Depression is significant comorbidity in patients with rheumatoid arthritis (RA), with many factors, such as disease activity, pain, and disability determining its development.
Materials and methodA cross-sectional study including patients with rheumatoid arthritis was conducted to determine prevalence of depression using the Patient Health Questionnaire (PHQ-9), and its relationship with disease activity using DAS-28. The HAQ-DI was used to determine functional disability and the number of painful and swollen joints.
ResultsDepression was observed in 42.9% of the patients. An association was found between depression, DAS-28 and HAQ-DI. It was also found that 70% of the patients with high activity disease and 38% of the patients with functional disability had moderate-severe depression. The number of painful and swollen joints was greater in patients with severe depression than in patients with mild depression. Patients with greater depression referred to more intense pain according to the visual analog pain scale.
La depresión es una importante comorbilidad en pacientes con artritis reumatoide; diversos factores como la actividad de la enfermedad, el dolor y la discapacidad contribuyen a su desarrollo.
Materiales y métodosSe realizó un estudio transversal en pacientes con artritis reumatoide para determinar la prevalencia de depresión, utilizando el cuestionario Patient health questionnaire (PHQ-9) y su relación con la actividad de la enfermedad mediante DAS-28 y la discapacidad funcional mediante HAQ-DI.
ResultadosEl 42.9% de los pacientes presentaron depresión. Se encontró una asociación entre depresión con DAS-28 y HAQ-DI, ya que el 70% de los pacientes con alta actividad de la enfermedad y el 38% de los pacientes con discapacidad funcional presentaron depresión de moderada a grave. El número de articulaciones dolorosas y tumefactas fue mayor en los pacientes con depresión grave que en aquellos con depresión leve. Los pacientes con mayor depresión referían un dolor más intenso según la escala visual del dolor.
Rheumatoid arthritis (RA) is an inflammatory chronic disease of unknown etiology that affects, mostly, people of working age and is characterized by bilateral and symmetrical polyarthritis of small and large joints.1 Depression is a significant comorbidity in patients with RA and it may occur either in a chronic or an intermittent manner. Its incidence is variable (10-25%) and it is more frequent in women.2 The incidence of depression in the general population is 10% in women and 6% in men3; people with chronic diseases have 5 to 10-fold higher risk of depression.4
The factors that contribute to depression in rheumatic patients are diverse. They include pain, suffering due to somatic symptoms, functional limitations, release of proinflammatory cytokines, progression of the disease and disability.5 Wolfe et al. determined that the disease activity, the changes in pain and the Health assessment questionnaire disability index (HAQ-DI) predict greater depression.6,7
Inflammation is the common pathophysiological pathway between depression and chronic diseases such as RA. An increase in proinflammatory cytokines, acute phase proteins and adhesion molecules has been observed in patients with depression. The studies of Lanquillon et al., together with those conducted by Alesci et al., found high concentrations of interleukin 6 (IL-6) and C-reactive protein (CRP) in patients with depression.8,9 Other studies described elevations of interleukin 1 beta (IL-1-β) and of the tumor necrosis factor alpha (TNF-α).10,11 Frommberger et al. found that IL-6 was elevated in patients during the acute phase of depression and that it decreased after reaching remission.12
The diagnosis of depression is made through a psychiatric interview and the clinical criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) which include: depressed mood, decreased interest or ability to feel pleasure, considerable weight loss or gain, insomnia or hypersomnia, psychomotor agitation or slowing, fatigue, feelings of worthlessness or guilt, decreased ability to concentrate or to think, and suicidal thoughts or attempts.13 Five or more of the above-mentioned symptoms must be present for at least 2 weeks for the diagnosis of major depression.
In rheumatology there are screening questionnaires that help to identify patients with depression, such as: Patient health questionnaire 9 (PHQ-9), Hospital anxiety and depression scale (HADS), Hamilton rating scale for depression (HAM-D), Mental health inventory (MHI-5), Center for epidemiologic studies depression scale (CES-D) and Beck depression inventory (BDI-II). These questionnaires have the advantage of being easy to answer and quick to interpret.14
The PHQ-9 is based on the DSM-IV diagnostic criteria and it allows measuring the mood of the patient in the weeks prior to consultation. Studies on depression in RA have used this questionnaire due to its reproducibility, validity, reliability and sensitivity to change.15 Milette et al. compared the PHQ-9 with the CES-D depression scale and they found that both are similar in terms of validity and reliability; however, the PHQ-9 has the advantage of being shorter and easier to apply.16
Due to the absence of similar data in our country, the objective of this study is to assess the presence of depression in patients with an established diagnosis of RA, by means of the PHQ-9 questionnaire and to evaluate its relationship with the disease activity.
