Autoimmune diseases are a group of chronic diseases in which genetic, environmental, and hormonal factors contribute to their appearance. In addition to having a broad clinical spectrum, the interpretation of the various autoantibodies and techniques used in the laboratory is also a clinical challenge. Given the complexity of these diseases, it is very important to rely on the results of laboratory tests to establish a correct diagnosis and follow-up and, in some cases even to establish a prognosis or prediction of autoimmunity. Taking all this into account, it is intended to improve the quality of life of patients by decreasing the increased morbidity and mortality in this group of diseases, especially by early diagnosis. Most rheumatological diseases are characterized by the high production of autoantibodies and acute phase reactants, which are involved in their pathophysiology, leading to systemic involvement. Among these, the most recognized are, systemic lupus erythematosus, rheumatoid arthritis, and Sjögren's syndrome. For these reasons, the objective of this project is to present a review that will help both physicians and laboratory personnel in the interpretation of the different autoantibodies in autoimmune diseases.
Las enfermedades autoinmunes son un grupo de patologías crónicas en las que factores genéticos, ambientales y hormonales, contribuyen a su aparición. Además de tener un amplio espectro clínico, la interpretación de los diversos autoanticuerpos y técnicas utilizadas en el laboratorio también son un reto clínico. Dada la complejidad de estas enfermedades, es muy importante apoyarse en las pruebas de laboratorio para establecer un correcto diagnóstico, seguimiento y, en algunos casos inclusive, establecer pronósticos o predicción de la posible aparición de autoinmunidad. Con todo esto, se pretende mejorar la calidad de vida de los pacientes disminuyendo la gran morbimortalidad de este grupo de patologías, especialmente al diagnosticarlas en etapas tempranas. La mayoría de enfermedades reumatológicas se caracterizan por la alta producción de autoanticuerpos y reactantes de fase aguda, los cuales estas implicados en su fisiopatología produciendo daño directo a nivel sistémico. Entre estas, el Lupus Eritematoso Sistémico, la Artritis Reumatoide y el Síndrome de Sjögren son las más reconocidas. Por tales motivos, el objetivo de este trabajo es hacer una revisión que permita guiar tanto a médicos como personal de laboratorio en la interpretación de los diferentes autoanticuerpos en enfermedades autoinmunes.
Autoimmune diseases are a complex and heterogeneous group of pathologies, which are the result of the interaction between genetic, epigenetic, immunological and environmental factors. About 5% of the world population is affected by organ-specific and systemic autoimmune diseases.1 It has been raised that there are different phases before the autoimmune disease occurs and the investigation on these phases points to a future where primary prevention that can delay or postpone the onset of the disease can be performed, based on models of predictability founded on the determination of autoantibodies.1,2. This is how it is considered that the appearance of autoantibodies has an important role in the evolution toward the clinical presentation of different autoimmune diseases.
The autoantibodies found in autoimmune diseases are the result of errors in the regulatory mechanisms or of a considerable increase in the self-antigens due to alteration in the regulatory mechanisms. Within the wide range of autoantibodies that have been studied, the vast majority of those that we determine in clinical practice have an important role as markers of autoimmunity and diagnosis in a specific clinical context. On the contrary, within this group of tests, very few are indicators of disease activity (for example, anti-double-stranded DNA antibodies in systemic lupus) and pathogenic.3 In this article we will describe the most important and commonly used antibodies in the clinical context, highlighting the most relevant characteristics and the role they play in the diagnosis, prediction, follow-up and prognosis of different autoimmune diseases.
MethodsLiterature search methodsA systematic literature search was conducted from June 2017 to October 2017, including articles published from 1970 until 2017. Different online databases such as Medline, Google Scholar, Scielo, Clinical Trials, Academic Search Ultimate, Clinics Review Article and Embase were consulted. The search in Pubmed was carried out using the MeSH terms: Autoinmunidad, Autoanticuerpos, Lupus eritematoso sistémico, Artritis reumatoide, Síndrome de Sjögren, Inmunofluorescencia indirecta, Enzimoinmunoanálisis (Autoimmunity, Autoantibodies, Systemic lupus erythematosus, Rheumatoid arthritis Sjögren's syndrome, Indirect immunofluorescence, Enzyme Immunoassay). These terms were linked with the Boolean connector AND. Those articles published before 1970 were excluded, and only articles published in English or Spanish were included.
Selection of articles and information extractionThe selected articles were stored in an electronic database; initially, those articles that had the keywords included in the abstract or in the title were taken into account. Subsequently, those articles which did not meet the inclusion criteria or the methodological quality were discarded, and a committee among the different authors was held in order to unify the database and choose those articles that were relevant for this publication.
Inclusion criteria- 1.
Types of study: Topic reviews, case-control, randomized and non-randomized studies, cohort studies, case reports and institutional protocols.
- 2.
Type of population: Adult healthy patients and with autoimmunity.
- 3.
Intervention: Studies describing history, laboratory techniques, applicability and clinical function of the antibodies.
- 1.
Articles without access to the full text.
- 2.
Duplicate articles.
- 3.
Studies that were not conducted in humans.
- 4.
Articles published before 1970.
At the end of the search, a total of 124 articles were found by all researchers. Excluding those duplicate or without access, a total of 77 articles were obtained. Due to the large amount of literature that currently exists and to the new changes in the interpretation of the different patterns of antinuclear antibodies (ANA), it was decided to conduct this work with the purpose of unifying the most relevant literature around this topic and to guide both physicians and laboratory personnel in the interpretation of the different autoantibodies in autoimmune diseases.
DiscussionAntinuclear antibodiesANAs are a broad group of autoantibodies that recognize macromolecules integrated in the structure of the cell nucleus and some cytoplasmic components.4 In 1948, the hematologist Malcolm Hargreaves described an observational finding present in bone marrow cells from patients with systemic lupus erythematosus (SLE), which he named “LE cells”.5 This would be the first step for the research on this phenomenon and for subsequent advances in antibody identification techniques. The ANAs were identified initially by immunofluorescence using mouse liver tissue; however, these initial identification attempts were not successful, due to the presence of technical difficulties such as the autofluorescence of the tissues used and the variability of the immunofluorescence patterns, among others. It was not until 1970 when HEp-2 cells, human epithelial lines derived from laryngeal carcinoma,5 began to be used in this test, since they allowed a better performance of the indirect immunofluorescence for the detection of ANA.
