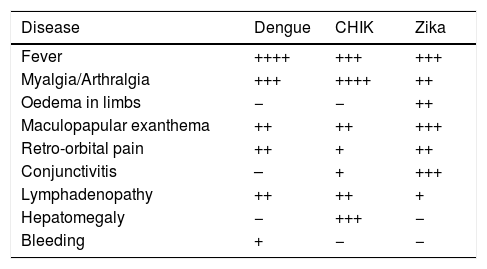
In Zika, symptoms are non-specific and the differential diagnosis represents a challenge. The most common symptom is maculopapular exanthema (90%) followed by fever (65%), arthralgia (65%), non-purulent conjunctivitis (55%), myalgia (48%), headache (45%), retro-ocular pain (39%), oedema (19%) and vomiting (10%).25 Other, rarer symptoms include haematospermia and lower urinary tract symptoms. Symptoms manifest in a period of 3–12 days, and only 1 in every 5 infected patients develops them.26 Despite these diseases’ similarities in terms of symptoms, there are differences between them that may guide the clinician towards a more accurate clinical diagnosis. These are grouped in Table 4.27
Clinical comparison of dengue, chikungunya and Zika.
| Disease | Dengue | CHIK | Zika |
|---|---|---|---|
| Fever | ++++ | +++ | +++ |
| Myalgia/Arthralgia | +++ | ++++ | ++ |
| Oedema in limbs | − | − | ++ |
| Maculopapular exanthema | ++ | ++ | +++ |
| Retro-orbital pain | ++ | + | ++ |
| Conjunctivitis | – | + | +++ |
| Lymphadenopathy | ++ | ++ | + |
| Hepatomegaly | − | +++ | − |
| Bleeding | + | − | − |
Adapted from: Ioos et al.27
Currently, the co-circulation of the three diseases in various endemic regions increases the probability of having simultaneous infections. In this regard, there are reports of isolated cases of co-infections in countries such as Colombia, Germany and New Caledonia. The authors did not observe a synergistic effect in co-infection in these reports. In Mexico, there is not yet any evidence that demonstrates this situation. It is important to consider probable simultaneous infection in clinical situations such as pregnant women and patients with comorbidities, where follow-up goes beyond acute signs and symptoms, or in the classification of patients with probable co-infection with dengue to detect and/or educate the patient in home monitoring of warning signs given the risk of having its severe form.28–30
ComplicationsThe most common complications in dengue include fluid overload (pleural effusion or ascites), acute pulmonary oedema, acute respiratory distress syndrome, severe metabolic acidosis, organ deterioration (acute liver failure, acute kidney failure, encephalopathy or encephalitis and cardiomyopathy) and electrolyte imbalances. Hyperglycaemia or hypoglycaemia may also occur.2,19 There are various individual risk factors that will influence the severity of the disease, such as age (small children may have a lower capacity to compensate for extravasation of plasma and are at a greater risk of having shock due to dengue); race; presence of chronic diseases; secondary infection due to a serotype other than that of the primary infection (this has been considered to be one of the most significant risk factors for severe disease and breast-feeding children of mothers who are immune to dengue may have this phenomenon, although severe cases associated to primary infection have also been reported).2,5
