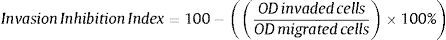Cosmos caudatus Kunth is a medicinal herb used traditionally in Latin America and South East Asia to retard aging, rigidify bones and for several cardiovascular uses.
ObjectiveIs to assess C. caudatus extract/fractions’ antioxidant and vascular smooth muscle cells (VSMC) migration and invasion inhibition capacity in vitro.
MethodsCosmos caudatus shoots were extracted by cold maceration in 50% ethanol to produce crude (CEE), and then the extract was fractionated to butanol (Bu.F), and aqueous fractions (Aq.f). Phenolics and saponins were quantified in extract and fractions by colorimetric methods and their antioxidant capacity was assayed in four different tests. Cytotoxic effect and safety level concentrations were determined for the fractions by using MTT assay. Migration and invasion inhibitory potential were measured in vitro at three different concentrations equivalent to (IC10, IC25, and IC50). Finally, invasion inhibitory index was calculated to obtain the best fraction(s) that show(s) the highest ratio of cell invasion inhibition to the total cell migration inhibition.
ResultsButanol fraction yield was the lowest; nevertheless, its phytochemical contents, antioxidant activities as well as its potency were the highest. Unlike other fractions, Bu.F was strongly correlated with all antioxidant assays experimented. In addition, it has the highest inhibitory effect at IC25 against VSMCs migration and invasion that accounts for 53.93% and 59.94% respectively. Unexpectedly, Bu.F and CEE at IC10 displayed the highest invasion inhibitory index (approx. 68%).
ConclusionButanol fraction of C. caudatus offers a potentiality for the discovery of new leads for preventing atherosclerosis.
Atherosclerosis is a group of cardiovascular diseases (CVD) characterized by a chronic inflammatory condition that affects arterial blood walls, leading to lipid deposition under tunica intima and finally, the atherosclerotic plaque formation.1 According to International Atherosclerosis Society (IAS), it comprises coronary and peripheral artery diseases, in addition to strokes and myocardial infarctions. Atherosclerotic cardiovascular diseases account for 40–50% of deaths in the developed countries, which are more than all cancer forms’ mortalities. Similarly, atherosclerosis has a growing number of morbidities and mortalities in developing countries as reported by a survey done on behalf of the European Atherosclerosis Society.2
The physiological migration and invasion takes place by the de-differentiated VSMCs to perform developmental processes and in response to vascular injury. When those de-differentiated VSMCs fail to switch to the differentiated phenotype after angiogenesis or tissue repairing, a pathological cell migration/proliferation (synthetic cells) will lead to atherosclerosis development. Differentiated VSMCs in normal blood vessels function as contractile cells that regulate vascular blood flow and diameter.3 It is noteworthy that the key step in atherosclerosis is the migration of de-differentiated SMC as well as the secretion of protease enzymes that break down the ECM in order to reach the intima layer in a process called invasion.4 Therefore, it can be considered that measuring cell migration and invasion inhibition capacity by drugs and plant extracts is a beneficial in vitro model to quantify their effects in reducing atherosclerosis.
In folk medicine, C. caudatus has been used in Southeast Asia for many uses, such as burns and muscular strains and spasms.5 Moreover, people in the Philippines incubate C. caudatus leaves with rice in order to prepare yeast.6 In addition to being consumed as an appetizer in salads and food, Malay and Javanese use C. caudatus leaves to tone up blood circulation, rigidify bones and for bad breath.7 These traditional uses are ascribed to a wide range of phytochemical constituents screened in several studies such as phenolic acids, flavonoids, carotenoids, sesquiterpene lactone, vitamins and phenylpropanoids.8–13 Although C. caudatus has demonstrated pharmacological benefits related to atherosclerosis risk factors, such as antihyperlipidemic, antihypertensive and antidiabetic effects,14,15 its SMCs migration and invasion reduction effects have not yet been studied. The present study was primarily designed to determine cell migration and invasion inhibitory effects in vitro for C. caudatus extract and derived fractions, in addition to assessing their antioxidant activities and phytochemical contents.
Materials and methodsPlant extraction and fractionationThe fresh whole plant of Cosmos caudatus (Kunth) was procured from Selangor wet market, Malaysia in November 2014. Plant samples were identified by a botanist at the institute of bioscience (IBS), Universiti Putra Malaysia, Specimen voucher number is (SK 2574/14). Extraction and fractionation were carried out according to Chan et al.16 with slight modifications. In brief, fresh shoots were picked up, oven-dried (Lab-Dryer Protech, FSD-380, Malaysia) for 3h at 50°C and mechanically powdered. The shoots powder was extracted twice for 48h each with intermittent shaking by cold maceration in 50% ethanol, wherein the ratio of the plant to solvent was 1:15 (W/V), then filtered through Whatman filter paper no.1. Thereafter, the filtrate was evaporated under reduced pressure (Hei-VAP value Heidolph – Germany), and the resulting semisolid mass was freeze-dried (Virtis Benchtop K, USA) in order to produce the crude alcoholic extract (CEE). The Crude extract was defatted with n-hexanol for three times and fractionated with n-butanol and water, followed by evaporation under reduced pressure (Hei-VAP value Heidolph – Germany) for 5 times to produce the butanol fraction. At the end, the aqueous layer was freeze-dried to yield the aqueous fraction. The resulting extract/fractions were stored at −80°C for further analysis.
Determination of total phenolic contentsTotal phenolic content (TPC) was determined for each fraction by Folin-Ciocalteu (FC) reagent assay, as described by Chan et al.17 Gallic acid was used as a standard. Briefly, 100μL of (2mg/mL) samples were mixed with 500μL of 10 times diluted (FC) reagent. After 1min, 400μL of (7.5mg/mL) sodium bicarbonate solution was added and allowed to stand for 30min at 40°C (Binder incubator, BD53 – GmbH). Each sample was measured at 760nm (Shimadzu, UV-1700, Japan). The TPC was calculated from the calibration curve Figure S1, and the results were expressed as (mg GAE/g extract).