The ANAs can be classified depending on the different structures they recognize: nucleosome, non-histone proteins associated with DNA, non-histone proteins associated with RNA or extractable nuclear antigens (ENAs), nucleolus and cytoplasmic antigens.6
The method used for the identification of the ANAs in HEp-2 cells is, by convention, the indirect immunofluorescence (IIF), although new techniques for the detection of these antibodies have been developed currently, the former is still considered the gold standard.7 IIF is a method accessible in many laboratories and easy to reproduce. For this test is required the serum of the patient, which is initially diluted in 1/40 or more, and then is added to the preparation of HEp-2 cells, thus allowing the patient's antibodies to bind with the target antigens present in these cells. A wash is carried out then with a buffer solution, and subsequently, a solution with anti-human IgG coupled to a fluorochrome that binds to the antigen/antibody complex present in the sample is applied. Finally, after a second wash that removes the fluorescent antibodies that did not bind, the result can be observed by means of an ultraviolet light microscope. HEp-2 cells are ideal for this type of test, since they are easy to grow and they grow forming a monolayer, which allows their visualization through the fluorescence microscope. In addition, they have a bigger nucleus than the one of any normal epithelial cell, which makes easier the visualization of the nuclear and cytoplasmic patterns. Moreover, these cells allow to detect antibodies against cell cycle-dependent antigens. However, it is important to take into account that the detection of ANA by IIF is a screening test, which after being positive requires a second test that increases its specificity, by means of radioimmunoassay, ELISA, electroimmunotransfer or Western blot, in order to determine the antigenic specificity to which the antibodies are addressed.8,9
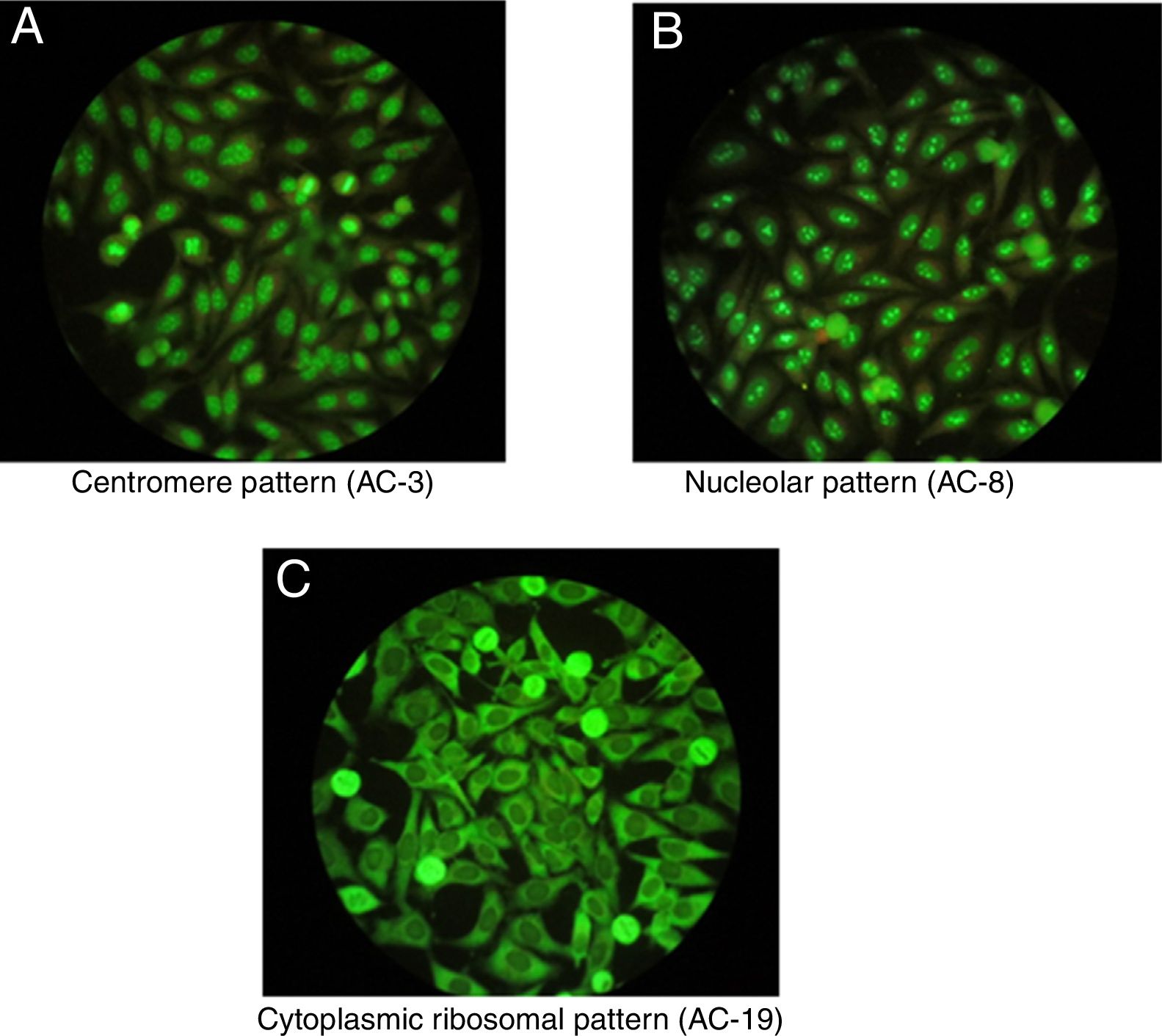
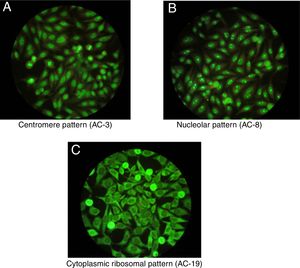
One of the most important characteristics of the IIF test is that it allows to identify different conventional patterns that will guide the clinical interpretation of the test and the management to follow. Due to the great variety of patterns and the complexity of some of them, an expert consensus met in 2016 to discuss a universal nomenclature that allows to standardize the reading and interpretation of the ANA by the IIF method. Table 1 summarizes the most important patterns based on this consensus, which gives each of them a numbering from AC-1 (anti-cell) to AC-28, separated in nuclear, cytoplasmic and mitotic patterns.10 The most common patterns are: (1) the homogeneous or diffuse pattern, in which a homogeneous staining is observed in the nucleus of the cell, when this pattern occurs is due to the presence, generally, of antibodies against the deoxyribonucleoprotein or histones6; (2) the peripheral pattern, showing a stain that is regular around the nucleus, with the center less stained than the periphery; this pattern indicates, in general, the presence of anti-double-stranded DNA, which has a high specificity for SLE6; (3) the speckled pattern is the most common and there are 2 types, the coarse speckled pattern and the fine speckled pattern which indicates de presence of anti-ENA antibodies8; (4) the nucleolar pattern characteristically stains intensely the cell nucleoli and indicates the presence of antibodies against the components of the nucleolus; the possible antigens to take into account are: Scl-70 and RNA polymerases I, II, and III. This pattern has clinical relevance since it is more frequent in the diffuse forms of scleroderma and is associated with greater renal and pulmonary involvement11; (5) the cytoplasmic pattern indicates the presence of antibodies against cytoplasmic components such as mitochondria, ribosomes and cytoskeletal proteins. Finally, (6) the centromere pattern which is mainly associated with the form of limited scleroderma previously known as CR syndrome.