Materials and methodsA prospective, descriptive study was conducted in a population of patients with diagnosis of RA, coming from the outpatient service of the Luis Vernaza Hospital and from a private rheumatology center (CERER) of the city of Guayaquil. The duration of the study was one year.
The study included men and women over 18 years of age who had an established diagnosis of RA according to the criteria of the American College of Rheumatology 1987,17 excluding patients with other connective tissue diseases and known psychiatric disorders. It was created a database which included: clinical manifestations, demographic data, ethnicity (self-defined by each patient), comorbidities (hypertension, diabetes mellitus, thyroid, gastric, allergic and neoplastic diseases), habits (alcohol, tobacco and drugs), usual treatment, pain visual analog scale (VAS) completed by the patient and by the physician, disease activity index DAS-28—using CRP as inflammatory marker,18—HAQ-DI disability index validated to Spanish in 199319 and the PHQ-9 depression questionnaire validated to Spanish in 2001.20
The data were collected through questionnaires that were filled out individually by the patients after having been assessed by the rheumatologist. Standard values for remission and disease activity were considered using the DAS-28 scale.
The HAQ-DI and PHQ-9 questionnaires were filled out exclusively by the patients in their native language. The first one consists of 8 categories and has a scoring range from 0 to 3, according to the degree of difficulty that the patient presents in each activity. A HAQ-DI score higher than 1.25 indicates disability and 3 indicates severe disability. The PHQ-9 has 9 questions and a scoring scale from 0 to 27. Scores from 0 to 4 indicate absence of depression; from 5 to 9, mild depression; from 10 to 14, moderate depression; from 15 to 19, moderately severe depression; and from 20 to 27, severe depression.
The data were uploaded and analyzed in the statistical software SPSS v. 22. The qualitative variables were studied using descriptive statistics with frequencies and percentages; while measures of central tendency, maximum and minimum were used for the quantitative variables. The Spearman correlation coefficient was calculated for the ordinal data and ANOVA for the comparison of the means. The statistical significance used was 0.01, with a reliability of 99%.
ResultsThe study included 184 patients with an average age of 51 years (20-90 years), a mean age of onset of the disease of 40 years (18-49 years) and a mean follow-up of 56 months.
