Irreversible organ damage is a predictive factor of morbidity, mortality, increased accumulation of damage, and poor quality of life in patients with systemic lupus erythematosus.
ObjectivesTo describe the damage, and the factors that best explain it, in a population of Colombian patients.
MethodsA retrospective follow-up study of a patient cohort. The damage was measured using the Systemic Lupus International Collaborating Clinics (SLICC) and the American College of Rheumatology ACR index, and disease activity by SELENA SLEDAI. Descriptive statistics were used to describe the damage. The factors associated with the outcome were evaluated with Pearson's or Fisher's Chi2, Student's t or Mann–Whitney's U. The proportion of patients that accumulated damage was evaluated with the Friedman test, and the cumulative score with the Wilcoxon test. The determination of the factors independently associated with the outcome was performed using logistic regression.
ResultsA total of 161 patients with recent diagnosis, and followed for one year or more, were included, 28.9% of whom had suffered damage. The most represented domains were neuropsychiatric, renal and vascular. Anti-phospholipid antibodies, mean doses of prednisolone greater than 12.5mg/day, and suffering two or more relapses were independently associated with organ damage.
ConclusionsAnti-phospholipid antibodies, steroid doses and frequency of relapses are associated with organ damage in a Colombian population of patients with SLE.
El daño irreversible de órgano es predictor de morbilidad, mortalidad, mayor acúmulo de daño y mala calidad de vida en los pacientes con lupus eritematoso sistémico.
ObjetivosCaracterizar el daño y los factores que mejor lo explican, en una población de pacientes colombianos con lupus eritematoso sistémico.
MétodosEstudio retrospectivo de seguimiento a una cohorte. El daño se midió con el SLICC/ACR (índice de Systemic Lupus International Collaborating Clinics y del American College of Rheumatology) y la actividad de la enfermedad por SELENA SLEDAI. La caracterización del daño se hizo mediante estadística descriptiva, los factores asociados con el desenlace se evaluaron con Chi2 de Pearson o Fisher, t de Student o U de Mann-Whitney; la proporción de pacientes que acumularon daño se evaluó con el test de Friedman y el puntaje acumulado con el test de Wilcoxon. La determinación de los factores asociados independientemente con el desenlace se hizo con una regresión logística.
ResultadosSe incluyeron 161 pacientes con diagnóstico de novo y seguimiento mínimo de un año; el 28,9% sufrió daño. Los dominios más representados fueron el neuropsiquiátrico, renal y vascular. Los anticuerpos antifosfolípido, las dosis promedio de prednisolona mayores a 12,5mg/día y presentar 2 o más recaídas se asociaron independientemente al daño orgánico.
ConclusionesLos anticuerpos antifosfolípido, la dosis de esteroides y la frecuencia de recaídas se asocian al daño orgánico en una población colombiana de pacientes con lupus eritematoso sistémico.
Although the survival of the patients with systemic lupus erythematosus (SLE) has improved significantly, mortality remains high1 and irreversible organ damage, especially renal2,3 and neurological,4,5 affects this outcome.
The accumulation of damage determines greater morbidity, morbility,6,7 harm, poor quality of life,8–10 hospitalizations, requirement of dialysis and kidney transplant, and costs for the healthcare systems.11,12
The factors that have been associated with the accumulation of organ damage in patients with SLE are the race,13 age of onset,14,15 male gender,16,17 diagnosis after 50 years of age,18 duration13 and activity of the disease,8,19 relapses,20 doses of steroids,21,22 antiphospholipid (APL), anti-DNA and anti-Ro antibodies23,24 and use of immunosuppressants.25,26 Antimalarials are protective factors against this outcome.27,28
The purpose of the study was to recognize which are the domains and specific manifestations that characterize the organ damage in a population of Colombian patients with SLE and to determine the factors that best explain it.