Conventional patterns of ANA and their relationship with autoantibodies according to the first international consensus of standardized nomenclature.
| Pattern | Related specific antigen | Related disease or clinical condition |
|---|---|---|
| Nuclear | ||
| Homogeneous (AC-1) | Double stranded DNA, chromatin, histones, nucleosomes | SLE, drug-induced lupus, juvenile idiopathic arthritis |
| Speckled (AC-2, 4, 5) | ||
| Dense fine speckled (AC-2) | DFS-70, LEDGF | Prevalent in healthy individuals |
| Fine speckled (AC-4) | SS-A/Ro, SS-B/La, RNA polymerase ii and iii, Ku, Ki, Mi-2, topo 1(Scl-70) | SLE, SS, SSc, inflammatory myopathies, MCTD |
| Coarse speckled (AC-5) | hnRNP, U1RNP, Sm, RNA polymerase iii | SLE, MCTD, SSc |
| Discrete nuclear dots | ||
| Centromere (AC-3) | Anticentromere CENP-A and CENP-B | Limited cutaneous sclerosis, PBC |
| Multiple nuclear dots (AC-6) | Sp100, promyelocytic leukemia protein | PBC, systemic autoimmune diseases, polymyositis, dermatomyositis |
| Few nuclear dots (AC-7) | p80-coilin, SMN | SLE, SSc, polymyositis, SS, asymptomatic individuals |
| Nucleolar (AC-8, 9, 10) | ||
| Homogeneous (AC-8) | PM/Scl-75, PM/Scl-100, Th, To, nucleolin, U3-snoRNP/fibrillarin | SSc, polymyositis with SSc overlap |
| Clumpy (AC-9) | U3-snoRNP/fibrillarin | SSc |
| Punctate (AC-10) | RNA polymerase I | SS, SSc |
| Nuclear membrane (AC-11, 12) | ||
| Smooth nuclear membrane (AC-11) | Nuclear lamins A, B, C or lamin-associated proteins | SLE, SS, seronegative arthritis |
| Punctate nuclear membrane (AC-12) | Nuclear pore associated proteins | PBC |
| Pleomorphic (AC-13, 14) | ||
| PCNA-like (AC-13) | PCNA | SLE |
| CENP-F-like (AC-14) | CENP-F | Cancer |
| Cytoplasmic | ||
| Fibrillar (AC-15, 16, 17) | ||
| Actin/linear (AC-15) | Actin, myosin not related with the muscle | MCTD, active chronic hepatitis, hepatic cirrhosis, myasthenia gravis, Crohn's disease, PBC, hemodialysis |
| Filamentous/microtubules (AC-16) | Vimentin, cytokeratin | Inflammatory conditions or infection, hemodialysis, alcoholic liver disease, psoriasis, healthy controls |
| Segmental (AC-17) | Alpha-actin, vinculin, tropomyosin | Myasthenia gravis, Crohn's disease, ulcerative colitis |
| Speckled (AC-18, 19, 20) | ||
| Discrete dots (AC-18) | GW182, Su/Ago2, Ge-1 | PBC, autoimmune diseases of the CNS |
| Dense fine speckled (AC-19) | PL-7, PL-12, ribosomal p protein | Antisynthetase syndrome, polymyositis, dermatomyositis, SLE, neuropsychiatric lupus |
| Fine speckled (AC-20) | Jo-1/histidyl-tRNA synthetase | Antisynthetase syndrome, polymyositis, dermatomyositis, limited cutaneous sclerosis |
| Reticular/AMA (AC-21) | PDC-E2/M2, BCOADC-E2, OGDC-E2, E3BP/protein X | PBC, SSc |
| Polar/Golgi-like (AC-22) | Giantin/macrogolgin, golgin-95/GM130, golgin-160, golgin-97, golgin-245 | SS, SLE, AR, MCTD, idiopathic cerebellar ataxia, viral infections |
| Rods and rings (AC-23) | IMPDH2 | Hepatitis C post-therapy with IFN/ribavirin, SLE, Hashimoto's thyroiditis |
| Mitotic | ||
| Centrosome (AC-24) | Pericentrin, ninein, Cep250, Cep110, enolase | SSc, Raynaud's phenomenon, infections (viral and mycoplasma) |
| Spindle fibers (AC-25) | HsEg5 | SS, SLE |
| NuMA-like (AC-26) | Centrofilin | SS, SLE, other |
| Intracellular bridge (AC-27) | Aurora kinase B, CENP-E, MSA-2, KIF-14, MKLP-1 | SSc, Raynaud's phenomenon malignancy |
| Mitotic chromosome layer (AC-28) | Modified histone H3, MCA-1 | Discoid lupus, chronic lymphocytic leukemia, SS, polymyalgia |
RA: rheumatoid arthritis; PBC: primary biliary cirrhosis; MCTD: mixed connective tissue disease; IFN: interferon; SLE: systemic lupus erythematosus; CNS: central nervous system; SS: Sjögren's syndrome; SSc: systemic sclerosis.
Once positive ANAs are detected in a patient, not only their presence and specificity should be addressed, but also their titles and their association with the clinical symptoms the patient presents. It is important to highlight that certain ANAs have such high specificity that are considered predictive for certain autoimmune diseases, even before developing signs or symptoms, such as the nucleolar pattern that is usually found in systemic sclerosis in its diffuse form.12 In addition, to interpret the data of the test it should be taken into consideration that the general healthy population presents positive ANAs in up to 32% with titers 1:40, 13% with titers 1:80 and 5% with titers 1:160,3,7,12 which is why the American College of Rheumatology (ACR) considers titers higher than 1:160 as positivity.13Fig. 1 shows some patterns by IIF.
Anti-double stranded DNA antibodiesAnti-DNA antibodies are immunoglobulins directed against the DNA, pure or in complex with proteins such as histones. These antibodies are a heterogeneous group of immunoglobulins that have different specificities, and are classified as anti-ssDNA (single-stranded DNA) and anti-dsDNA (double stranded DNA). Anti-ssDNA antibodies are the most commonly identified, however, due to their low specificity they have very little clinical relevance. In this way, anti-dsDNA antibodies have greater importance given by their high specificity in the diagnosis of SLE, specifically in those patients with lupus nephritis.14,15
Different special techniques have been developed for the detection of these antibodies, among which the most commonly used are IIF using the flagellate organism Crithidia luciliae (CLIFT), Farr immunoprecipitation, and ELISA. Each of these tests has different sensitivity and specificity, being CLIFT the most specific (97.2%), but with the lowest sensitivity (33.6%). ELISA presents an intermediate sensitivity (55.8%) with the lowest specificity (92.5%) and in turn, Farr immunoprecipitation has the highest sensitivity (up to 85%) and a specificity relatively comparable with that of CLIFT (96.7%).16–20 Despite this, in many laboratories is preferred the use of CLIFT because this test is easy to carry out in a laboratory equipped for autoimmune serological tests, and it also allows to determine the classes of anti-DNA antibodies and it does not produce cross-reaction with anti-ssDNA antibodies.5 Due to the differences in the techniques, with variable results in terms of sensitivity and specificity, it is advisable to determine this antibodies at least by 2 techniques.21 The clinical usefulness of the anti-dsDNA is based on the diagnostic support facing a patient with suspected SLE, as a follow-up method or as a marker of future relapses of the disease.3 Therefore, a patient with suspected SLE and with an ANA positive result requires a test of specificity that evaluates anti-dsDNA antibodies, which may be present in two thirds of patients (60–83%) with SLE. Another aspect to take into account within the clinical usefulness of these antibodies is that they can be present up to a year or more before the first clinical manifestations. In addition, they are highly associated with the disease activity, especially with lupus nephritis, and hepatic and neurological commitment.21–23
Anti-ribosomal P antibodiesAnti-ribosomal P antibodies are directed against 3 types of phosphoproteins (P0, P1 and P2) which are found in the 60S ribosomal subunit.22 These antibodies are considered specific for SLE and are detected in 12–16% of patients with this disease, including a subgroup of patients with negative anti-dsDNA.23 There are different methods for the detection of these antibodies such as ELISA, IIF, analysis of solid phase antibodies and Western blot. It is important to emphasize that this different tests, such as ELISA and IIF which are the most commonly used, cannot be compared with each other and are dependent on the titers of these antibodies, so when the titers are higher, there is greater correlation between different methods. Anti-ribosomal P antibodies are especially related with the presence of neuropsychiatric manifestations of SLE, such as psychosis and depression.24 In 1987 Bonfa et al. documented the association between this antibody and psychiatric manifestations in patients with psychosis secondary to SLE.21 Starting from this first description, multiple studies have confirmed this association. In addition to the relationship with psychosis, this antibody is associated with an increased risk for future lupus psychosis in recently diagnosed patients.24 It has also been reported a relationship of this antibody with lupus nephritis, especially when it is found positive along with the anti-dsDNA antibody and in patients with an onset of the disease at an early age.22 Although an association of this antibody with liver disease has been reported, the literature is scarce and more research regarding this topic is required.