Determination of total saponin contentsTotal saponin content (TSC) was determined for each fraction according to Hiai et al.18 Diosgenin was used as a standard. In brief, 100μL of (10mg/mL) sample was mixed with equal amounts of vanillin (8mg/100mL), and 1mL of 72% (V/V) H2SO4 was mixed with them, and then incubated for 10min at 60°C. Optical density (OD) was recorded at 540nm (Shimadzu, UV-1700, Japan). The TSC was calculated from the calibration curve Figure S2, and the results were expressed as (mg DE/g extract).
Determination of total steroidal saponin contentsTotal steroidal saponin contents (TSSC) was determined for each fraction according to Baccou et al.19 Diosgenin was used as a standard. Briefly, 500μL of different concentrations for each fraction were mixed with 250μL of anisaldehyde (0.5mL/100mL ethyl acetate). The resulting mixture was added to 250μL sulfuric acid (50mL/100 ethyl acetate). Subsequently, it was allowed to stand at room temperature for 30min, and the absorbance was measured spectrophotometrically at 430nm (Shimadzu, UV-1700, Japan). The TSSC was calculated from the calibration curve Figure S3, and the results were expressed as (mg DE/g extract).
Antioxidant activity assaysDPPH scavenging activity assayThe DPPH scavenging activity was measured for each fraction according to Chan et al.20 with mild modifications. Briefly, 50μL of (0.5mg/mL) triplicate samples were added to 195μL methanolic solution of (0.2mM) 2,2-diphenylpicrylhydrazyl (DPPH) (Sigma–Aldrich, USA) in 96-well plate (TPP, Switzerland), and gently shaken for 1min prior to be allowed to stand in the dark for 1h at room temperature. Finally, absorbance was measured at 540nm by a microplate reader (BioTek, Synergy H1, US). Trolox was used as a standard and the radical scavenging activity was expressed as mg TEAC/g extract (Figure S4).
ABTS+ scavenging activity assayABTS+ scavenging activity was measured for each fraction as explained by Chan et al.17 with modifications. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) ABTS (Sigma–Aldrich, USA) test reagent was prepared by reacting 10mL of both 7.5mM ABTS solution with 2.45mM potassium persulfate, and the resulting dark blue solution was allowed to stand in the dark at room temperature for 16h. Subsequently, the solution was diluted with absolute ethanol to an absorbance of 0.7±0.02 at 734nm. Thereafter, 950μL of ABTS test reagent was reacted with 50μL of different concentrations of each fraction with mild shaking and then allowed to stand at room temperature for 1h. Triplicate samples were transferred into a 96-well plate and the absorbance was measured at 734nm by a microplate reader. Trolox was used as a standard and the radical scavenging activity was expressed as mg TEAC/g extract (Figure S5).
Ferric ion chelating activity assayFerric ion chelating activity was measured for C. caudatus extract/fractions as explained by Chan et al.16 In brief, 1mL of (0.5mg/mL) samples were reacted initially with 50μL of 2mM FeCl2 (Sigma–Aldrich, USA), and followed by adding 100μL of 5mM ferrozine (Sigma–Aldrich, USA). The final mixture was vortexed and allowed to stand for 10min at room temperature. Triplicate samples were transferred to a 96-well plate and the absorbance was measured at 562nm. EDTA was used as a standard and the radical scavenging activity was expressed as (mg EDTA/g extract) (Figure S6).
Beta carotene bleaching activity assayβ-Carotene bleaching activity (BCB) was measured for C. caudatus extract/fractions as explained by Wettasinghe et al.21 with minor modifications. Briefly, 3mL of β-carotene solution (1.2mg/mL chloroform) (Sigma–Aldrich, USA) was mixed with 180mg linoleic acid and 1800mg tween 20 (Fisher Scientific, USA), then they were shaken vigorously. Afterward, chloroform was evaporated rapidly using rotary evaporator (Hei-VAP value Heidolph – Germany) at 40°C and 100mBar. In order to prepare β-carotene–linoleic acid emulsion, 100mL of distilled water was immediately added to the latter solution. Samples were tested by reacting 20μL of each sample (2.5mg/mL), with 1.5mL of the emulsion and incubated for 60min at 50°C. Triplicate samples were transferred to a 96-well plate and the absorbance was measured at 470nm. Trolox was used as a standard and the BCB activity was expressed as (mg TEAC/g extract) (Figure S7).
Cell cultureA-10 cell line is an adherent, non-differentiated vascular smooth muscle cells that have been derived from medial layer of embryonic rat aorta, but have significant resemblance with neointimal cells. At the same time, it has a myoblast morphological characteristic. (A-10) cell line was purchased from American type culture collection (ATCC® CRL-1476, Rockville, USA). It was maintained in Dulbecco modified Eagle medium supplemented with 10% FBS and 2% penicillin-streptomycin at 37°C in 5% CO2, cells were digested by 0.25% (W/V) trypsin/EDTA (Biowest, Nuaillé – France).
Cell viability assay (MTT)VSMCs viability were quantified by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. When culture flask reached 80% confluency, cells were harvested, and seeded at a density of 5×103/well in a 96-well plate and incubated overnight. Serially diluted C. caudatus extract/fractions in 200μL media were added to each well and incubated for 24h at 37°C in 5% CO2. Thereafter, 20μL of MTT (Amresco, Ohio, USA) solution of (5mg/mL PBS) was added to each well and incubated for 4h at 37°C in 5% CO2. Subsequently, well contents were aspirated and 100mL of DMSO (Amresco, Ohio, USA) was added to wells to dissolve formazan formed by cells, followed by gentle shaking for 10min. Finally, the absorbance was measured by microplate reader (TECAN, Infinite 200 pro-Männedorf, Switzerland) at wavelength 570nm and reference subtraction at 650nm. The assay was repeated three times and each time four wells were allocated for each concentration.