Patients and methodsIt was conducted a retrospective cohort study which included all patients over 16 years of age who met at least 4 ACR (American College of Rheumatology) classification criteria at the time of diagnosis of SLE and who were followed-up for more than one year in a high complexity hospital in the city of Medellin (Colombia). Only those with de novo diagnosis in the institution were included to properly assign the organ damage; those with drug-induced lupus and those with systemic sclerosis or dermatomyositis overlap syndromes were excluded.
The lupus activity was measured with SELENA SLEDAI29 and the organ damage with the Systemic Lupus International Collaborating Clinics Index (SLICC)/ACR SDI.30 Relapse of SLE was defined by the minimal increase of 3 points in SELENA SLEDAI, by the need to increase the dose of steroids or to start a new immunosuppressant or by the hospitalization due to lupus.20
The studied variables were the gender, age at diagnosis, follow-up time, lupus nephritis, arterial hypertension, SELENA SLEDAI score at diagnosis and during follow-up, number of relapses, APL (IgG and IgM anticardiolipin antibodies, IgG and IgM anti-β2 glycoprotein 1 antibodies and lupus anticoagulant), anti-Ro and anti-DNA, daily and cumulative dose of prednisolone and use of pulses of methylprednisolone, cyclophosphamide, antimalarials, azathioprine and mycophenolate.
Statistical analysisThe population was characterized with descriptive statistics. The association between risk factors and organ damage was evaluated with the Pearson's Chi2 or Fisher's exact test and the Student's t or Mann–Whitney's U, according to the case; the Friedman test was used to evaluate the proportion of patients with cumulative damage, and the Wilcoxon test was used for the accumulation of damage during the follow-up. In the multivariate logistic regression analysis were included the variables with p<0.25 that had significant crude ORs in the univariate analysis. Collinearity was determined by clinical criteria, the variables that were considered collinear were excluded from the final statistical model.
The organ damage was categorized as mild (1–2 points) and severe (≥3 points),29 the SELENA SLEDAI score as ≤10 and greater than 10 points at the onset of the disease and in 3–8 and ≥9 points during the follow-up. The daily dose of prednisolone was categorized as ≤7.5; 7.6–12.5 and higher than 12.5mg. Imputation of the missing data of the autoantibodies was not made.
The SPSS software version 21, license CES University, was used for the analysis.
The research was approved by the Ethics Committees of the Hospital Pablo Tobon Uribe and the CES University.
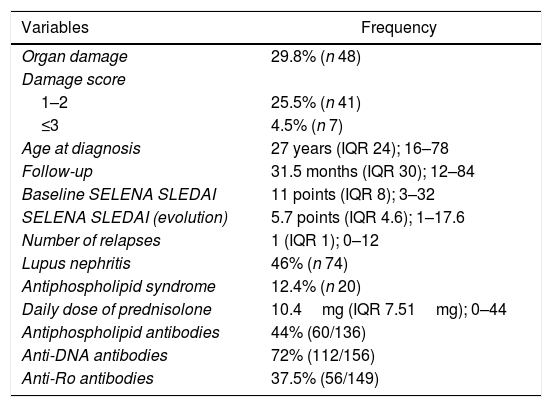
ResultsThe population was constituted by 161 patients with de novo diagnosis of SLE, 93.7% were women, followed-up for 31.5 months (IQR 30, from 12 to 84 months) (Table 1). The diagnosis was made between 16 and 25 years in 46% of cases, between 26 and 50 years in 39.8%, after the age of 50 years in 14.2% of them. 44% of the subjects presented lupus nephritis, 26.1% had arterial hypertension and 5% died.