Anti-extractable nuclear antigen antibodiesENA antibodies have their name because the identification of ANAs was initially made by purifying nuclear proteins through extraction techniques that used saline solutions. There are more than 100 antigens identified in this group, but those with greater clinical relevance are SSA (anti-Ro), SSB (anti-La), RNP-U1/Sm, Sm, Scl70 and Jo-1.3,25 The main characteristic of these antibodies is that they guide the clinician to discriminate between different types of systemic autoimmune diseases and thus reach the diagnosis and even, in some cases, they provide information about the prognosis of the patient.26
Anti-Ro antibodiesAnti-Ro or SSA (anti-Sjögren's syndrome related antigen A) antibodies are immunoglobulins against proteins of 52kD and 60kD associated with RNA.3,9 These autoantibodies are found in several autoimmune diseases such as Sjögren's syndrome (SS), SLE, and rheumatoid arthritis (RA), among others. The anti-Ro antibody is found in approximately 40-95% of patients with SS, and it is mainly directed against the 52kD antigen, which is part of the classification criteria of this autoimmune disease.26 On the other hand, it is present in 25–50% of patients with SLE3 and is related to the presence of subacute cutaneous lupus, sicca syndrome, nephritis and cytopenias. Furthermore, the patients with RA that have this antibody have an increased risk of sicca syndrome. These antibodies are also associated with neonatal lupus since they have been found positive in up to 100% of mothers who have this complication.6 They are also associated with congenital atrioventricular block, so it is recommended to carry out this screening to pregnant patients with autoimmune diseases. Fortunately, the probability of presenting this complication is very low, around 2–3% of cases.6,27
Anti-La antibodyThe anti-La or SSB antibody is an immunoglobulin against the La protein of 45kD, which is part of the Ro/La antigenic complex, which is constituted by 52kD Ro, 60kD Ro and 45kD La. The biological function of the La protein is to act as a chaperone of the RNA and participate in the metabolism therein, especially in the termination of the RNA polymerase III. These autoantibodies are mainly associated with SS and are found in 50–87% of the patients. In SLE they are associated to a lower percentage, 10–20%, and in other connective tissue disease they are much less frequent.22–28 It should be noted that these antibodies are rarely found alone, since in the majority of cases they appear positive along with the anti-Ro, generated by an immune response with epitope spreading. That is why is indicated to measure these antibodies when there is a desire for conception by women with a diagnosis of SLE, as well as in patients with suspected SLE with negative ANAs or with suspected SS.
Anti-Sm and anti-RNP antibodiesAnti-Sm antibody is an immunoglobulin directed against small nuclear ribonucleoproteins (snRNP) which are part of the spliceosome (multiprotein complex responsible for RNA splicing). It has been found that it is the most specific antibody for SLE, with a specificity close to 97%.3 However, it is found only in 5–30% of the patients with this disease, so its absence does not rule it out.9 Due to its high specificity it is part of the classification criteria of the Systemic Lupus International Collaborating Clinics (SLICC), as well as of the ACR criteria for the classification of SLE.13 These antibodies are considered clinically relevant in the context of a patient with suspected SLE and who has a negative anti-dsDNA.5 In most cases these antibodies are found positive along with the anti-RNP.
Anti-RNP antibodies react against associated proteins such as U1RNA and the U1 snRNP form, that are also part of the spliceosome.29 These antibodies are found in 25–50% of the patients with SLE, but they can also be found in different autoimmune diseases. However, it is considered that high titers of this autoantibody are associated with mixed connective tissue disease (MCTD), especially when the presence of some other autoantibody has been ruled out, being part of the classification criteria for this entity. On the other hand, it has been found an association of this antibody with Raynaud's syndrome, edema in the fingers of the hands and leukopenia.30
Anti-Scl-70 or anti-topoisomerase I antibodyAnti-topoisomerase i or Scl-70 antibodies are a subtype or ENA, that have been described since 1979, which are characterized by being directed against a non-histone nuclear protein of 70kD.31 They produce a nucleolar or fine speckled pattern by the measurement of ANA by IIF.4 Their clinical importance lies in the fact that they are found in patients with systemic sclerosis in its diffuse form in 40-64%, as well as in its limited form, the CREST syndrome, in 10–34%. Therefore, they are a great help in the diagnosis of this disease, since they have a high specificity for it (99.6%).5,32 In addition, they have been associated with other manifestations of the entity such as pulmonary fibrosis, intestinal symptoms, heart block, diastolic dysfunction and renal crisis of the sclerosis.31,32 This antibody is associated with poor prognosis and increased mortality due to right heart failure secondary to pulmonary fibrosis and restrictive lung disease.3
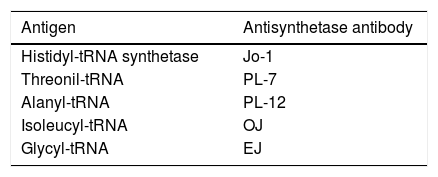
Anti-Jo-1 antibodiesThe anti-Jo-1 antibody is directed against the enzyme histidyl-tRNA transferase. This antibody, associated with the presence of other anti-tRNA synthetase antibodies, is found in patients with myositis that characteristically present with arthritis, Raynaud's phenomenon, fever and interstitial lung disease, defining a subtype of pathology known as antisynthetase syndrome.33 The latter has been recognized in recent years as an important cause of inflammatory myopathies. The severity and extent of the disease usually vary, with myositis being a little less severe in the absence of this syndrome. This disease is more frequent in women, with an average age of onset at 45 years. The morbidity and mortality usually depend on the pulmonary involvement.34 In Table 2 are described the most important antibodies related to this syndrome and their respective antigens.