Data revealed by this assay was used to calculate inhibitory concentrations (IC) for 10%, 25%, and 50% of the cell population for each extract/fraction from the dose–response curves.
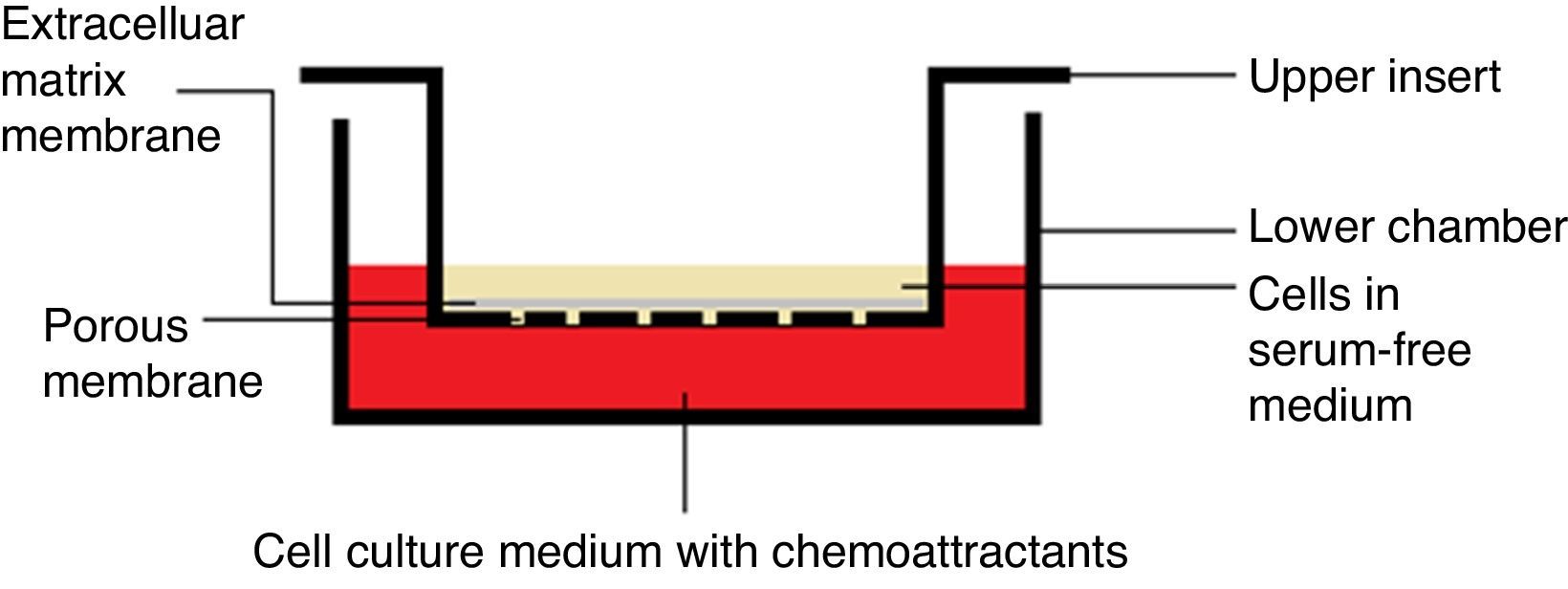
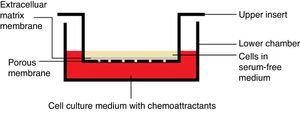
Chemotaxis assayChemotaxis assay was carried out according to the manufacturer instructions (Merck Millipore Darmstadt, Germany), catalog number “ECM 508”. When culture flask of VSMCs reached 80–85% confluency, it was starved with serum-free media for 18h, and then harvested using trypsin/EDTA. Cells were treated with each extract/fraction in free–serum media for 30min prior to being decanted in the insert. Each insert contains 300μL free-serum media with 15–30×104cells/insert.
After adding 500μL media with 20% FBS to the lower chamber (24-well plate), insert was hanged on the lower chamber, covered and were incubated for 6h at 37°C and 5% CO2 (Fig. 1). Subsequently, media with cells were pipetted out, followed by staining with dye provided with the kit for 20min. Afterward, the interior surface of the insert was washed with water and dried with cotton-tipped swab prior to extracting the dye by the extraction buffer. Finally, 100μL of the extracted stain was transferred to a 96-well plate and absorbance measured at 560nm (TECAN, Infinite 200 pro-Männedorf, Switzerland).
The positive control insert was considered as 100% reference, where cells were left without treatment, while the negative control insert was considered as 0% reference, where the lower chamber was left without chemoattractants. The formula used to calculate migration inhibition percentage is:
Chemoinvasion assayChemoinvasion assay was carried out according to the manufacturer instructions (Merck Millipore Darmstadt, Germany), catalog number “ECM 550”. When culture flask of VSMCs reached 80–85% confluency, it was starved with serum-free media for 18h, and then harvested by using trypsin/EDTA. Cells were treated with each extract/fraction in free-serum media for 30min prior to being decanted in the insert. Each insert contains 300μL free-serum media with 15–30×104 cells/insert.