According to demographic data, 94.6% were mestizos; 3.8%, white and 1.6%, Afro-Ecuadorians. Of them, 10.3% came from public health care, 29.3% from private health care and 60.3% from mixed health care (public-private). 86.4% of patients came from urban areas and 13.6% from rural areas; in addition, 5.4% smoked tobacco and 4.9% consumed alcohol.
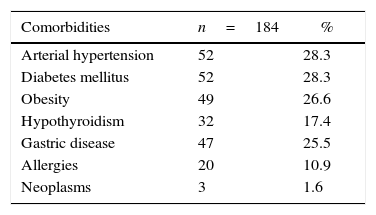
The most frequent comorbidities in the patients were hypertension and diabetes mellitus, each one with 28.3%, as evidenced in Table 1.
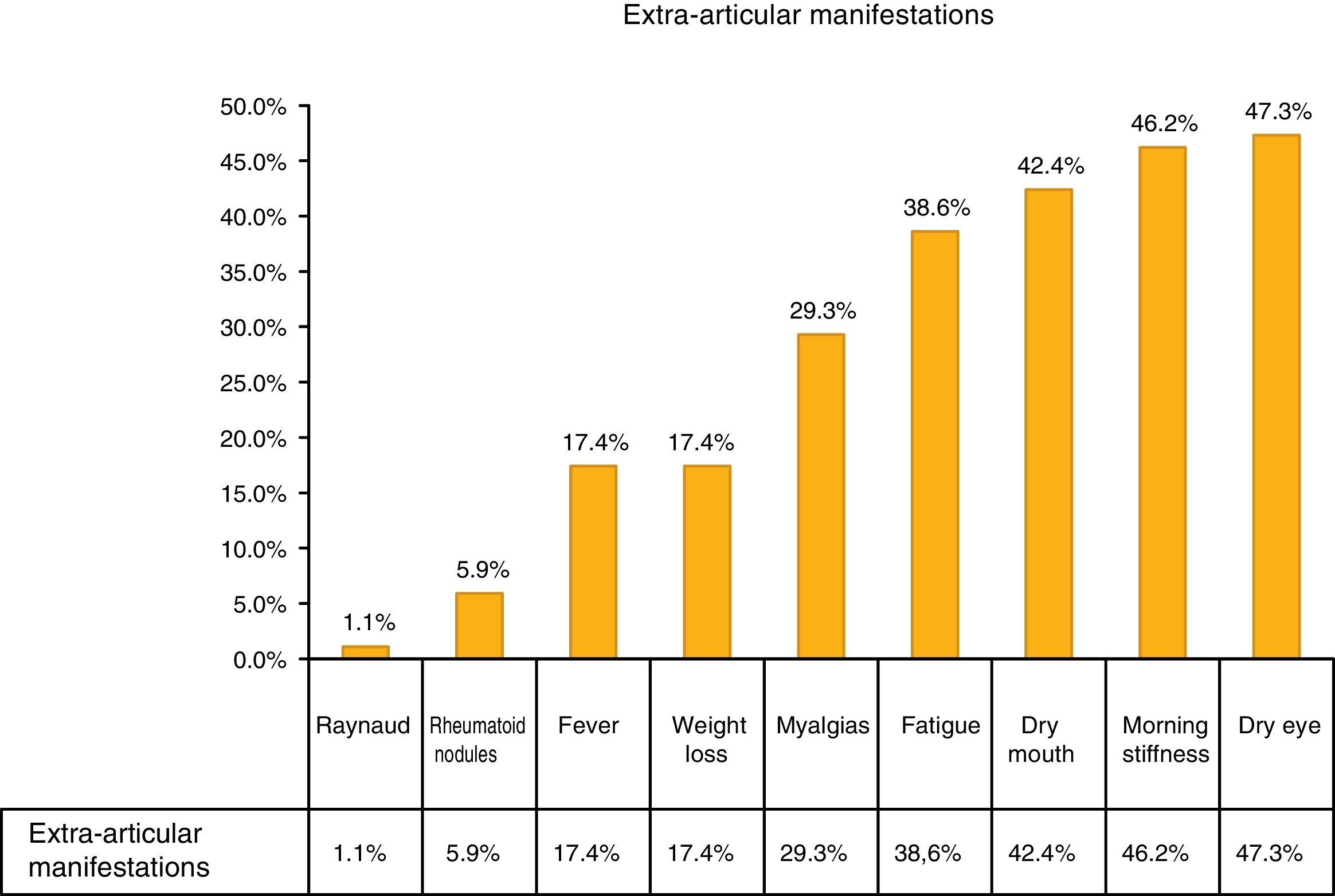
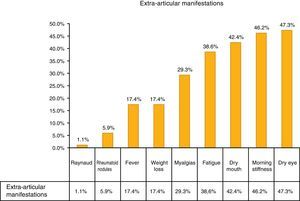
The most common extra-articular manifestation was dry eye (47.3%), other manifestations included: fever, weight loss and myalgias, as shown in Fig. 1.
The mean of the DAS-28 was 3.11 (0.7-7.6), with 44% in remission, 16.8% with low activity, 32.1% with moderate activity and 7.1% with high activity.
The mean of the HAQ-DI questionnaire was 0.8 (0-3), 27.0% of the studied population presented functional disability and 1.6% had severe disability.
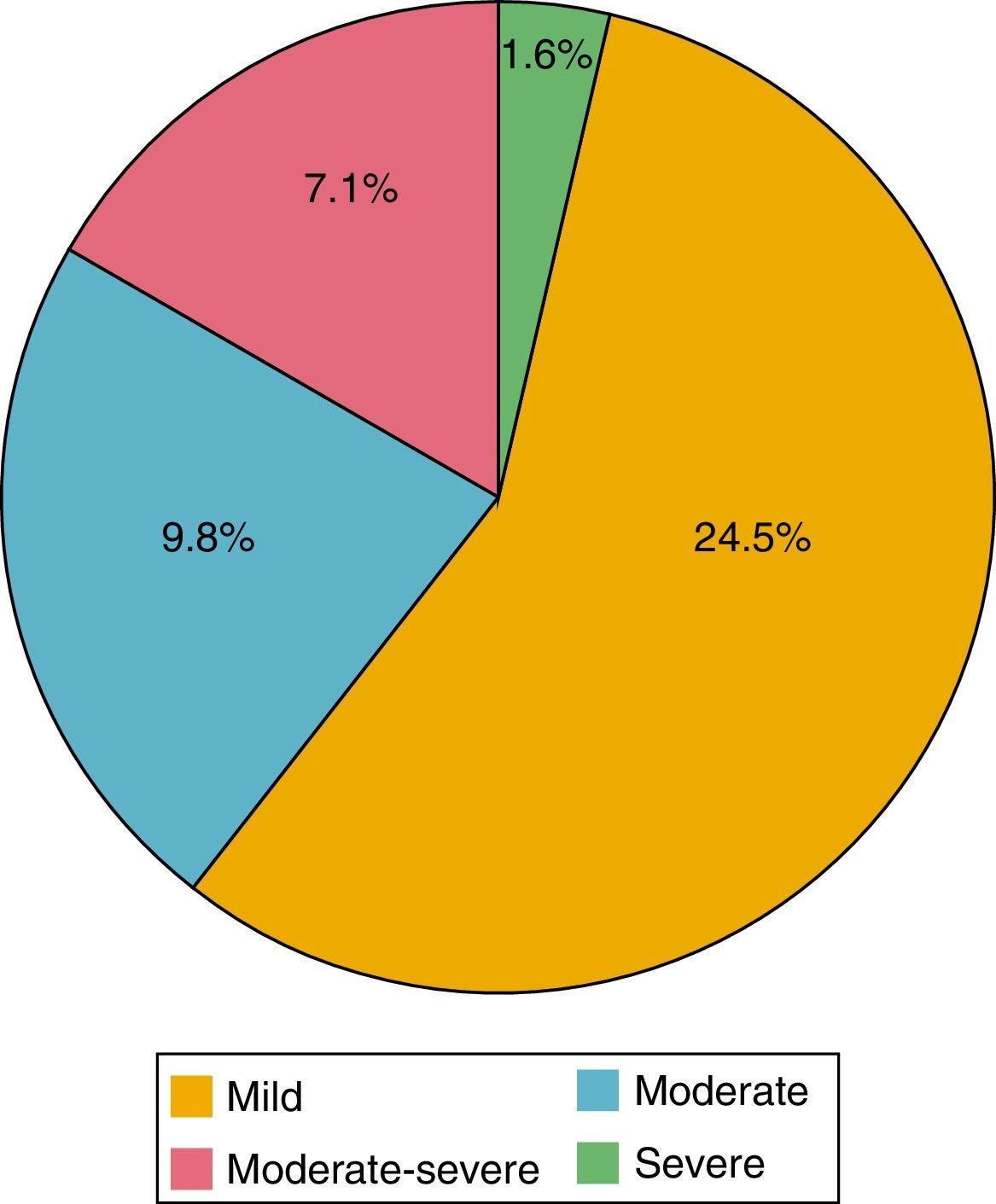
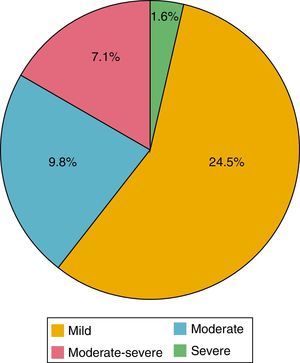
Of the 184 patients studied, the mean of the PHQ-9 questionnaire was 5.39 points (0-24). According to the evaluation of this questionnaire, depression was found in 42.9%; of these patients, 24.5% had mild depression; 9.8% moderate, 7.1% moderately severe and 1.6% severe (Fig. 2). 89.9% were women and 10.1% men; 44.3% had a HAQ-DI>1.25 and 46.8% a DAS-28>3.2.
