The preparation of 131I-trazodone hydrochloride and its biological evaluation as a promising brain imaging radiopharmaceutical using two routes of administration.
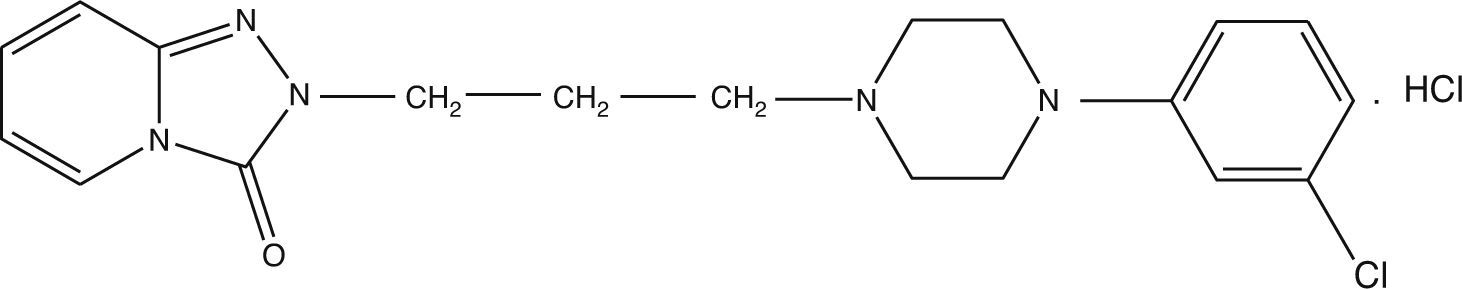
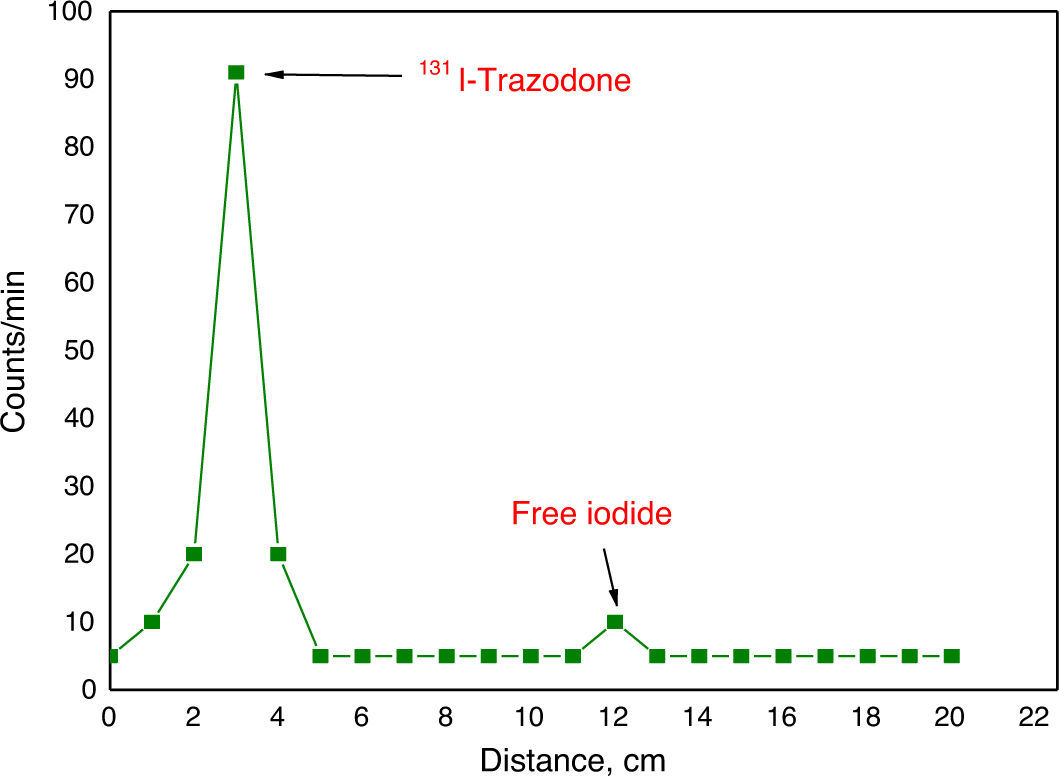
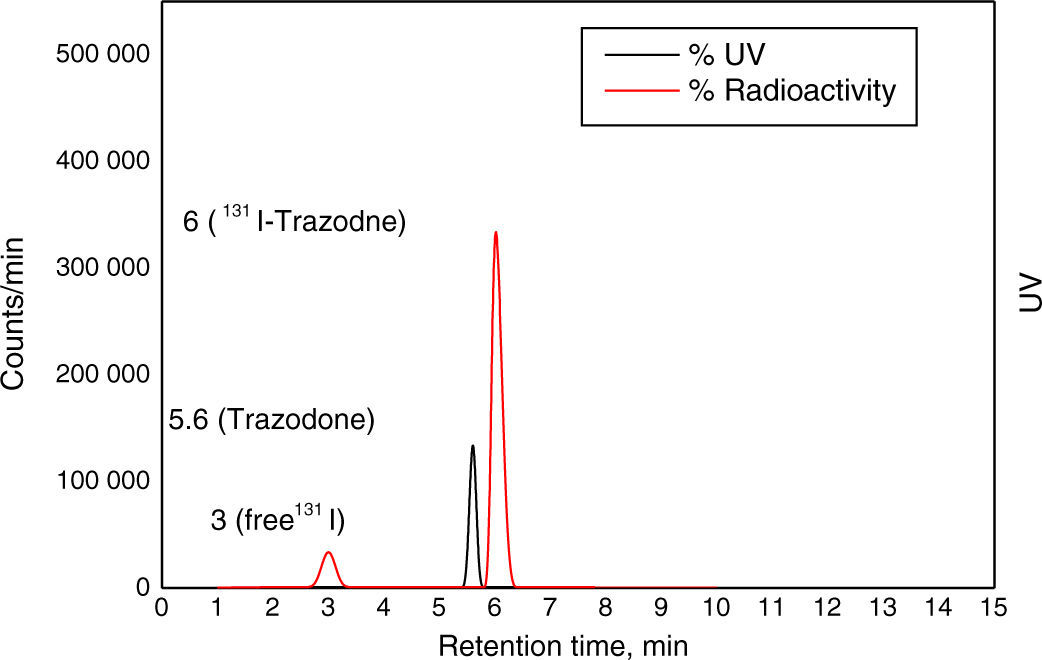
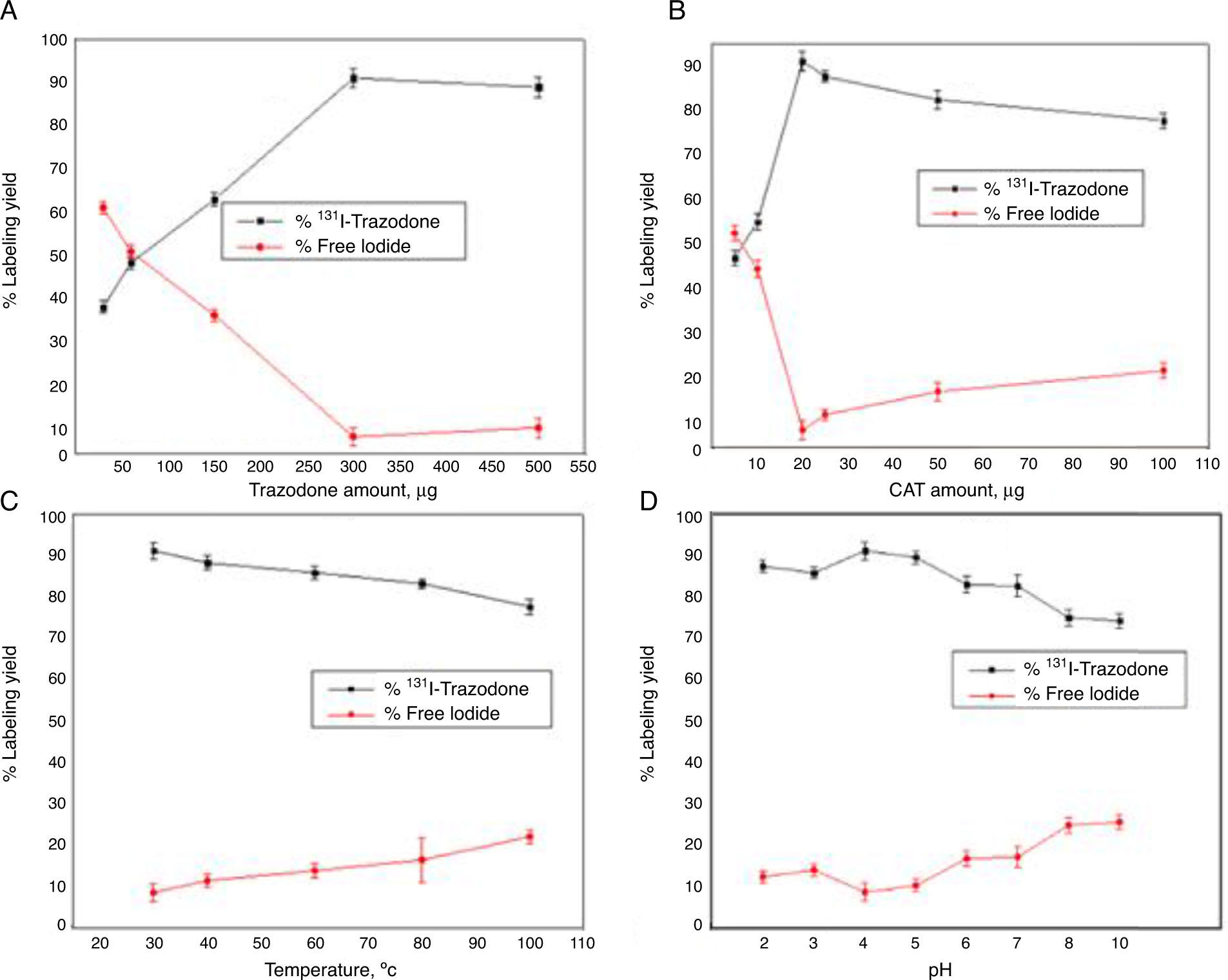
Material and methodsTrazodone (TZ) was radiolabelled with 131I using direct electrophilic substitution, and different factors affecting labelling yield were studied. Quality control of 131I-TZ was carried out using ascending paper chromatography, paper electrophoresis, and high pressure liquid chromatography (HPLC). In vivo biodistribution of 131I-TZ was evaluated in Swiss albino mice using 3 methods: intravenous 131I-TZ solution (IVS), intranasal 131I-TZ solution (INS), and intranasal 131I-TZ microemulsion (INME).