After the acute phase of infection with CHIKV, the most common complications are joint-related, followed by neurological and cardiovascular.23,24 The proportion of patients who develop chronic post-CHIK musculoskeletal disorders (pCHIK-MSDs) is 25–34%, and the proportion of patients who develop chronic arthritis is 14%, given that symptoms decrease over time after the onset of CHIK (88–100% during the first 6 weeks and >50% after 3–5 years). Diagnosis is generally late, which involves a strong economic and functional impact on the life of patients. Some individuals remain symptomatic for 6–8 years post-infection.24
Neonatal manifestations due to CHIK include central nervous system abnormalities, bleeding disorders and cardiac manifestations. The CHIMERE study reported severe manifestations of infection acquired in utero in neonates who had seizures, required mechanical ventilation or had encephalitis confirmed through magnetic resonance imaging during the acute phase of the disease.31
ZIKV is characterised by being highly neurotropic, and so its main complications manifest primarily in the nervous system. An example of this is Guillain–Barré syndrome (GBS). The first country to report this association was French Polynesia with 42 cases. Studies revealed that 98% of patients with GBS had anti-Zika IgM and 100% had anti-Zika neutralising antibodies, and that 37 patients (88%) had experienced symptoms similar to Zika in the preceding 6 days.32
Abnormalities in neonates are even more significant. As a result, the WHO/PAHO published an epidemiological alert on 1 December 2015. This alert reported in Brazil an increase 20 times the expected rate in cases of microcephaly: 99.7 cases in every 10,000 pregnant women in 2015, as opposed to an expected rate of 5.7 cases in 2010 and 5.5 cases in 2000.3 French Polynesia also determined that the risk of microcephaly was 95 cases per 10,000 pregnant women compared before 2013 to 2 cases per 10,000 pregnant women.33 Abnormalities that have been identified as part of congenital Zika syndrome include intrauterine growth restriction, intraventricular calcifications, decrease in placental blood flow, foetal death and arthrogryposis.
DiagnosisDiagnosis of dengue, chikungunya and Zika is mainly clinical. In regions where the risk of dengue is significant, it is important to intentionally look for warning symptoms, with a comprehensive medical history and physical examination in addition to a complete blood count and transaminases to determine the phase and severity of the disease.2,19 Serological tests are not required to start the patient's management and are only done to confirm the differential diagnosis between these diseases and other infectious diseases and to perform epidemiological monitoring and research.2,19
Specific tests include the enzyme-linked immunosorbent assay (ELISA) to detect the NS1 antigen, which is abundant in the first five dates of infections with any of the four serotypes of dengue. This technique reportedly has a sensitivity of 80–100% and a specificity of 100%. It should be noted that this glycoprotein is produced by all flaviviruses.2,19 Detection of anti-dengue IgM antibodies (MAC-ELISA) is the serological test of choice. These antibodies may be detected after 4–5 days of onset of fever. It is not possible to detect serotypes of the virus with these tests.2,16