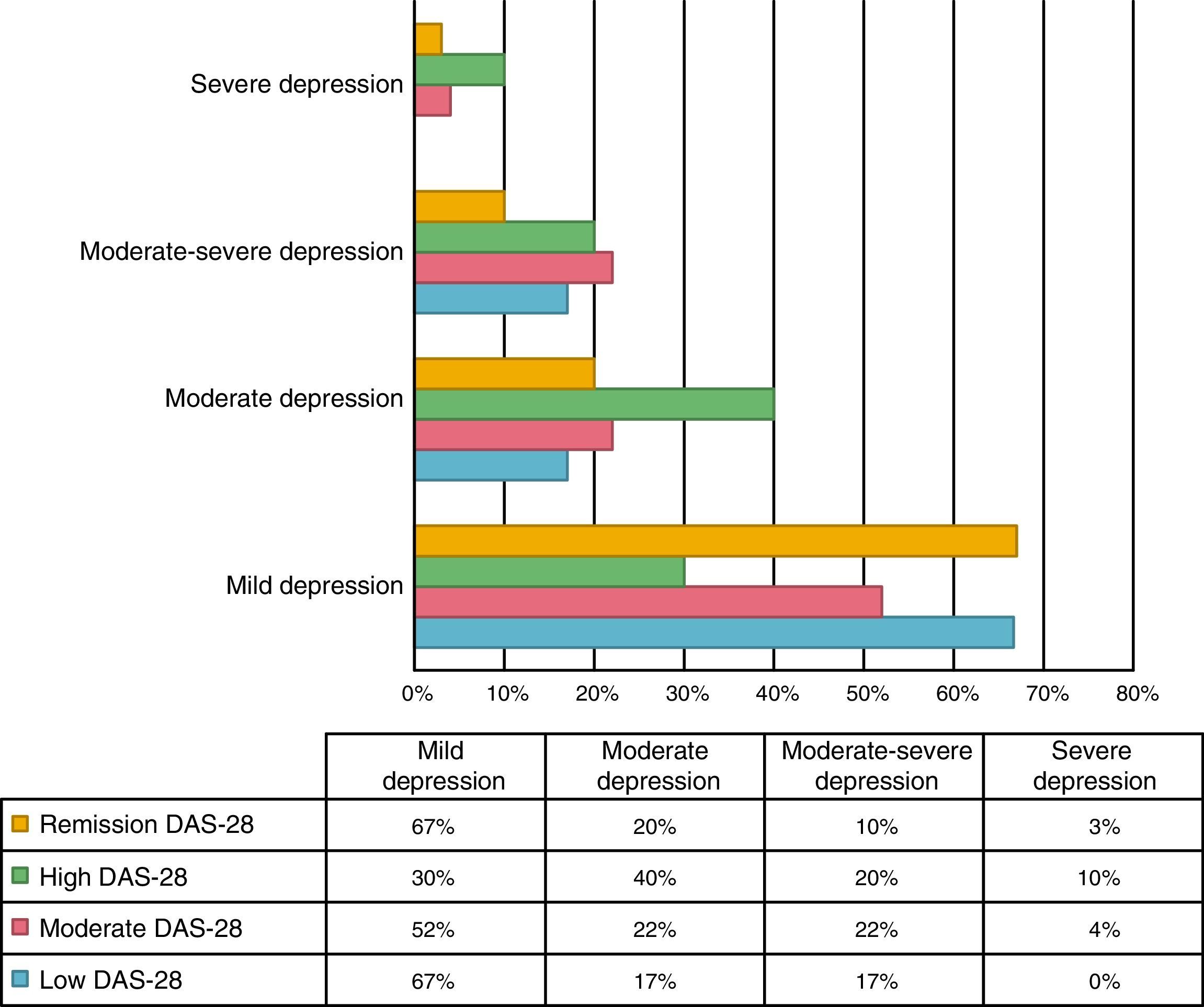
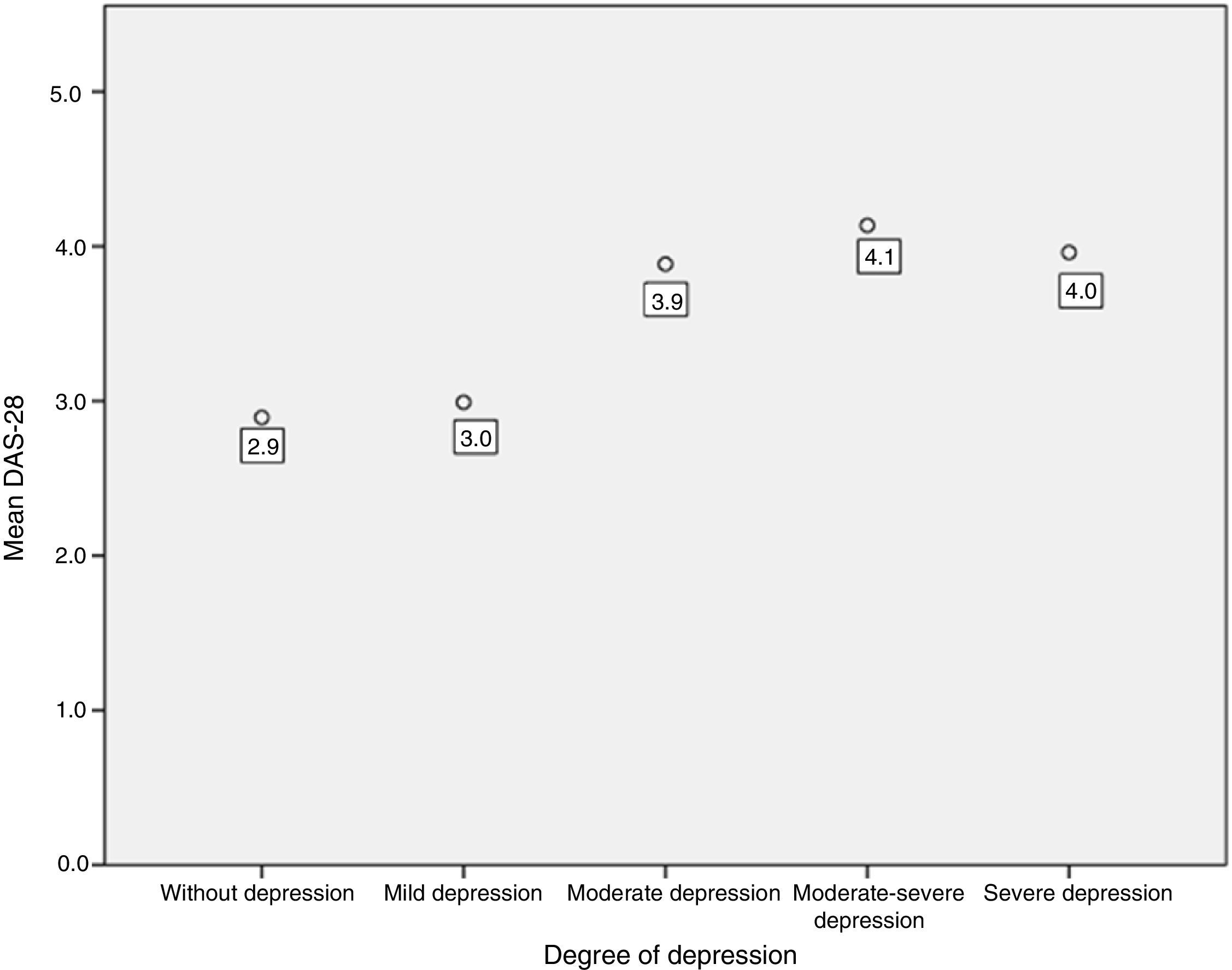
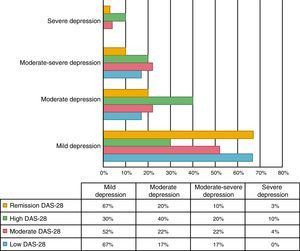
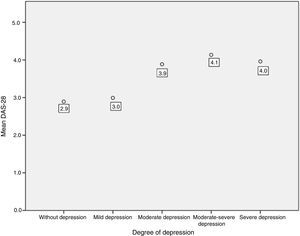
An association between depression levels and disease activity by DAS-28 of CRP was found. 66.7% of the patients in remission had mild depression, whereas 70% of the patients with high activity had moderate to severe depression (p=0.009) (Fig. 3). The mean DAS-28 for the patients with moderate to severe depression was higher than in those with mild depression (4.0 vs. 2.9; p=0.004) (Fig. 4).
Disability was also related with the levels of depression, since 38.0% of the patients with functional disability (HAQ-DI>1.25) had moderate to severe depression levels, compared with 11.19% of the patients without disability (p=0.000).
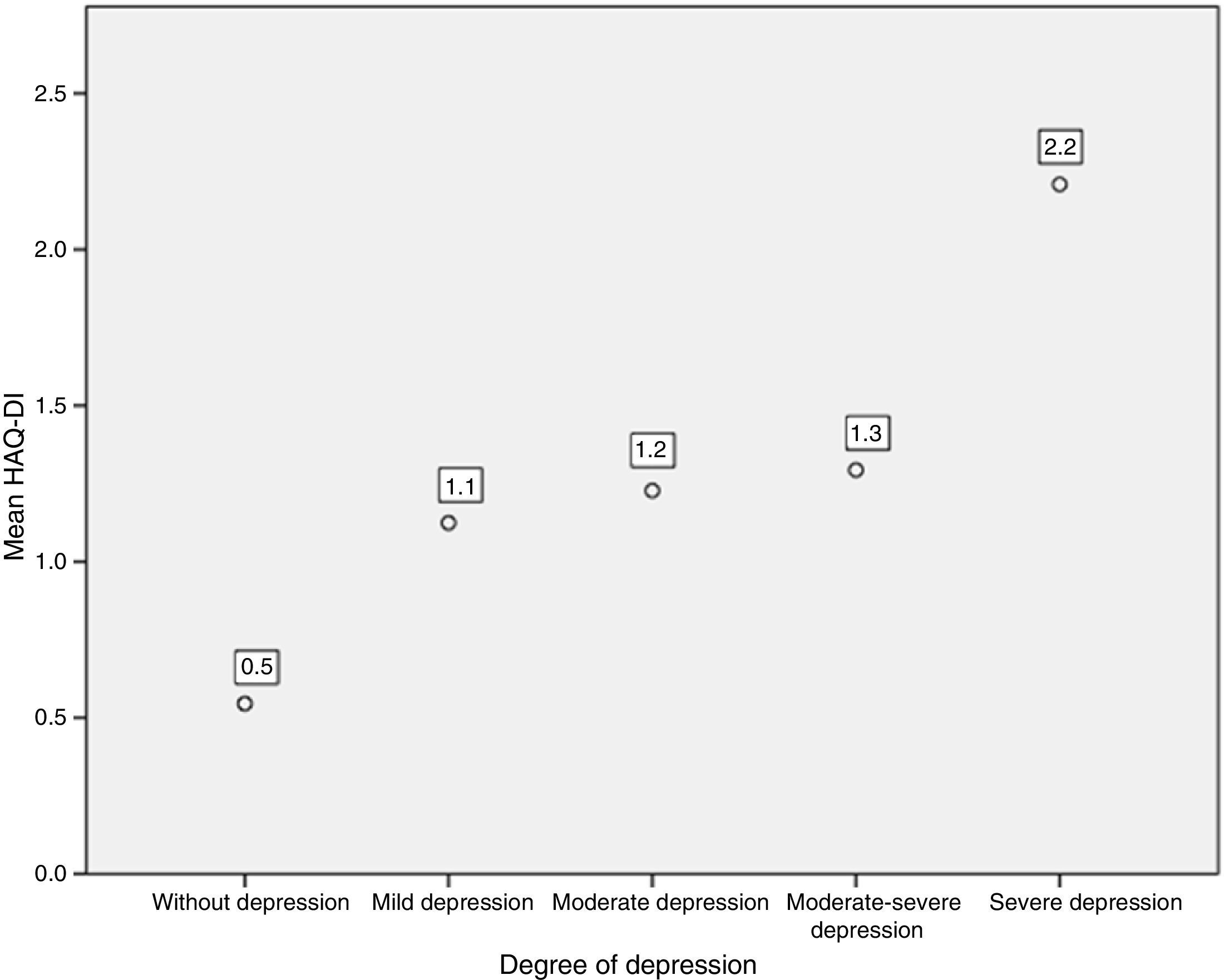
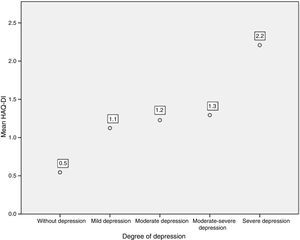
Likewise, when comparing the HAQ-DI in patients with depression, it was found that patients with severe depression had the highest mean value of HAQ-DI (with 2.2; p=0.000). The 3 patients who presented severe disability with HAQ-DI had high levels of depression measured by PHQ-9 (Fig. 5).