After adding 500μL media with 20% FBS to the lower chamber (24-well plate), insert was hanged on the lower chamber, covered and were incubated for 24h at 37°C and 5% CO2 (Fig. 1). Using a cotton-tipped swab, non-invading cells, as well as the ECM gel, were gently removed from the interior surface of the inserts. This has been followed by staining with the dye provided with the kit for 20min. Afterward, the interior surface of the insert was washed with water prior to extracting the dye by 10% acetic acid. Finally, 100μL of the extracted stain was transferred to a 96-well plate and absorbance was measured at 560nm. Positive and negative controls and calculations for the invasion inhibition percentage were exactly calculated as mentioned in the chemotaxis assay.
Statistical analysisData were expressed as a mean±standard deviation for triplicate samplings. One way (ANOVA) was used to compare differences between groups. Post hoc comparisons were performed with Tukey test to indicate the significant variations between groups (p<0.05). Pearson test was carried out to identify the correlation between phytochemical contents of the tested fractions and their antioxidant activities. In bar charts, different letters indicate statistically significant differences between groups.
Results and discussionYields of extraction and fractionation, total phenolics, saponin and steroidal saponin contentsCrude extraction yield was 8.10%. This yield was lower than what was reported in other studies carried out on C. caudatus, as they accounted for approximately 23%.10,22 This can be ascribed to the implementation of heat reflux and sonication techniques. Both extraction methods are known to give higher yields compared to maceration. In particular, ultrasonic waves destroy the cell wall and allow solvents to penetrate inside and extract phytochemical constituents.23
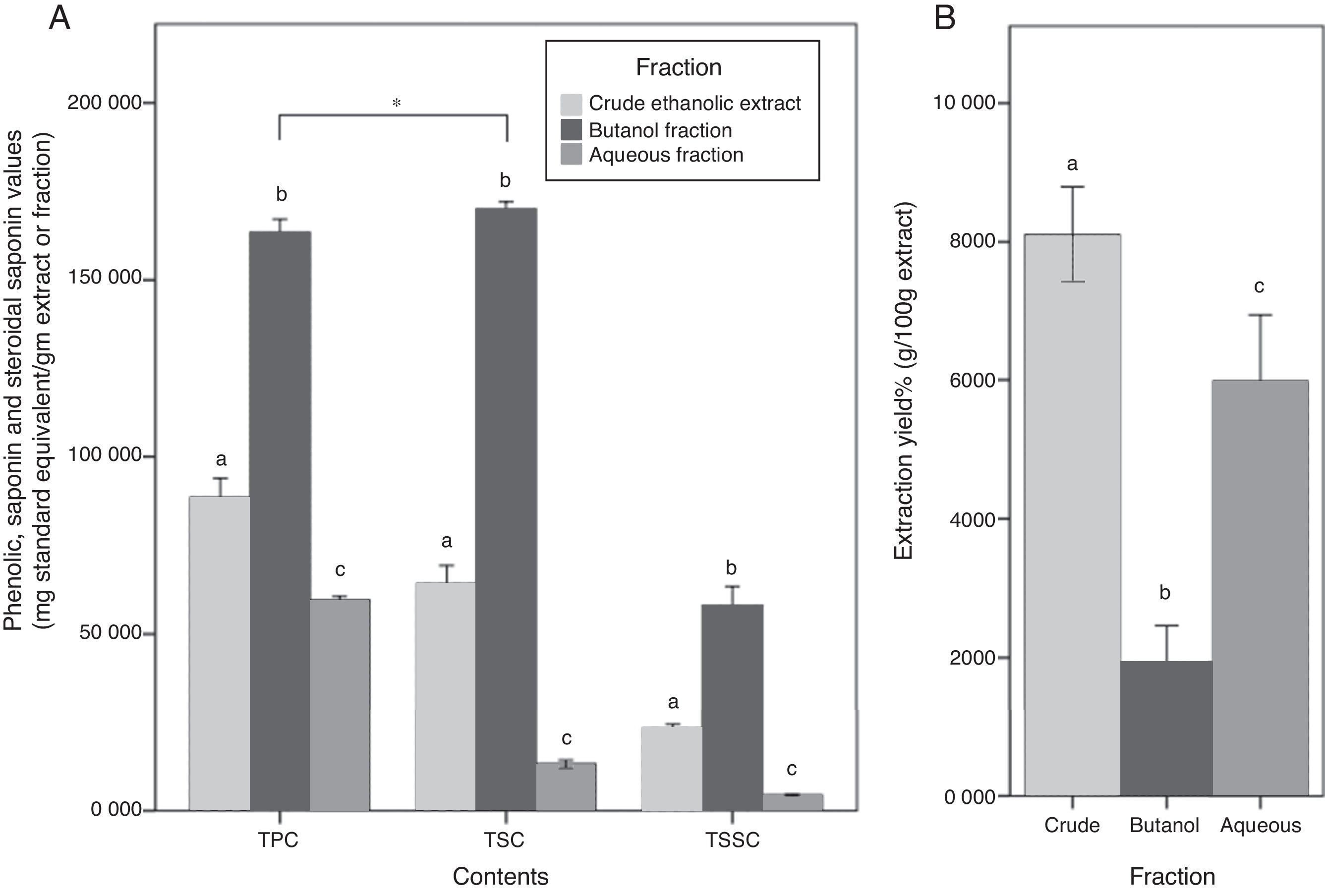
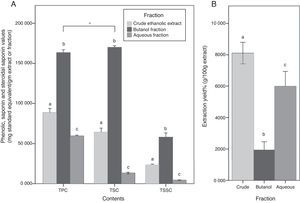
Although butanol yield was the lowest among other fractions (1.9%), its TPC was approximately two times more than CEE, and almost 3 times more than the Aq.f (p<0.05). Likewise, TSC of the butanol fraction was approximately 3 times more than the CEE, and 13 times more than the Aq.f (p<0.05). In the TSSC determination, it has demonstrated the same trend of the previous results, all C. caudatus extract/fraction followed a descending order as follows Bu.F> CEE>Aq.f (p<0.05) Fig. 2. For this reason, the Bu.F was considered the phenolics-saponins rich fraction (PSRF). Up to our knowledge, this is the first time saponins and steroidal saponins were quantitatively determined in C. caudatus extract/fractions. Remarkably, TSC was the highest content in Bu.F compared with TPC and TSSC (p<0.05), and this might affect the antioxidant power of this fraction.