Baseline clinical characteristics of the population.
| Variables | Frequency |
|---|---|
| Organ damage | 29.8% (n 48) |
| Damage score | |
| 1–2 | 25.5% (n 41) |
| ≤3 | 4.5% (n 7) |
| Age at diagnosis | 27 years (IQR 24); 16–78 |
| Follow-up | 31.5 months (IQR 30); 12–84 |
| Baseline SELENA SLEDAI | 11 points (IQR 8); 3–32 |
| SELENA SLEDAI (evolution) | 5.7 points (IQR 4.6); 1–17.6 |
| Number of relapses | 1 (IQR 1); 0–12 |
| Lupus nephritis | 46% (n 74) |
| Antiphospholipid syndrome | 12.4% (n 20) |
| Daily dose of prednisolone | 10.4mg (IQR 7.51mg); 0–44 |
| Antiphospholipid antibodies | 44% (60/136) |
| Anti-DNA antibodies | 72% (112/156) |
| Anti-Ro antibodies | 37.5% (56/149) |
53.4% of the patients had a SELENA SLEDAI baseline score higher than 10 points, and 18%, a score higher than 9 points during follow-up. 99.4% of the patients received prednisolone, 31.7% cyclophosphamide, 28.6% pulses of methylprednisolone, 89.4% antimalarials (69.6% chloroquine and 21.1% hydroxychloroquine), 25.5% azathioprine and 32.9% mycophenolate.
Organ damage occurred in 29.8% of the patients, with an incidence rate of 1/10 patients/year and it was severe in 14.5% of the cases. It was observed an increase in the proportion of patients with damage between the first and the fifth year (p=0.03), especially at 12, 36 and 48 months (p=0.000, 0.005 and 0.011).
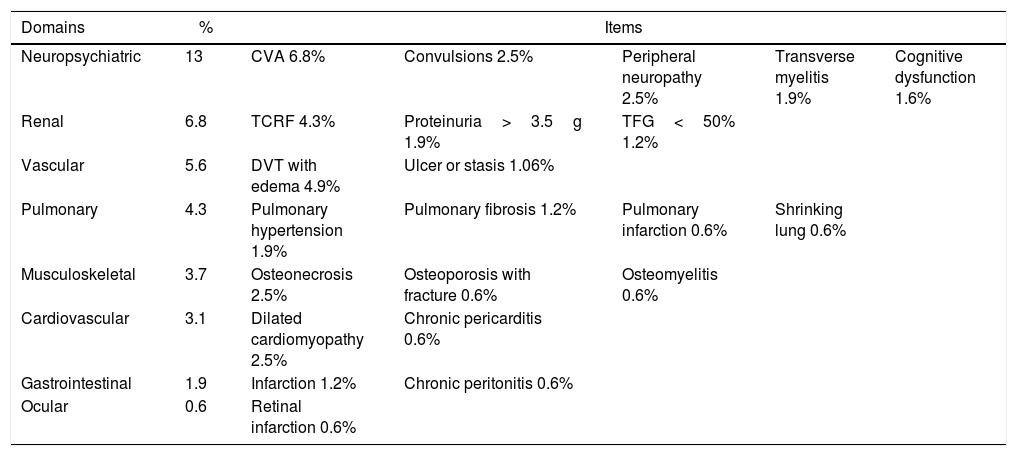
The neuropsychiatric domain was the most compromised (13%), followed by the renal (6.8%), vascular (5.6%) and pulmonary (4.3%). The most frequent manifestations in each of them were cerebrovascular disease (6.8%), terminal chronic renal failure (4.3%), deep venous thrombosis with residual edema (4.9%) and pulmonary hypertension (1.8%), respectively (Table 2).
Characterization of the organ damage in a population of Colombian patients with SLE.
| Domains | % | Items | ||||
|---|---|---|---|---|---|---|
| Neuropsychiatric | 13 | CVA 6.8% | Convulsions 2.5% | Peripheral neuropathy 2.5% | Transverse myelitis 1.9% | Cognitive dysfunction 1.6% |
| Renal | 6.8 | TCRF 4.3% | Proteinuria>3.5g 1.9% | TFG<50% 1.2% | ||
| Vascular | 5.6 | DVT with edema 4.9% | Ulcer or stasis 1.06% | |||
| Pulmonary | 4.3 | Pulmonary hypertension 1.9% | Pulmonary fibrosis 1.2% | Pulmonary infarction 0.6% | Shrinking lung 0.6% | |
| Musculoskeletal | 3.7 | Osteonecrosis 2.5% | Osteoporosis with fracture 0.6% | Osteomyelitis 0.6% | ||
| Cardiovascular | 3.1 | Dilated cardiomyopathy 2.5% | Chronic pericarditis 0.6% | |||
| Gastrointestinal | 1.9 | Infarction 1.2% | Chronic peritonitis 0.6% | |||
| Ocular | 0.6 | Retinal infarction 0.6% | ||||
CVA: cerebrovascular accident; TCRF: terminal chronic renal failure; DVT: deep venous thrombosis.
