Several studies provide evidence on the effectiveness of the application of EMLA cream in reducing pain in lumbar puncture in infants. However, as there are very few studies in adults, is quite scarce, it cannot be determined that its use is also effective in adults.
ObjectiveTo determine if the application of the EMLA anaesthetic cream reduces the pain compared to the application of placebo when performing lumbar punctures in an adult population.
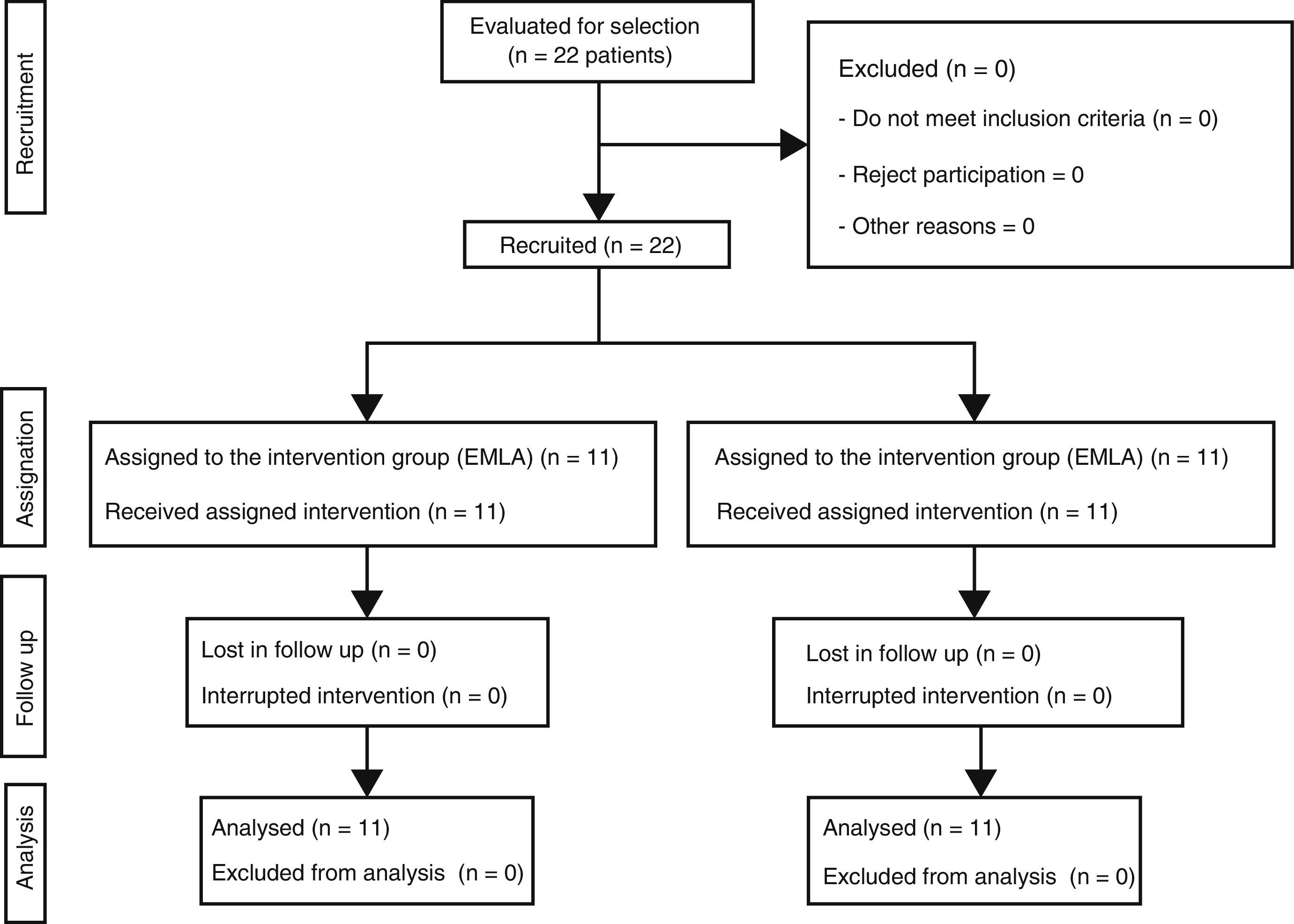
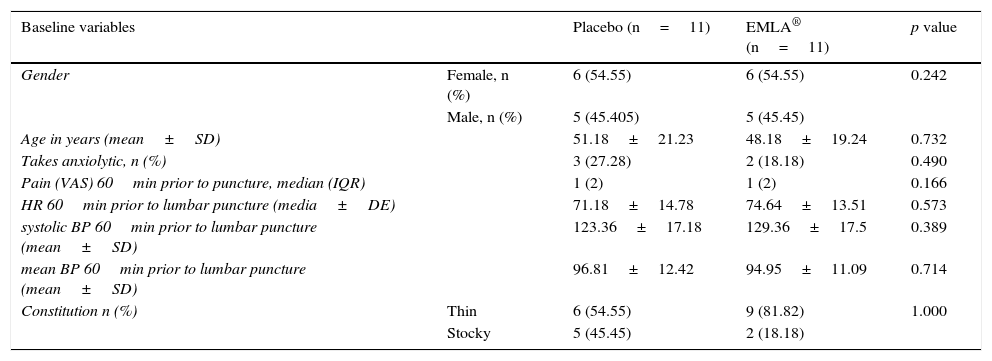
Material and methodA clinical trial was conducted with 22 patients using lidocaine-procaine cream (EMLA) versus placebo (moisturising cream). Eleven subjects were assigned to each group, randomising the first patient and alternately distributing the others into both groups.