(A) Total phenolic contents (TPC), total saponin contents (TSC) and total steroidal saponin contents (TSSC) for C. caudatus fractions. (B) The extraction yield for crude (CEE), butanol (Bu.f) and aqueous (Aq.f) fractions. Data were presented as a mean±standard deviation, n=3. a-c Different letters within the same group indicate significant difference (p<0.05) according to ANOVA and post hoc with Tukey tests. *Significant difference between groups at (p<0.05).
Total saponin content of the Bu.f was (0.83%). This value was comparable to saponin contents in some kinds of legumes which is known for high saponin contents such as soybean, chickpeas, and lentils (0.1–2%).24 This indicates that C. caudatus leaves have a considerable amount of saponins and can be used as a nutritive and economic resource that can be employed in the medicinal and cosmetic industry. In accordance with Kerem et al.25 findings, the result of this study shows that water-butanol purification for saponins from C. caudatus ethanolic extract is the most suitable system to concentrate saponin contents. Specifically, the high extractability of this solvent system can be explained by that the diverse polarity indices of its components; water (9.0) and butanol (4.0), is compatible with saponins’ amphiphilic nature that have both a hydrophobic nucleus and a hydrophilic glycoside. On the other hand, the high TPC that was yielded by butanol fractionation suggests that phenolics’ polarity in C. caudatus constituents is compatible with the intermediate polarity of butanol and this is supported by low water extractability to phenolics. Besides, the high extraction and fractionation yields for the CEE and the Aq.f propose that high percentage of C. caudatus constituents are polar with a non-phenolic nature. Therefore, they have been substantially extracted by polar solvents; ethanol and water. Taken together, the high TPC, TSC, and TSSC in Bu.f distinctively propose the comprehensive extractability of n-butanol for phenolics and saponins in C. caudatus.
Saturated n-butanol was employed to concentrate saponins and phenolics from the crude extract of C. caudatus, and indeed, it had proved its effectiveness in this work in addition to other studies.16,17 After fractionation with butanol, the fraction was subjected to evaporation under reduced pressure in order to rid all solvent residues, and consequently prevent any interference with cell homeostasis or antioxidant power in the further assays.
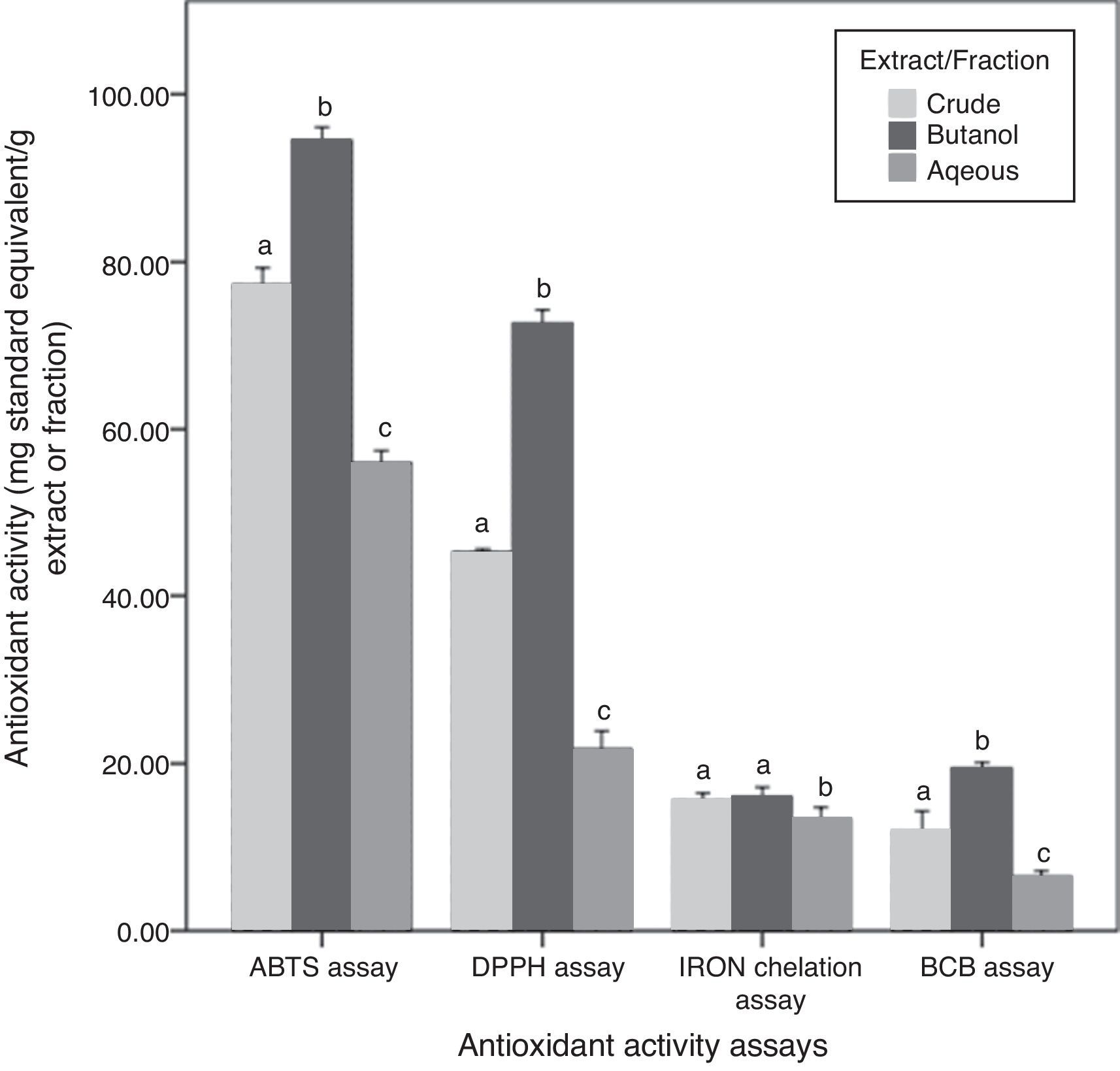
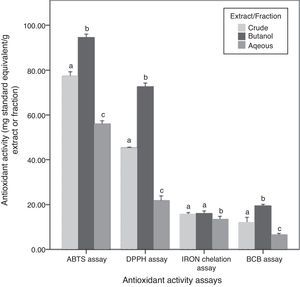
Antioxidant activities and their correlation with phytochemical contentsAntioxidant activities of the three fractions of C. caudatus were assessed by four different assays based on different mechanisms of action. Results obtained are shown in Fig. 3. DPPH and ABTS antioxidant activity assays were employed to evaluate the free radical scavenging activities of C. caudatus extract/fractions. Both assays showed that Bu.f has the strongest antiradical activity, followed by CEE and the least power was found for the Aq.f (p<0.05).