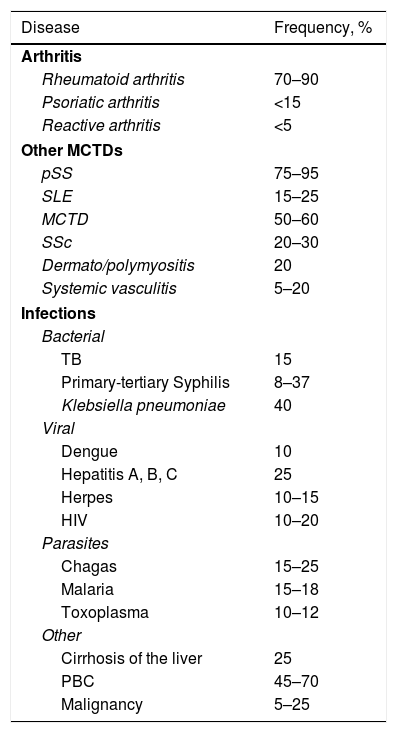
Rheumatoid factorThe rheumatoid factor (RF) is an autoantibody directed against the Fc portion of the IgG immunoglobulins. Although several isotypes have been described, including IgG, IgA, IgE, IgD and IgM, the IgM isotype is the most commonly measured in clinical practice, and corresponds to the classical RF. The RF was detected initially in patients with RA, and therefore received its name; however, it was later found in patients with other autoimmune and non-autoimmune diseases and even in healthy subjects.35. In Table 3 are shown the different diseases that occur with RF positivity.35
Frequency of positive rheumatoid factor in different clinical conditions.
| Disease | Frequency, % |
|---|---|
| Arthritis | |
| Rheumatoid arthritis | 70–90 |
| Psoriatic arthritis | <15 |
| Reactive arthritis | <5 |
| Other MCTDs | |
| pSS | 75–95 |
| SLE | 15–25 |
| MCTD | 50–60 |
| SSc | 20–30 |
| Dermato/polymyositis | 20 |
| Systemic vasculitis | 5–20 |
| Infections | |
| Bacterial | |
| TB | 15 |
| Primary-tertiary Syphilis | 8–37 |
| Klebsiella pneumoniae | 40 |
| Viral | |
| Dengue | 10 |
| Hepatitis A, B, C | 25 |
| Herpes | 10–15 |
| HIV | 10–20 |
| Parasites | |
| Chagas | 15–25 |
| Malaria | 15–18 |
| Toxoplasma | 10–12 |
| Other | |
| Cirrhosis of the liver | 25 |
| PBC | 45–70 |
| Malignancy | 5–25 |
PBC: primary biliary cirrhosis; MCTD: mixed connective tissue disease; SLE: systemic lupus erythematosus; SSc: systemic sclerosis; pSS: primary Sjögren's syndrome; TB: tuberculosis; HIV: human immunodeficiency virus.
Under physiological conditions, the antibodies with RF activity perform functions such as clearance of immune complexes, improve the presentation of antigens and neutralize certain pathogens (herpes simplex virus and trypanosome).36 In healthy patients the prevalence can be as high as 30%, although it varies according to the racial population. In healthy population, the titers of RF are low and are produced mainly by CD5 B lymphocytes (BL) of low affinity.35
In non-autoimmune conditions where a positive RF is detected, such as in some acute and chronic infections, the RF detected is transient and not harmful. This is attributed to the fact that under physiological conditions, these antibodies have the ability to increase the clearance of immune complexes and the RF-producing BLs can behave as antigen-presenting cells in response to infectious microorganisms. The infection agent that has been most related with high levels of RF is the hepatitis C virus35; although other microorganisms related with the presence of RF are tuberculosis, syphilis and leprosy.37
In addition, it has been implicated in the pathogenesis of several autoimmune diseases because it forms immunocomplexes and efficiently activates the complement.36 The autoimmune diseases in which the presence of RF has been documented are: SS, cryoglobulinemia, SLE, and MCTD, among others; and those that are most frequently associated with high titers of RF, in addition to RA, are mixed cryoglobulinemia and SS. It is even believed that the activation of RF-producing BL clones is involved in the pathogenesis and development of lymphoproliferative disorders in about 5% of patients with this condition.38
In RA the RF has a sensitivity of 60-90% and a specificity of 75%.35 The 3 isotypes (IgM, IgA and IgG) can be detected in up to 52% of patients who suffer from this autoimmune disease, but in other connective tissue diseases they are present in only 5% of the patients. The presence of RF of IgA and IgG isotypes in the absence of IgM is more prevalent in MCTD than in RA. On the other hand, the presence of RF of both IgM and IgA isotypes is something almost exclusive of RA.35
As for RA, different aspects in which the measurement of RF titers had a clinical impact have been detected, including:
- •
The presence of high titers of RF is correlated with a higher risk of developing RA. The risk can increase up to 26-fold if the initial titers were >100IU/mL.39
- •
The presence of the IgA isotype is related with extra-articular manifestations, including vasculitis39.
- •
The presence of RF in patients with RA is associated with more aggressive forms of the disease and greater severity of functional disability, given by a more erosive disease and presence of rheumatoid nodules.39
- •
Some studies have demonstrated that the immunosuppressive therapy can decrease the levels of RF, but is clinically useless to measure the titers of RF for the follow-up of the disease.35,39
- •
The measurement of the titers of RF to predict the response to treatment is considered limited and it has been found variability between different studies, so it continues under research.40,41
- •
Patients with high titers of RF are benefited from the BL-reducing therapy, such as rituximab.35,42
Due to the large number of diseases in which the presence of RF can be found, it is important to be clear about the clinical scenarios in which the test should be requested; and only perform it in a clinical context.3 The test should be performed on a patient with:
- •
Early inflammatory arthritis without apparent cause.
- •
Clinical suspicion of RA.
- •
Before starting anti-BL therapy in a patient with RA.
- •
Dry symptoms (dry syndrome).
- •
Pediatric patient with chronic polyarthritis.
There are several methods for the measurement of the RF. Agglutination techniques were used initially and among them, the most widely used was the latex agglutination test, which is an inexpensive and easy to carry out test that provides qualitative information. Subsequently, methods that provide quantitative information were developed, such as the measurement by nephelometry, turbidimetry and ELISA. Nephelometry and turbidimetry do not allow to identify isotypes, unlike the measurement by ELISA which allows to identify the different isotypes of immunoglobulins. However, the ELISA test reaches a sensitivity of 53% and a specificity of 99% in the diagnosis of RA.43
New diagnostic methods using immunoassay techniques have been developed currently; within these, there is the measurement of RF by electrochemiluminiscence, which provides a quick measurement, a high sensitivity and specificity, and a wide range of measurement with a reduced sample volume.
The result of the measurement of the RF depends on the technique with which the measurement is carried out, which varies according to the laboratory. However, the World Health Organization suggested to standardize the result, and every laboratory should report the result in international units: values above 20IU/mL are positive and values above 50IU/mL are considered high.3 But at present, the interpretation of the results depends on the laboratory and the reagents used, considering normal values up to 14IU/mL and in other laboratories <8IU/mL.