As for the patient's VAS, it was found that the mean value in people with mild depression was 3.7, while in moderate to severe depression it was 5.4 (p=0.000). In the physician's VAS, the mean value in people with mild depression was 3.1, while in moderate to severe depression it was 4.6 (p=0.000).
The number of painful joints was greater in the patients with severe depression than in those with mild depression (7 vs. 1; p=0.000), as well as the number of swollen joints (8 vs. 1; p=0.000).
DiscussionRA is an inflammatory, disabling and crippling disease that has a great impact on the emotional and physical life of the patient; patients affected by RA face constant psychological stress, and for this reason, depression is a frequent comorbidity in them. In the meta-analysis conducted by Matcham et al., it was found that the prevalence of depression in patients with RA was 38.8%.21 This is slightly lower than what was found in the present study, which was 42.9%. Dickens et al. published that there was greater depression in patients with RA than in those with osteoarthritis; however, it was lesser than in people with fibromyalgia.22 The prevalence of depression in patients with RA is even higher than the observed in patients with diabetes (12%),23 Parkinson disease (17%)24 and cancer (24%).25
The mean of the PHQ-9 in this study was 5.39, figure which, as in the study of Margaretten in which the mean was 7.0, corresponds to mild depression.26 The PHQ-9 questionnaire has demonstrated to be useful, reliable and reproducible to assess depression. Englebrecht et al. demonstrated that the PHQ-9 has a reliability of r=0.68 (p<0.001), convergent validity r=0.61 (p<0.001) and discriminant validity r=−0.13 (p=0.074) vs. other questionnaires that assess depression such as the Beck depression inventory II, the WHO-5 general well-being index and the Montgomery-Asberg depression scale.14
Wolfe demonstrated that depression is associated with disability due to the disease, age, pain and time between visits.7 Rathbun coincided with this, since, in his study, the measures of disease activity were associated with previous depressive symptoms, having a greater correlation with the patient's VAS, pain and HAQ-DI.2 The present study demonstrated a relationship between the levels of depression, the patient's VAS, the physician's VAS, the HAQ-DI, the DAS-28 and the number of swollen and painful joints.
Kekow demonstrated that patients who achieved clinical remission with DAS-28 had fewer symptoms of depression.27 The relationship was similar in this study, since only 33.3% of the patients in remission showed moderate to severe depression, compared with 70% of the patients with high activity.