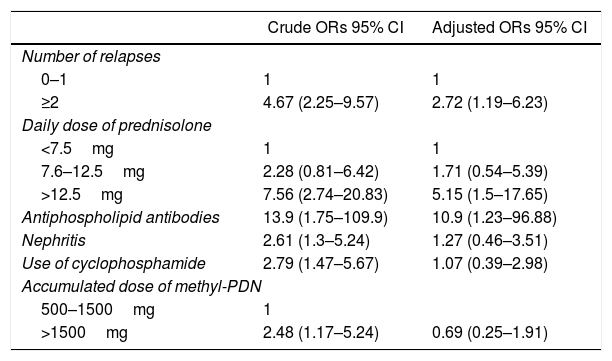
Male gender, lupus nephritis, arterial hypertension, SELENA SLEDAI at baseline and during the evolution, APL, number of ACR criteria and relapses, use of azathioprine and cyclophosphamide and daily doses of prednisolone and accumulated of methylprednisolone were associated with the damage in the univariate analysis and were included in the logistic regression model.
Having presented 2 or more relapses during the evolution (adjusted OR: 2.72; 95% CI: 1.19–6.23), having received a mean dose of prednisolone higher than 12.5mg/day in the course of the disease (adjusted OR: 5.15; 95% CI: 1.5–17.65) and the presence of APL (adjusted OR: 10.9; 95% CI: 1.23–96.88) were the independent determinants of irreversible organ damage in the population (Table 3).
Variables that explain the organ damage in the population.
| Crude ORs 95% CI | Adjusted ORs 95% CI | |
|---|---|---|
| Number of relapses | ||
| 0–1 | 1 | 1 |
| ≥2 | 4.67 (2.25–9.57) | 2.72 (1.19–6.23) |
| Daily dose of prednisolone | ||
| <7.5mg | 1 | 1 |
| 7.6–12.5mg | 2.28 (0.81–6.42) | 1.71 (0.54–5.39) |
| >12.5mg | 7.56 (2.74–20.83) | 5.15 (1.5–17.65) |
| Antiphospholipid antibodies | 13.9 (1.75–109.9) | 10.9 (1.23–96.88) |
| Nephritis | 2.61 (1.3–5.24) | 1.27 (0.46–3.51) |
| Use of cyclophosphamide | 2.79 (1.47–5.67) | 1.07 (0.39–2.98) |
| Accumulated dose of methyl-PDN | ||
| 500–1500mg | 1 | |
| >1500mg | 2.48 (1.17–5.24) | 0.69 (0.25–1.91) |
29.8% of patients suffered damage, with an incidence rate of 1 per every 10 patients/year; in 15% of cases the damage was severe. The most affected domains were neuropsychiatric, renal and peripheral vascular. APLs, mean doses of prednisolone higher than 12.5mg/day and the number of relapses were the explanatory variables of the outcome. The broad confidence interval of the OR for the APLs is attributed to the sample size.
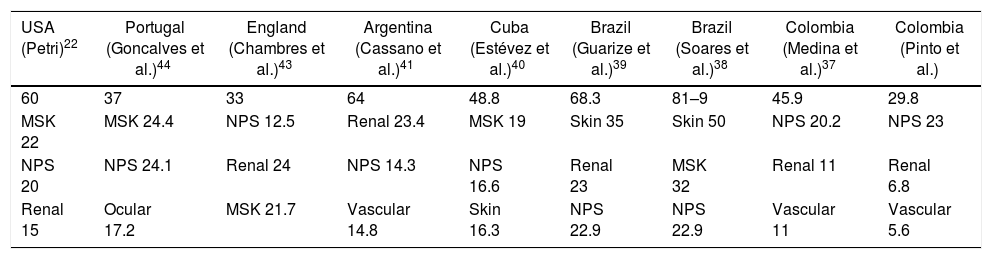
The rate of damage accumulation and the affected domains in the population are different from those observed in other cohorts in which the musculoskeletal system is the most involved,31–33 followed by the skin, kidneys and central nervous system.34–36 The findings of organ damage in the studied population and other cohorts are compared in Table 4.