Anti-cyclic citrullinated peptide antibodiesConsidering that RA is a chronic, autoimmune disease that affects 1–2% of the world population and has a high impact on the morbidity and mortality of the individuals who suffer from it, it is necessary to make sure an accurate and timely diagnosis.44 Initially, the RF was the only diagnostic laboratory test available for this disease. However, due to the difficulties associated with the interpretation of the RF mentioned above (mainly the low specificity), from the sixties of the past century it was started to look for antibodies with greater specificity for the diagnosis of this condition. In this way, and after finding several antibodies that showed a higher specificity (such as the perinuclear factor and the anti-keratin antibodies) it was described in 1998 that in patients with RA was found the production of antibodies against proteins with high content of citrulline (which corresponded to the antigen of the previously mentioned antibodies). These descriptions allowed for the development of a more specific diagnostic test using synthetic cyclic citrullinated peptide by means of the ELISA technique, which is why they were called anti-cyclic citrullinated peptide (CCP) antibodies. These antibodies can be detected in 80% of patients with RA with a specificity greater than 98%.44 The anti-CCPs are antibodies directed against citrullinated proteins such as filaggrin, vimentin, enolase A, fibrinogen, collagen type I and II, actin, histones and heat shock proteins HSP90, among others.45 The anti-CCP antibodies are mostly of the IgG isotype, although IgA, IgM and IgE isotypes can also be found.46,47
The first anti-CCP test that was created used as antigen a cyclic peptide derived from filaggrin, but it was not present in the swollen joints. For this reason it was created the second generation anti-CCP test, in which about 12 million of peptides were isolated, choosing the best citrullinated peptides and thus generating the test. Subsequently, it was introduced the term of anti-citrullinated peptide antibodies (ACPA), which makes reference to the antibodies of RA for which the detection was carried out using citrullinated proteins/peptides of putative autoantigens associated with RA instead of filaggrin. In the second-generation anti-CCP test, the vast majority of ACPA present in patients with RA can be detected by ELISA.48 However, other tests that measure specific ACPA, such as anti-MCV (antibodies directed against mutated citrullinated vimentin) and anti-CCP3, among others, have been developed. Although new ACPA tests have been developed, the second generation anti-CCP test continues to have the highest sensitivity and specificity, which is why it is considered the gold standard and is preferred for the diagnosis of RA.49,50
The clinical impact of these antibodies is so relevant that they have been included in the 2010 classification criteria for RA and are part of a classification of RA patients: they can be classified as ACPA positive and ACPA negative.45,49,50 However, recent works indicate that although anti-CCP has a high specificity for the diagnosis of RA, it can be a prognostic factor in other diseases whose clinical evolution leads to connective tissue diseases, as is the case of palindromic rheumatism, in which approximately 30–50% of patients evolve into AR.44 In the same way, it has been found a prevalence of up to 15% in a cohort of patients with scleroderma.51
As mentioned above, the clinical utility of the ACPAs goes from the timely diagnosis and the choice of the treatment, until the prognosis in the patients who suffer from RA. Several characteristics that confer the great clinical importance of the detection of these antibodies in the spectrum of this disease have been described. First, the most significant characteristic of the presence of these antibodies from the clinical point of view is their early appearance.44 The diversification of the ACPA is an early event in the pathophysiology of RA, which occurs before the disease is clinically recognized in many cases.44,45,48,52 It has been described that 40–70% of patients with signs and symptoms of arthritis, with less than 12 weeks of evolution, have positive anti-CCP antibodies in the rheumatology consultation.44 In addition, they are associated with rapid progression to RA during the first year of follow-up, when they are detected in patients with undifferentiated arthritis.45 It has been observed than more than 90% of the patients with arthralgias without a diagnosis of RA who have a positive anti-CCP develop RA in the following 3 years.4.
Another clinical characteristic that has been described is that the presence of anti-CCP in patients with diagnosed RA is associated with the development of a more erosive disease, according to radiographic findings.45,48 Previously, it has been established a relationship between the positivity of IgM RF as a predictor of the radiographic progression of bone erosions, however, it has been demonstrated today that the positive RF is coexpressed with positive ACPA and the positive ACPA is which is associated with an erosive course.48 The RF alone does not contribute to the progression of the disease, compared to the ACPAs that indeed contribute by themselves.
On the other hand, the presence of ACPA in patients with RA has been associated with polymorphisms of the HLA-DRB1*04 gene (the main genetic risk factor involved in RA) and the presence of anti-peptidyl arginine deiminase (PAD)4 antibodies.45,53 PAD is the enzyme responsible for the citrullination of the extracellular proteins containing arginine. It is related to the pathogenesis of RA, because during an inflammatory state the cells undergo apoptosis or necrosis, releasing proteins that are susceptible to the effect of PAD. The presence of anti-PAD4 antibodies can be detected in up to 20–40% of patients with anti-CCP positive RA. In addition, it has been seen that the risk of developing ACPA is associated with environmental factors such as tobacco consumption and that the presence of the antibodies, in turn, increases the risk of developing ischemic coronary heart disease.48,54,55
The last characteristic of clinical usefulness is the capability of the anti-CCP to predict the prognosis of the RA. Certain studies confirm that patients with ACPA positive RA have higher remission rates when they are treated with methotrexate compared with ACPA negative patients.45,48 The ACPAs are the more powerful predictors of the prognosis of RA, so there are at least 4 reasons for which the test should be done and compared: because they have the ability to confirm and predict the development to RA, the radiographic progression, the remission and the response to the synthetic and biological modifying treatment. The ability to predict prognosis in patients with RA is possible because it has been described that in patients with anti-CCP positive RA there is presence of more germinal centers in synovial tissue infiltrates.48 Therefore, the patients with positive anti-CCP RA are associated with a worse prognosis compared with those with negative anti-CCP.
In summary, the clinical impact that the ACPAs have had in the scenario of the RA has been very significant, and therefore they were included within the 2010 ACR/EULAR classification criteria for RA.49,50 The aspects that should be taken into account when measuring these antibodies are:
- •
ACPAs are the most specific antibodies for the diagnosis of RA.
- •
Their presence is useful for the diagnosis and classification of RA, and is not useful for follow-up. So they should not be requested again, once the test is positive.
- •
The titers do not correlate with the disease activity.
The anti-neutrophil cytoplasmic antibodies (ANCA) were initially described in patients with pauciimmune glomerulonephritis in 1982.56 However, their clinical importance lies in their association with 3 diseases: granulomatosis with polyangiitis, microscopic polyangiitis, and they have also been associated with a large percentage of patients with eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome).