Detection of IgM and IgG through the ELISA technique may be used for CHIKV from 4 days after the onset of fever to 2 months in the case of IgM. A sensitivity of 81.8% is reported for late stages of the disease. IgGs may be detected in samples in the convalescence stage and persist throughout life.9,17,34
For ZIKV, detection of IgM by means of ELISA may be used in the convalescence phase (after 6 days since onset of symptoms). This test can only detect whether there is an infection with flaviviruses, and so the plaque reduction neutralisation test (PRNT) is more reliable, since the antibodies may give results of cross-reactivity in people previously infected with other flaviviruses. The specificity and sensitivity of this test may reach figures of 81–100%. This is reduced if the cross-reaction is caused by flaviviruses other than DENV (for example, yellow fever or Japanese encephalitis).35
Mainly for the purposes of epidemiological monitoring, the serotype of the viruses in the acute phase of any of these diseases is determined using the reverse transcription polymerase chain reaction (RT-PCR) test.17,18 The sensitivity of this test for CHIKV in the early stages of infection is 88.3%.34 In the case of Zika this is the test of choice due to its high sensitivity and specificity, which in some cases have both reached values of 100%. Serum or blood samples are used and it is specified that urine could be the ideal sample type. However, the availability of this test is limited.18,36,37
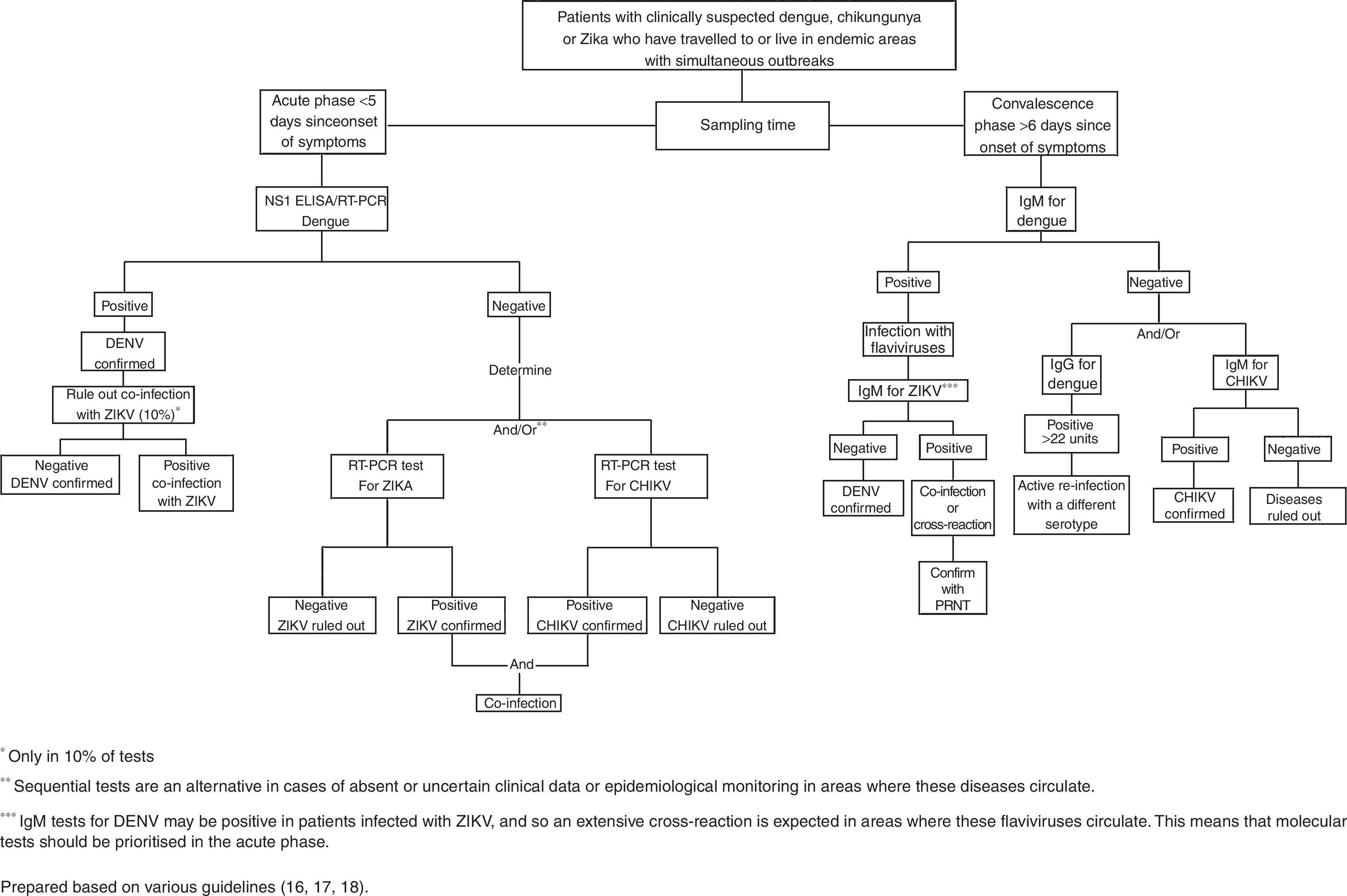
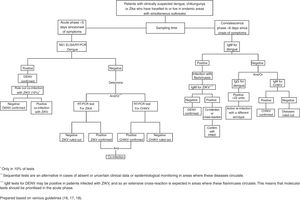
In the standardised guidelines for epidemiological monitoring and laboratory diagnosis of infection with Zika virus in Mexico, it is proposed that 10% of patients with confirmed Zika virus infection will be tested for dengue and chikungunya to identify co-infections.18Fig. 1 shows the flow chart proposed to make the diagnosis in patients with suspected infection with arboviruses.16–18
In areas of active Zika virus transmission, the CDC guidelines propose that pregnant women with Zika symptoms should be analysed with RT-PCR. If the results are positive or inconclusive, a series of foetal ultrasounds and amniocenteses for PCR in amniotic fluid must be performed. If the results are negative, an ultrasound must be performed to look for microcephaly or intracranial calcifications. In normal studies, routine prenatal care is continued. It is advisable to perform the test in pregnant women without symptoms.38–39
TreatmentThese diseases are generally self-limiting with no need for hospitalisation. In Zika, chikungunya and dengue fever without warning signs, hospitalisation is only indicated to manage supportive measures such as ensuring appropriate fluid intake and bed rest with a mosquito net (viraemia phase) and keeping the temperature below 39°C, whether through physical measures or paracetamol, which is the only authorised antipyretic agent and which also decreases joint pain. Acetylsalicylic acid and other non-steroidal anti-inflammatory drugs (NSAIDs) are contraindicated in dengue and Zika, and should be used with caution to prevent bleeding events and Reye's syndrome in paediatric patients. For Zika fever, the use of antihistamines is indicated to treat pruritus.2,19,39