Several authors have demonstrated that the relationship between depression and disease activity is bidirectional; that is, the activity of RA favors the onset of depression and, in turn, the depression influences the activity of RA.28 In the work of Hider et al., it was seen that patients with depression had higher DAS-28 scores and those who were persistently depressed had a smaller reduction in this score over time despite being treated with anti-TNF therapy.29
This relationship between depression and disease activity is based on the proinflammatory state in which people with chronic diseases are. The cytokines released in the periphery reach the brain through different pathways. Maier and Watkins established that the communication between the immune system and the brain is given by the entrance of cytokines through circumventricular organs, binding to carrier molecules expressed in the cerebral endothelium and activation of vagal afferent fibers that transmit signals to specific brain nuclei such as the solitary tract.30 Once there, they exert effects on neurotransmitters, which cause behavioral alterations.31 Dunn et al. studied the effects of cytokines on neurotransmission and found that IL-1, IL-6 and TNF-α alter the metabolism of serotonin, norepinephrine and dopamine in regions implicated in emotion regulation such as the limbic system.32
Magni et al. found that pain increases depression and that depression contributes to the persistence and increase of pain33; in the studies of Conner et al., along with those of Zautra et al., the patients who had recurrent depression reported higher levels of pain than those who did not have depression.34,35 This can be attributed to the fact that negative thoughts in depression affect the way in which patients perceive their somatic symptoms.
Likewise, functional limitations lead to depression, which increases the disability. According to Katz and Yelin, a reduction of 10% in the ability to carry out activities of daily living causes a 7-fold increase in the risk of depression in the following years.36
Several authors have demonstrated that depression is more common in single, white and young women.2,37,38 Wolfe demonstrated that the changes in the degree of depression are related, although to a lesser extent, to the educational level, the morning stiffness and the duration of the disease.7 In this study, depression was more common in women; however, no relationship was found with the other demographic variables.
In addition to the disease activity and the disability, Covic established that low self-esteem, emotional stress, fatigue and pain are strong predictors of depression.39 Other predictors found were the presence of myalgias and feeling depressed. In the studies of Kojima et al., the depression scores correlated with the levels of CRP and both, in turn, with the pain levels.40 In another study, CRP was also associated with disease activity, depression and disability, to a greater extent that ESR.41
The consequences of depression in patients with RA are diverse and they cause great deterioration in the quality of life. Sleep alterations are more common in patients who have recurrent depression, as evidenced in the study conducted by Jindal et al.; suicide and suicidal thoughts are also higher.42 Treharne et al. demonstrated that 11% of patients with RA had had suicidal thoughts, which are more common in women.43 As well, in another study was demonstrated that the odds ratio for suicidal thoughts in individuals with arthritis is 2 times higher than in those without arthritis.39
Timonen et al. analyzed the records of suicides in the North of Finland from the year 1988 until 2000 and they found 19 cases of suicide in patients with RA. Of these, 52.6% were women, 90% had presented depression prior to the suicide and 50% have had a previous suicide attempt.44
Rathbun et al. found that depression decreased the efficacy of pharmacological and non-pharmacological treatment of RA.45 DiMatteo et al. found that depression was associated with a lack of adherence to treatment.46 In addition, it is related to an increase in the rate of interruption, as seen in the study of Mattey.47
The majority of patients with RA and depression have not consulted a mental health professional. Fuller and Shaked determined that only 45% of people with both comorbidities had attended a consultation for depression, 29% had had more than 4 visits, one third used antidepressants and 25% had discussed their mental problems with their family doctor.37
The health status and quality of life of the patient can be improved with a proper treatment of the depression. Lin et al. demonstrated that in 12 months, the patients with arthritis and depression who received therapy showed a reduction in depressive symptoms, reduction in pain scores and functional improvement due to less interference with their daily activities.48
Recognition and treatment of depression in patients with RA are important, since this entity affects their health status and the ability to manage their disease. Ang et al. determined that depression increases the risk of mortality in patients with RA with a hazard ratio of 2.2.28 As for the economic factor, the health costs of patients with RA and depression increase significantly. Joyce et al. found that the annual costs of the disease in patients with RA and depression ($12,225) were higher than in those who had only RA ($11,404).49 Other repercussions of depression are work absenteeism, suicidal thoughts and poor adherence to medical treatment.
The presence of depression in Ecuadorian patients with RA was high, similar to that of other populations, women were the most affected, and the depression was associated with greater disability and disease activity.
The main limitation of this study is the use of the PHQ-9 questionnaire which, despite having excellent reliability to detect depression, it does not constitute the diagnosis of choice with the DSM-IV criteria; in addition, no information about suicidal thoughts or the use of antidepressants was collected.
As far as it is known, this is the first study on depression in RA in our country; the disease is closely related to depression, so it would be advisable that patients receive psychological evaluation in the event that they present clinical manifestations that indicate depression. In addition, it should be remembered that depression and RA have in common symptoms such as fatigue, insomnia and loss of appetite, which make the diagnosis difficult.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare they do not have any conflict of interest.
Please cite this article as: Maldonado G, Ríos C, Paredes C, Ferro C, Intriago MJ, Aguirre C, et al. Depresión en artritis reumatoide. Rev Colomb Reumatol. 2017;24:84–91.