In addition to DPPH and ABTS assays, β-carotene bleaching activity is also considered an assay that measures the primary antioxidant activities. However, BCB was assayed to assess C. caudatus fractions’ capacity in preventing lipid peroxidation.26 Extract and fractions derived from C. caudatus were tested to minimize β-carotene oxidation by hydroxy peroxides generated from linoleic acid under heat conditions. In agreement with the above-mentioned results, Bu.f showed higher BCB activity (19.43mg TEAC/g extract) than CEE (11.90), and the least activity was for the Aq.f (6.48) (p<0.05).
Iron ion chelating activity was carried out in this work to determine the secondary antioxidant activity for C. caudatus extract/fractions. It is important to mention that secondary antioxidants work indirectly by retarding the oxidation process by many ways such as quenching singlet and triplicate oxygen, repairing primary antioxidants, chelating metal ions or by absorbing ultraviolet radiation.27 In line with other antioxidant assays, Bu.f has demonstrated higher iron chelating strength (16.03mg EDTA/g extract) than CEE (15.59) and Aq.F (13.39) (p>0.05). This indicates that Bu.f is the strongest primary and secondary (preventive) antioxidant fraction. This can be attributed to that; n-butanol attracted both phenolics and saponins with multiple polarities ranging from non- to semi-polar compounds. Furthermore, Bu.f contains more saponin contents than phenolics (p<0.5) Fig. 2, and this could substantiate the secondary antioxidant mechanism as well as the primary one.
A very strong correlation was observed between the phenolic content and DPPH, ABTS and BCB antioxidant activities (0.949–0.976), except for iron chelation activity as there was no significant correlation. On the other side, TSC and TSSC have strong correlations with all antioxidant activities including the iron chelation assay (0.692–0.989) (Table S1). Thus, it can be said that saponins and steroidal saponins have more contribution in the secondary antioxidant mechanism than phenolics. In addition to that, the high saponin contents can reasonably interpret Bu.f higher iron chelating activity than other extract/fractions. Moreover, butanol fraction as a whole has a strong correlation with all antioxidant assays tested in this work, which could not be applied to crude and aqueous extract/fraction.