These antibodies are directed against antigens present in the granules of the cytoplasm of the neutrophils and the lysosomes of the monocytes; although there are many antigens, those that have clinical importance are myeloperoxidase (MPO) and proteinase 3 (PR3).57
During several decades, it has been questioned the pathogenic capability of the ANCA, however, this property has been demonstrated in a number of studies. What has been observed is that when the ANCA bind their target antigen, they activate the neutrophils and induce degranulation, with the consequent release of enzymes, proinflammatory cytokines and the generation of a respiratory burst that leads to endothelial damage and, eventually, to the vasculitic process.58
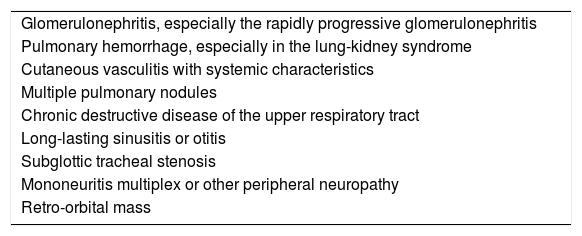
The test for ANCA measurement should be performed only as long as there is a clinical suspicion, otherwise the test has very low sensitivity. For this reason, the Clinical ANCA Test-ordering Guidelines proposed as part of the international consensus statement on taking and reporting ANCA tests of 2003 to take the test in patients who at least meet one of the clinical conditions proposed in the criteria (Table 4).57,59 When performing the test only to the patients who meet any of the criteria described, the sensitivity and specificity for the detection of the ANCA-associated vasculitis increase 95% and 90%, respectively.60
Clinical indications for the measurement of anti-neutrophil cytoplasmic antibodies.
| Glomerulonephritis, especially the rapidly progressive glomerulonephritis |
| Pulmonary hemorrhage, especially in the lung-kidney syndrome |
| Cutaneous vasculitis with systemic characteristics |
| Multiple pulmonary nodules |
| Chronic destructive disease of the upper respiratory tract |
| Long-lasting sinusitis or otitis |
| Subglottic tracheal stenosis |
| Mononeuritis multiplex or other peripheral neuropathy |
| Retro-orbital mass |
There are two techniques by which the measurement of the antibodies can be made: IIF and ELISA. Taking into account that the test by IIF is more sensitive and by ELISA is more specific, the guidelines recommend the combination of both tests for the detection of the presence of ANCA in patients with suspected ANCA-associated vasculitis.60 When the test is performed using the IIF technique, it is possible to identify certain specific patterns that have been associated with the specific antigen that the antibody recognizes. The cytoplasmic pattern (c-ANCA), which is observed under the fluorescence microscope as a diffuse staining of the granules present in the cytoplasm of the cell which is more prominent in the center between each lobe of the neutrophil, is associated with the presence of anti-PR3 antibodies. On the other hand, the perinuclear pattern (p-ANCA) shows perinuclear staining throughout the cell nucleus and is associated with the presence of anti-MPO antibodies. An atypical pattern (a-ANCA), which is described as a perinuclear staining that does not involve the entire extension of the nucleus or as a diffuse flat cytoplasmic staining has also been observed, but it is the combination of both patterns: perinuclear and cytoplasmic.61 The pattern identified by the IIF technique is related to a specific antibody which can be identified with the ELISA technique: anti-MPO for p-ANCA and anti-PR3 for c-ANCA. In the clinical context, the identification of each pattern has been associated with a specific disease. For example, the granulomatosis with polyangiitis, formerly known as Wegener's granulomatosis, has been associated with c-ANCA in 90% when the disease is active and systemic (40% in the localized form), while the microscopic polyangiitis and the eosinophilic granulomatosis with polyangiitis have been associated with p-ANCA.61 On the other hand, the a-ANCAs are not associated with vasculitis, but have been associated with exposure to drugs, inflammatory bowel disease and RA; and when performing the ELISA test, there is no presence of specific antigens.61
The international consensus established for the test obtention and reporting of the ANCAs recommends to take both tests, since, although there is a concordance of up to 85% between the 2 tests, 10% of the patients are positive only by IIF and 5% only by ELISA.57
In addition to the diagnostic value of the performance of the test, it has been studied the possibility that it would have clinical utility in the detection of the disease activity, once the diagnosis has been established. Some studies make reference to the relationship between the increase in the titers of ANCA and the relapse of the disease, however, further studies are required to corroborate this relationship.62,63 Despite this, there is currently a clinical score called BVAS, which is very useful to detect the disease activity.64
Antiphospholipid antibodiesAntiphospholipid antibodies (aPL) are a heterogeneous group of antibodies of IgG, IgM and IgA isotypes directed against phospholipids, phospholipid-protein complexes or phospholipid binding proteins, located in the membrane of endothelial cells, platelets and other cells involved in the coagulation cascade.65,66 The antigens that are recognized by these antibodies include: beta-2-glycoprotein i, cardiolipins, prothrombin, phosphatidylserine, phosphatidylinositol, annexin v, C protein, S protein, tissue plasminogen activator, factor VII, factor XI, factor XII, complement C4 component and complement factor H.67
The aPLs are part of the pathogenesis of the antiphospholipid antibodies syndrome (APS), an autoimmune systemic disease characterized by recurrent thrombosis and morbidity in pregnancy in patients with aPL.
The presence of these antibodies is essential for the diagnosis of APS, because it is required to have at least one clinical criterion and one laboratory criterion to consider the diagnosis of the disease. The above mentioned antibodies which are included in the international classification criteria for the APS are those directed against the beta-2 glycoprotein i and cardiolipins of IgG and IgM isotypes, in addition to a modified coagulation test called lupus anticoagulant (LA).59 The APS is classified as primary, when there is no other related autoimmune disease, and secondary when it is related to other autoimmune diseases such as SLE, systemic sclerosis, SS, RA, and idiopathic thrombocytopenic purpura, among others.67,68
It should be taken into account that the aPLs can also be found in people without clinical manifestations. It has been seen that anticardiolipin antibodies (aCL) are found in low and transient titers in up to 10% of normal blood donors,69 but the presence of persistent antibodies against cardiolipin/B2GPI or LA can be found at moderate or high titers in less than 1% of healthy patients. The prevalence of positive aPL increases with age. It has been documented that in asymptomatic patients who have positive aPL persistently for decades, the probability of developing APS is relatively low.70
Cardiolipin was the first target antigen for the aPLs that was identified in 1941.68 However, it was reported in 1990 that the aCLs are directed against the B2GPI/cardiolipin complex and not against cardiolipin alone.71 Although the aCLs are positive in 80% of patients with APS, they can also be found in infectious diseases such as syphilis, Q fever and HIV infection. In this infectious conditions, the titers are usually low, the dominant isotype is IgM and are generally not associated with thrombotic phenomena.67 The method used for the measurement of these antibodies is ELISA, the test is sensitive but not specific for the diagnosis of APS. Although it has still a great clinical relevance, so that is included within the classification criteria, the presence of aCL of IgG and IgM isotypes, in intermediate or high titers (>40GPL or MPL, or above the 99th percentile) should not replace the measurement of anti-B2GPI.59
In addition to the criteria for APS, they are also clinically useful because they are included in the SLICC criteria for SLE, including the IgA isotype,13 since the presence of aCL of IgA isotype in patients with systemic autoimmune disease indicates that they are in a subgroup of patients at risk of developing specific clinical manifestations. It is associated with clinical manifestations such as thrombocytopenia, oral ulcers and vasculitis.59,72 With respect to aCLs of IgA isotype and the diagnosis of APS, although they are not included in the international classification criteria, they are clinically useful when there is a patient with a high clinical suspicion of APS but with negative LA, anti-B2GPI IgG and IgM, and aCL IgG and IgM on several occasions, condition in which it is indicated to perform the test.57