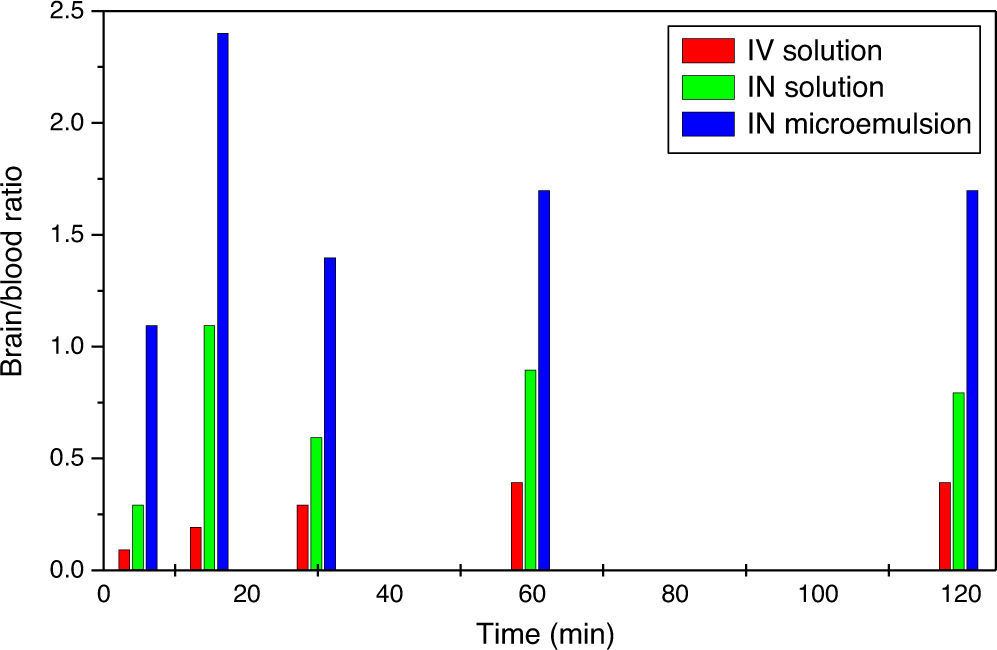
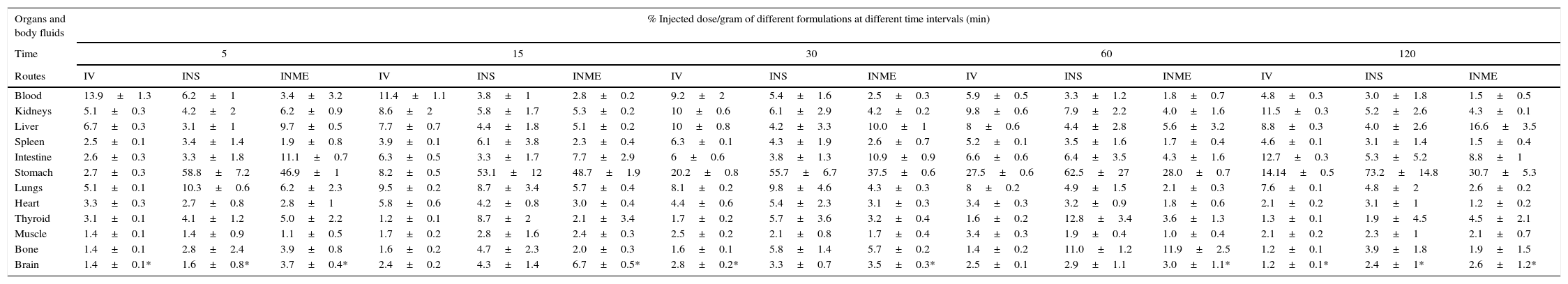
ResultsOptimum labelling yield of 91.23±2.12% was obtained with in vitro stability of 131I-TZ up to 6h at room temperature. The biodistribution results showed a notably higher and sustained brain uptake for INME compared to IVS and INS at all time intervals. In addition, heart and blood uptake levels for INME were lower than those for IV solution which, in turn, could decrease the systemic side effects of trazodone. Also, the 131I-trazodone INME brain uptake of 6.7±0.5%ID/g was higher than that of 99mTc-ECD and 99mTc-HMPAO (radiopharmaceuticals currently used for brain imaging).
Conclusion131/123I-trazodone formulated as INME could be used as a promising radiopharmaceutical for brain imaging.