Patients with dengue with warning signs, a state of shock or comorbidities should be referred for hospital management. Patients with severe dengue in the critical phase of the disease require emergency treatment and urgent transfer to a hospital.2 The most effective treatment to prevent deaths due to extravasation of plasma is the use of intravenous rehydration with isotonic crystalloid solutions and generally this is the only intervention that is required. Colloid solutions may be used in the event of shock with hypotension.2 It is necessary to record and evaluate vital signs, temperature curves, haematocrit levels, diuresis and organ function (liver, kidney and coagulation tests). It is advisable to assess the condition of the patient up to 48h after the fever has disappeared. Blood transfusion is only indicated in cases with suspicious or massive bleeding.2
In patients whose joint pain due to CHIK is not relieved with NSAIDs, the use of corticosteroids or narcotic analgesics is indicated. Convalescence in this disease may be prolonged. Thus, treatment of pCHIK-MSDs is important and should be started by a rheumatologist. It consists of anti-inflammatory drugs in conjunction with physiotherapy.9
PreventionThe measures recommended by the different international guidelines are aimed at preventing mosquitoes from biting and reproducing. They are listed below2,9,11:
- •
Wear long-sleeved clothing.
- •
Use repellents with DEET (N,N-diethyl-meta-toluamide), IR3535 or icaridin. Avoid their use in infants under 2 months of age. These should be used in strict compliance with the instructions on the label.
- •
Stay in places with air conditioning.
- •
Avoid standing water and mosquito-breeding areas.
- •
Place mosquito nets over windows, doors and beds. Domestic insecticides in aerosol form, mosquito coils and other insecticides in vapourisers within the home can also be used.
- •
Treat clothing and equipment with permethrin.
The WHO and the PAHO have not issued any travel restriction due to outbreaks of the Zika, chikungunya or dengue virus.
Currently, the only vaccine that exists against these diseases, CYD-TDV, is for dengue and was approved for use in Mexico in December 2015. It is a recombinant, live-attenuated tetravalent virus vaccine. It is recommended for people 9–45 years of age who live in endemic areas.40 The vaccine has higher efficacy for protecting against infections by serotypes 3 and 4 of DENV, has lower efficacy for protecting against DENV 1 and does not protect against DENV 2. It has also managed to decrease the risk of hospitalisation and severe dengue in children between 9 and 16 years of age, but not younger children, who had a higher rate of hospitalisation due to dengue.40
Ethical disclosureProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that they have no conflict of interests.