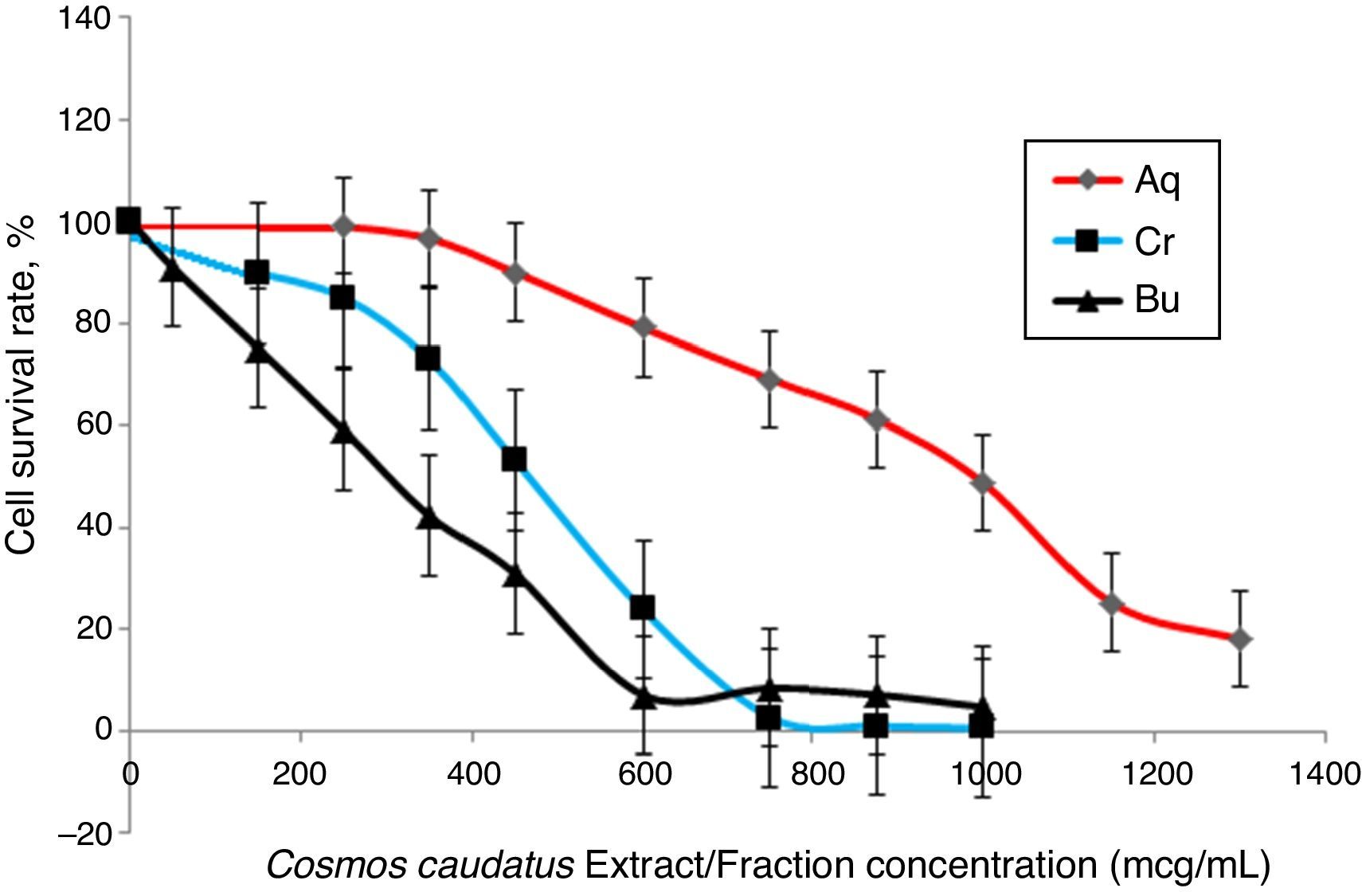
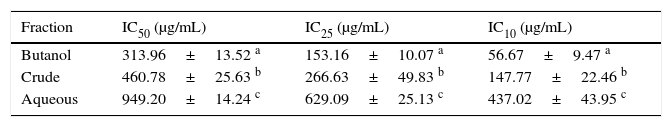
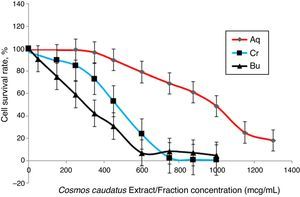
Cell viability assayThe effects of Bu.f, CEE, and Aq.f on vascular smooth muscle cells viability were detected by MTT assay. A-10 cells were treated with different concentrations of C. caudatus extract and derived fractions for 24h. A significant decrease in the cell viability after adding treatments have been shown in a dose- and time-dependent manner (p<0.05) (Fig. 4). Table 1 shows the inhibitory concentrations for each extract/fraction at IC50, IC25, and IC10. Butanol fraction described in the above-mentioned assays as a highly dense fraction of phytochemicals has the highest cytotoxic effect that decreases the viability of A-10 cells, thus, it has the lowest IC values, followed by CEE and lastly the Aq.F.
Cosmos caudatus extract and derived fractions inhibitory concentrations for 10, 25, and 50% of the cell count.
| Fraction | IC50 (μg/mL) | IC25 (μg/mL) | IC10 (μg/mL) |
|---|---|---|---|
| Butanol | 313.96±13.52 a | 153.16±10.07 a | 56.67±9.47 a |
| Crude | 460.78±25.63 b | 266.63±49.83 b | 147.77±22.46 b |
| Aqueous | 949.20±14.24 c | 629.09±25.13 c | 437.02±43.95 c |
a-c Different letters within the same column indicate significant difference (p<0.05).
Potent fraction generally reduces cell viability in the MTT assay in a lower concentration than non-potent ones. Similarly, it gives more pronounced pharmacological effects at lower doses that are usually used to treat cells in culture.28,29 However, butanol and crude fraction/extract in this study cannot be considered cytotoxic due to that the American national cancer institute states that plant fractions are considered cytotoxic to cancer cells when their IC50 is less than 20μg/ml.30 Moreover, concentrations are commonly employed in pharmacological assays should demonstrate a slight reduction in cell viability that does not reach a statistical significance.31 Exceptionally, we tested three concentrations of C. caudatus extract/fractions (IC10, IC25, and IC50), which could cause an effect on cell viability, especially at IC25 and IC50 in order to shed some light on the effects of highly concentrated phytochemicals on cell behavior in vitro. Further explanation for the effect of each concentration on cells in the following pharmacological assays will be discussed later in this article.