In relation to the LA, this term was used for the first time in 1972, but the phenomenon has been studied since 1952, when Conley and Hartmann reported the prolongation of prothrombin time in patients with SLE.71 In 1972, it was described as a direct inhibitor against the phospholipids of the coagulation cascade, which mainly affected the conversion of prothrombin into thrombin.72 In 1983 was described in detail a syndrome that includes thrombosis or pregnancy morbidity that occurs in patients with laboratory evidence of the presence of aPL, official description of the APS.73 Since then, several data that confirm the importance of this antibody in the pathogenesis, diagnosis and prognosis of PSA have been described. The LA is the strongest aPL test in predicting pregnancy complications and a more specific predictor of thrombosis when compared with aCL.67 Unlike the other aPL tests that are performed by ELISA, the measurement of LA is a functional test that uses plasma instead of serum and requires a 4-step process: (1) the demonstration of a prolonged phospholipid-dependent coagulation test, such as a partial thromboplastin time or the diluted Russel's viper venum test; (2) non-correction of the prolonged coagulation test when adding normal platelet-poor plasma, demonstrating the presence of an inhibitor; (3) correction of the prolonged coagulation test, when adding platelet-rich plasma (the platelets have phospholipids in their membrane, which demonstrates the phospholipid dependence of the inhibitor that prolongs the clotting times); and finally, (4) other inhibitors must be excluded.74 Taking into account that it is a complex and meticulous procedure, which is susceptible to errors that affect the result, the International Society of Thrombosis and Homeostasis created in 1995 the guidelines for the detection of the presence of LA, to standardize the test and decrease possible errors.75
It should be considered that there is the possibility of obtaining false positives and false negatives when the patient is on anticoagulant therapy with heparin or warfarin. Although there is no guide that determines the value of positivity of the test and it differs between the different laboratories, the international classification criteria for the APS mention a clotting time (test/control) >1.1 for dRVTt and >1.2 for the kaolin clotting time, these values are accepted by many laboratories.59
The last aPL test included in the international APS criteria was the measurement of anti-B2GPI antibodies in 2006. However, since 1990, it has been recognized the B2GPI antigen, which when it binds to cardiolipin forms complexes that are recognized by aPL.76,77 Under physiological conditions, the B2GPI antigen participates as an inhibitor of the activation of the intrinsic pathway of the coagulation cascade, is involved in platelet aggregation, and affects fibrinolysis, angiogenesis, apoptosis and atherogenesis.78 Therefore, when there are antibodies that act against this antigen, some of the pathogenesis of the above mentioned diseases can be known. In international classification criteria of APS, the B2GPI is identified as the most clinically important autoantigen in APS,59 since it represents an independent risk factor for thrombosis and complications during pregnancy. Anti-B2GPI antibodies are measured by immunological analyses (such as ELISA or chemiluminiscence) and 3 isotypes: IgA, IgG and IgM can be identified. According to the international criteria for APS, the IgG and IgM isotypes are included and are considered positive when they are above the 99th percentile.59 As for the IgA isotype, its clinical importance has been recognized and, although it has not been included within the criteria for APS, it should be measured when the patient has a clinical picture highly suggestive of this condition and LA, aCL IgM and IgG, and the anti-B2GPI IgM and IgG are negative.57,59 In addition, these antibodies have gained clinical relevance in the diagnosis of SLE, and they were even included within the classification criteria of the SLICC in 2012.13
After mentioning the 3 tests included in the criteria for APS, it is important to highlight that the 3 procedures must be performed to confirm the presence of aPL. In addition, the above-mentioned guidelines make emphasis in repeating the test 12 weeks later, since these antibodies may be present in non-autoimmune diseases such as infections, leading to a misdiagnosis. And as well as with the antibodies mentioned previously in the article, it is of utmost importance to perform the measurement tests for aPL in a clinical context.59,75 The clinical conditions in which the tests for aPL should be requested are:
- 1.
Recurrent fetal losses.
- 2.
Venous thrombosis without apparent cause.
- 3.
Arterial thrombosis with apparent cause.
- 4.
All patients with SLE.
- 5.
Incidental finding of prolongation of the partial thromboplastin time in asymptomatic patients.
For some time, the term seronegative APS has been a very controversial issue. The term is used to define patients with clinical findings suggestive of an APS, but in whom the routine tests used for the measurement of aPL are persistently negative. In these patients, the antibodies included in the diagnostic criteria are not detected, but other antibodies are found, such as B2GPI of IgA isotype, aCL of IgA isotype, and antiphosphatidylserine or antiphosphatidylcholine antibodies, among others.66
Conclusion: key points about the correct interpretation of the antibodies in autoimmunity and autoimmune diseases- 1.
The ANAs are a group of autoantibodies that recognize macromolecules integrated in the structure of the cell nucleus and some cytoplasmic components.
- 2.
The international consensus of standardized nomenclature (2016) must be taken into account for the correct interpretation of the ANA.
- 3.
Anti-dsDNA antibodies are of great importance in the diagnosis and follow-up of patients with SLE given their high specificity (>95%).
- 4.
Anti-ribosomal P antibodies are related with neuropsychiatric manifestations of SLE; as well as with lupus nephritis when they are found positive together with anti-dsDNA.
- 5.
Anti-Ro or SSA antibodies are found mainly in SS, so they are part of its classification criteria. They are also related with congenital heart block.
- 6.
The anti-La or SSB antibody is also found mainly associated with SS. In most cases is found along with anti-Ro, generated by an immune response with epitope spreading.
- 7.
The anti-Sm antibody, despite it is only found in up to approximately 30% of patients, is the most specific for SLE, with a specificity close to 97%.
- 8.
The anti-RNP antibody has also been associated with patients with SLE; however, high titers are associated with MCTD, making part of the classification criteria for this condition.
- 9.
Anti-Scl70 antibodies have a high specificity especially in systemic sclerosis of the diffuse and limited form and in CREST syndrome; as well as in certain clinical manifestations, especially pulmonary.
- 10.
The anti-jo1 antibody has been associated with a pathologic subtype of inflammatory myopathy known as the antisynthetase syndrome.
- 11.
The RF can be found at low titers in healthy populations. Non-autoimmune diseases can increase the RF transiently; the autoimmune disease in which it has greater clinical utility is RA.
- 12.
The anti-CCPs, due to their high specificity in RA, are part of the classification criteria, mainly because their presence is related to early onset of the disease and greater bone erosion.
- 13.
The sensitivity and the specificity of the ANCA (MPO and PR3) are quite high when there is a clinical suspicion of associated vasculitis.
- 14.
The aPLs can be found at low titers in healthy individuals. In addition to being part of the classification criteria for APS, it should be remembered that the determination of these antibodies should be repeated 12 weeks later since certain non-autoimmune diseases can increase them.
None of the authors declare any conflict of interest.
Please cite this article as: Mendez-Rayo T, Ochoa-Zárate L, Posso-Osorio I, Ortiz E, Naranjo-Escobar J, Tobón GJ. Interpretación de los autoanticuerpos en enfermedades reumatológicas. Rev Colomb Reumatol. 2018;25:112–125.