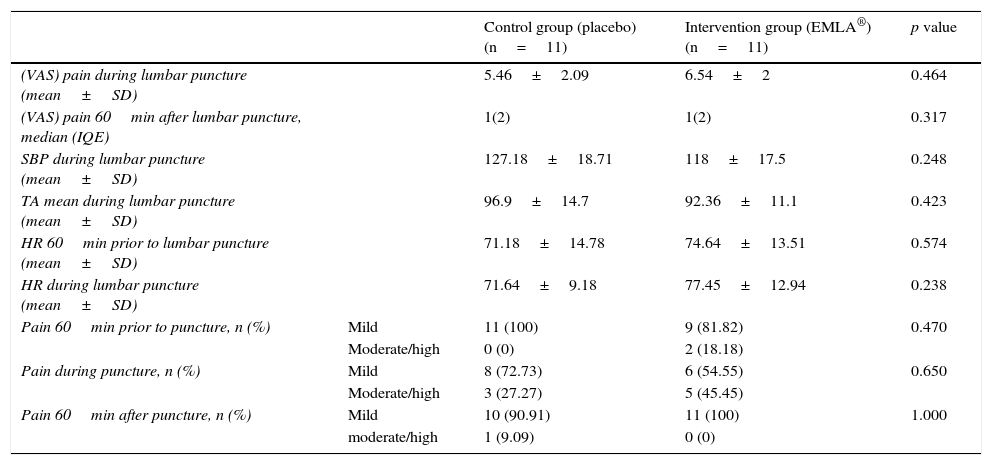
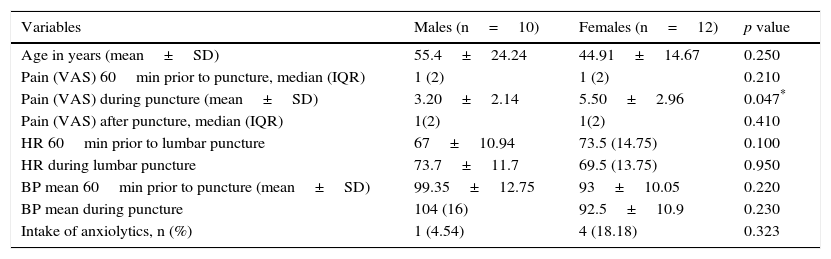
ResultsThe median in level of pain of both groups 60min before puncture, was 1 with an interquartile range of 2, according to a visual analogue scale (VAS). In both cases there was mild pain (less than 4). During puncture, both groups reported moderate pain (EMLA: 6.54±2 vs. Placebo: 5.46±2.09) p=0.464. One hour after the technique, both groups had a median in level of pain of 1, with an interquartile range of 2, p=0.317. No significant within or between group differences were detected in the level of pain or other variables, such as systolic blood pressure and heart rate, 1 hour before and during puncture.
ConclusionsThere were no significant differences in the patients who were treated with EMLA and patients treated with placebo, compared to the studies reviewed previously. More studies need to be carried out with larger patient sample, and to take into account other variables, such as anxiety level, skin thickness, and time of exposure to the product.
Varios artículos aportan evidencia sobre la efectividad de la aplicación de la crema anestésica EMLA® en la reducción del dolor en la punción lumbar en lactantes y niños. Sin embargo, la escasez de estudios en adultos no permite demostrar la efectividad del uso de EMLA en esta población.
ObjetivoComprobar si la aplicación de la crema anestésica EMLA® reduce el dolor frente a la aplicación de placebo, al realizar punciones lumbares en población adulta.
Material y métodoEnsayo clínico con 22 pacientes utilizando crema de lidocaína-procaína (EMLA®) frente a un placebo (crema hidratante). Se asignaron 11 sujetos a cada grupo, aleatorizando al primer paciente y distribuyendo de forma alterna, en ambos grupos, a los demás.
ResultadosLa mediana del nivel de dolor en ambos grupos, 60minutos antes de la punción, fue de 1, con un rango intercuartílico de 2 según la Escala visual analógica. En ambos casos dolor leve (menor a 4). Durante la punción ambos grupos refirieron dolor moderado (EMLA: 6,54±2 vs. placebo: 5,46±2,09) p=0,464. Una hora después de la técnica ambos grupos presentaron una mediana del nivel de dolor de 1, con un rango intercuartílico de 2; p=0,317. No se detectaron diferencias significativas inter e intragrupos en el nivel del dolor, ni en otras variables como la tensión arterial sistólica y la frecuencia cardiaca una hora antes y durante la punción.
ConclusionesNo existen diferencias significativas en los pacientes que fueron tratados con EMLA y los pacientes tratados con placebo, frente a los estudios revisados anteriormente. Es necesario realizar más estudios con una muestra mayor de pacientes y que tengan en cuenta otras variables como nivel de ansiedad, grosor de la piel y tiempo de exposición al producto.