Comparison of the main domains of organ damage committed in several cohorts.
| USA (Petri)22 | Portugal (Goncalves et al.)44 | England (Chambres et al.)43 | Argentina (Cassano et al.)41 | Cuba (Estévez et al.)40 | Brazil (Guarize et al.)39 | Brazil (Soares et al.)38 | Colombia (Medina et al.)37 | Colombia (Pinto et al.) |
|---|---|---|---|---|---|---|---|---|
| 60 | 37 | 33 | 64 | 48.8 | 68.3 | 81–9 | 45.9 | 29.8 |
| MSK 22 | MSK 24.4 | NPS 12.5 | Renal 23.4 | MSK 19 | Skin 35 | Skin 50 | NPS 20.2 | NPS 23 |
| NPS 20 | NPS 24.1 | Renal 24 | NPS 14.3 | NPS 16.6 | Renal 23 | MSK 32 | Renal 11 | Renal 6.8 |
| Renal 15 | Ocular 17.2 | MSK 21.7 | Vascular 14.8 | Skin 16.3 | NPS 22.9 | NPS 22.9 | Vascular 11 | Vascular 5.6 |
Data in percentages.
MSK: musculoskeletal; NPS: neuropsychiatric.
Medina et al.37 found damage in 45.9% of the cases in a cohort of Colombian patients; the most involved domains were: neuropsychiatric (20.2%), renal (11%), peripheral vascular (11%), pulmonary (9.2%) and musculoskeletal (6.4%), similar to what we observed. In addition, they found a correlation between the educational level, socio-economic stratum and physical component of SF-36, and the SLICC-ACR SDI score, but they do not evaluate the factors independently associated with organ damage.
In Brazilian patients, the most compromised domain is the cutaneous. Soares et al.38 found an association between damage and the number of ACR criteria, APL, age, methylprednisolone and duration of the use of steroids. The most affected domains were the skin, musculoskeletal and neuropsychiatric. Guarize et al.39 observed that the damage was mainly cutaneous, followed by renal and neuropsychiatric.
In a series of Cuban patients,40 48.8% presented damage, mainly musculoskeletal, neuropsychiatric and cutaneous, and the associated factors were high doses of prednisolone, leukocytopenia and duration of the disease.
36% of a population of Argentinian patients41 had damage one year after the diagnosis of SLE, 46% at 2 years, 64% at 5 years and 81% at 10 years. The most affected domains were the renal, neuropsychiatric, cardiovascular and musculoskeletal, while in a cohort of Mexican patients with severe SLE, 73% had damage and the most involved systems were the neuropsychiatric, renal, cutaneous and musculoskeletal.42
In the United Kingdom43 there is an incidence of damage of 10% in the first year of disease, 33% at 5 years and 50% at 10 years; the most involved domains are neuropsychiatric, renal and musculoskeletal. In a national cohort of Portugal,44 damage is observed in 37% of the patients, mainly musculoskeletal, neuropsychiatric and ocular. The advanced age, duration of the disease, nephritis, APL and use of steroids are associated with the damage.
An association between male gender and the accumulation and severity of the organ damage has been found in patients with SLE.16,38,45,46 However, Yee et al.23 did not observed more damage in men, similarly to that was presented in the cohort studied by us, although this finding could have been influenced by the little representation of men in the population.