Preparación del hidrocloruro de 131I-Trazodona y su evaluación biológica como radiofármaco prometedor de imagen cerebral utilizando dos vías de administración.
Material y métodosEl marcaje de la Trazodona (TZ) con 131I se realizó mediante sustitución electrofílica directa y se estudiaron diferentes factores que pueden afectar al rendimiento del marcaje. El control de calidad de 131I-TZ se realizó usando cromatografía en papel ascendente, electroforesis en papel y HPLC. La biodistribución in vivo de 131I-TZ se evaluó en ratones albinos suizos de tres maneras: solución intravenosa de 131I-TZ (IV), solución intranasal de 131I-TZ (IN) y microemulsión intranasal 131I-TZ (MEIN).
ResultadosSe obtuvo un rendimiento de marcaje óptimo de 91,23±2,12% con una estabilidad in vitro de 131I-TZ hasta 6 horas a temperatura ambiente. Los resultados de biodistribución mostraron una captación cerebral elevada y mayor para la MEIN en comparación con la solución IV e IN en todos los intervalos de tiempo. Además, la captación vascular y cardíaca para la MEIN fueron más bajos que los de la solución IV, lo que disminuiría los efectos secundarios sistémicos de la TZ. La captación cerebral de 131I-TZ MEIN fue de 6,7±0,5% ID/g, siendo mayor que la de 99mTc-ECD y 99mTc-HMPAO (radiofármacos utilizados actualmente para la imagen cerebral).
ConclusiónEl 131/123I-TZ formulado como MEIN podría ser utilizado como un radiofármaco prometedor para la imagen cerebral.
Article

Revista Española de Medicina Nuclear e Imagen Molecular (English Edition)