In the GLADEL cohort14 the patients with late-onset SLE have more ocular, pulmonary and cardiovascular damage, and higher mortality, but less cutaneous damage than those diagnosed before the age of 50 years. In the LUMINA cohort15 they have less activity, but higher mortality and damage accumulation, especially CVD, cataracts and pulmonary fibrosis.
Brazilian patients47 with late-onset SLE have lower activity, but greater ocular, renal, musculoskeletal, peripheral vascular and cardiovascular damage, diabetes and neoplasms than those diagnosed before the age of 50 years.
On the contrary, in a Canadian cohort48 the patients with late-onset SLE do not accumulate more organ damage, similarly to what was observed in the study population in which this subgroup did not show a higher frequency of damage or higher SDI score. The small proportion of patients with onset of SLE after the age of 50 years included in the series and the short follow-up time could influence this finding.
The number of ACR criteria49 has been associated with the frequency of organ damage. In our population, less damage was observed in the patients with 4–6 criteria than in those with 7 or more, but the difference was not significant.
There is a relationship between nephritis and irreversible damage in Asian,2,50 Hispanic,51 Caucasian10,52 and Afro-Caribbean53 patients with SLE. In the study population, the univariate analysis showed that patients with nephritis had a higher risk of organ damage, but there was no association with this outcome in the multivariate analysis. Probably the short follow-up time explains this finding, since the renal domain was the second most compromised in the cohort.
The use of azathioprine, cyclophosphamide4,5,11,12 and corticosteroids21,22,31,33 is associated with damage; antimalarials are protective factors5,28 showing that they reduce the risk of damage, thrombosis, terminal chronic renal failure and death.5,27,28 In the study population, a mean dose of prednisolone higher than 12.5mg/day was an independent risk factor for suffering organ damage. Antimalarials did not show up as protectors, probably because most patients were treated with these drugs. Azathioprine, cyclophosphamide and mycophenolate did not behave as risk factors.
The APLs predict organ damage, especially neuropsychiatric.54 In a population of Caucasian patients,24 54% accumulates damage at 5 years; APLs are the only antibodies associated with this outcome and antiphospholipid syndrome is the main predictor of damage and death.28 Similarly, the presence of APL was the main factor that explained the presentation of organ damage in the population studied by us. Probably this association would explain why the neuropsychiatric domain was the most compromised and the CVD its most frequent manifestation. The cases of CVD, pulmonary infarction, deep venous thrombosis, transverse myelitis, retinal infarction and infarction of the gastrointestinal tract could be related with states of hypercoagulability mediated by antibodies other than APLs.
Several antibodies have been associated with organ damage.7,15 Yee et al.23 found an association between anti-Ro antibodies and ocular damage; others find an association between anti-DNA, anti-Sm and anti-Ro and the damage score,55 something that was not observed in the population studied by us.
The activity and the relapses of SLE are associated with mortality and organ damage.20,32 In our population, having 2 or more relapses was independently associated with the presence of damage.
The limitations of the research are the inclusion of patients of a single center and the observational retrospective character, which implies information biases that do not allow the results to be generalized. The fact of not having all the antibodies uniformly adds an important limitation. It was decided not to impute the missing data and we assumed the loss of statistical power that this implies. The size of the population and the short follow-up time did not allow us to detect the late manifestations of organ damage, especially cardiovascular, and other possible associated factors.
Conclusions29.8% of the patients suffered organ damage, mainly neuropsychiatric, renal and vascular. APLs, doses of prednisolone higher than 12.5mg/day and suffering 2 or more relapses explained the presentation of organ damage. Prospective studies with a larger number of subjects and a longer follow-up time are required to better characterize the accumulation of damage in the long term and its predictive factors.
Conflict of interestThe authors declare they do not have any conflict of interest.
Please cite this article as: Pinto-Peñaranda LF, Echeverri-García AF, Velásquez-Franco CJ, Mesa-Navas MA, Muñoz-Grajales C, Zuluaga-Quintero M, et al. Daño de órgano en una cohorte de pacientes colombianos con lupus eritematoso sistémico: caracterización y factores asociados. Rev Colomb Reumatol. 2018;25:85–91.