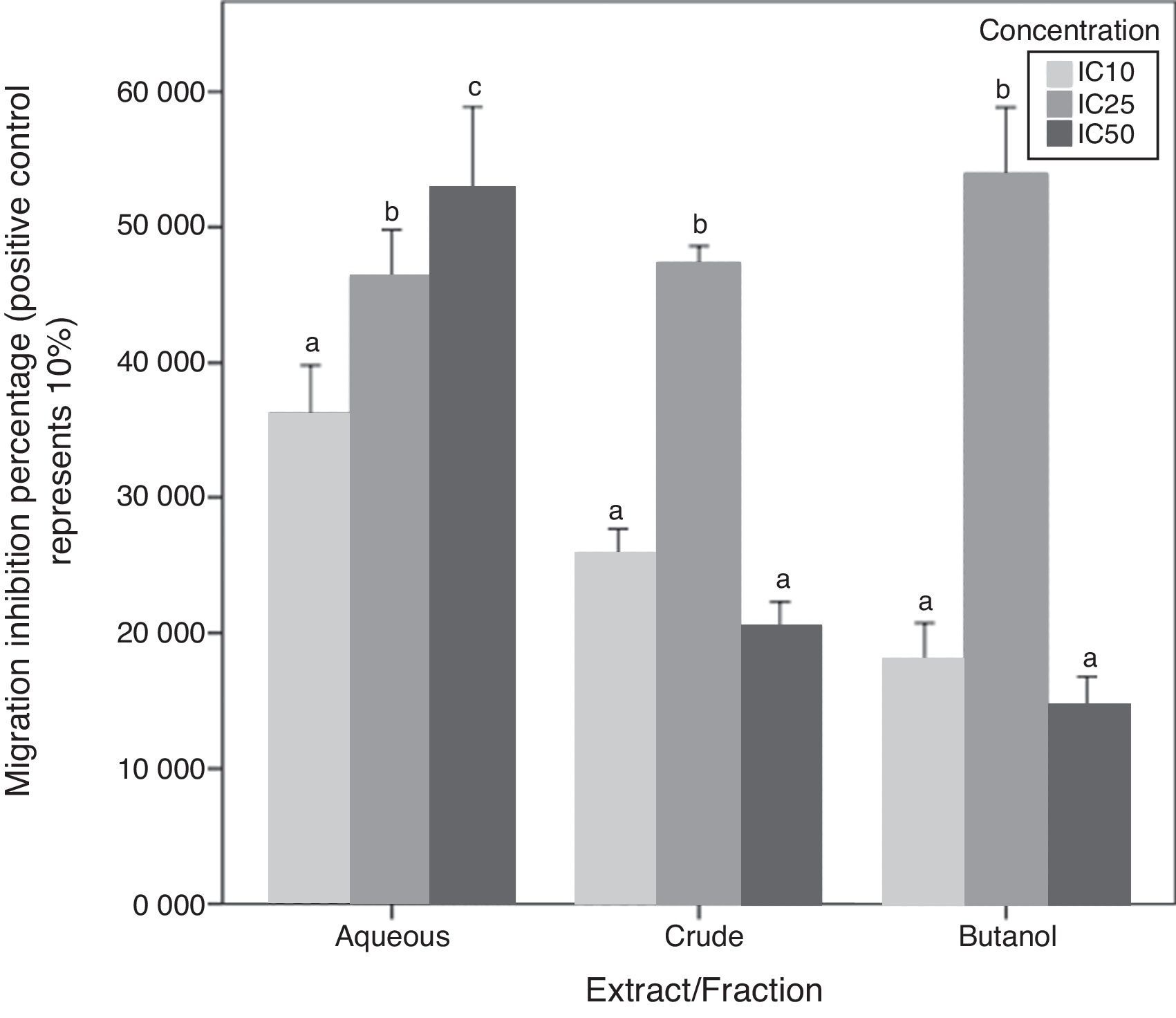
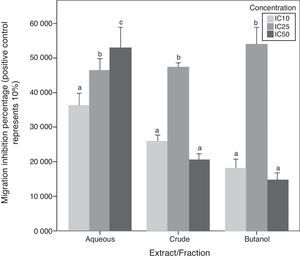
Chemotaxis inhibition activityA-10 cells migration inhibition activity for CEE, Bu.f, and Aq.F of C. caudatus at three different concentrations (IC10, IC25, and IC50), were determined by this assay. The increase of migration inhibition of VSMCs by Aq.F was in a dose-dependent manner; IC50 followed by IC25 have significantly inhibited cell migration more than IC10 (p<0.05). On the contrary, potent fractions (Bu.f and CEE) demonstrated a different mode of response; migration inhibition by IC25 was higher than IC10 in both fractions (p<0.05). However, the highest concentration (IC50) of Bu.f and CEE produced the lowest cell migration inhibition amongst all other concentrations (Fig. 5).
Butanol fraction and CEE induced a different pattern of the cellular migratory reaction in the chemotaxis assay compared to the aqueous fraction. Unexpectedly, the addition of high concentration (IC50) of Bu.f or CEE to cell culture media increased the migration more than other concentrations. This can be explained as the high dose increases oxidative stress due to increasing H2O2 generated in vitro in a dose-dependent fashion. High antioxidants’ concentration and the multiplicity of their compounds supposedly augment H2O2 concentration in cell culture so that they exceeded their antioxidant role and react as pro-oxidants.32 Furthermore, some flavonoids such as catechin, quercetin, and epigallocatechin were found to generate more H2O2 than others when added to cell culture media,33 and those flavonoids were previously screened in C. caudatus extracts.8,13 Moreover, it has been found that many polyphenols are not stable in DMEM, which is the culture media used in this study, and this increases the chance for hydrogen peroxide formation in vitro.34 Aqueous fraction is considered the fraction with the lowest phenolics and saponins’ concentration among C. caudatus fractions in this study. When cells treated with Aq.f at IC50, they showed the highest level of cell migration inhibition in the chemotaxis assay. This can be interpreted by that the concentration of phenolics and saponins found in this fraction was exactly enough to inhibit cell migration more than other fractions at the same concentration. Thus, the concentration of H2O2 might not reach the level whereby the antioxidants converted to pro-oxidants.
In order to prove that cell migration reduction was not caused by reduced cell viability, cells in inserts containing C. caudatus extract/fractions were tested with trypan blue exclusion assay to check their viability after the migration assay. In an unpublished data, 70–80% of cells were still viable after 6h of incubation with fractions/extract. Moreover, in crude and butanol fractions-containing inserts, more cells migrated to the lower chamber when they were treated with the higher concentration (IC50) compared with cells treated with the intermediate one (IC25). This means that cell viabilities have not been affected by treatments’ cytotoxicity due to that cells were exposed to treatments only for 6h, and more importantly, it seems that cells had migrated away from the highly-concentrated treatment in the first few hours before getting affected by it.
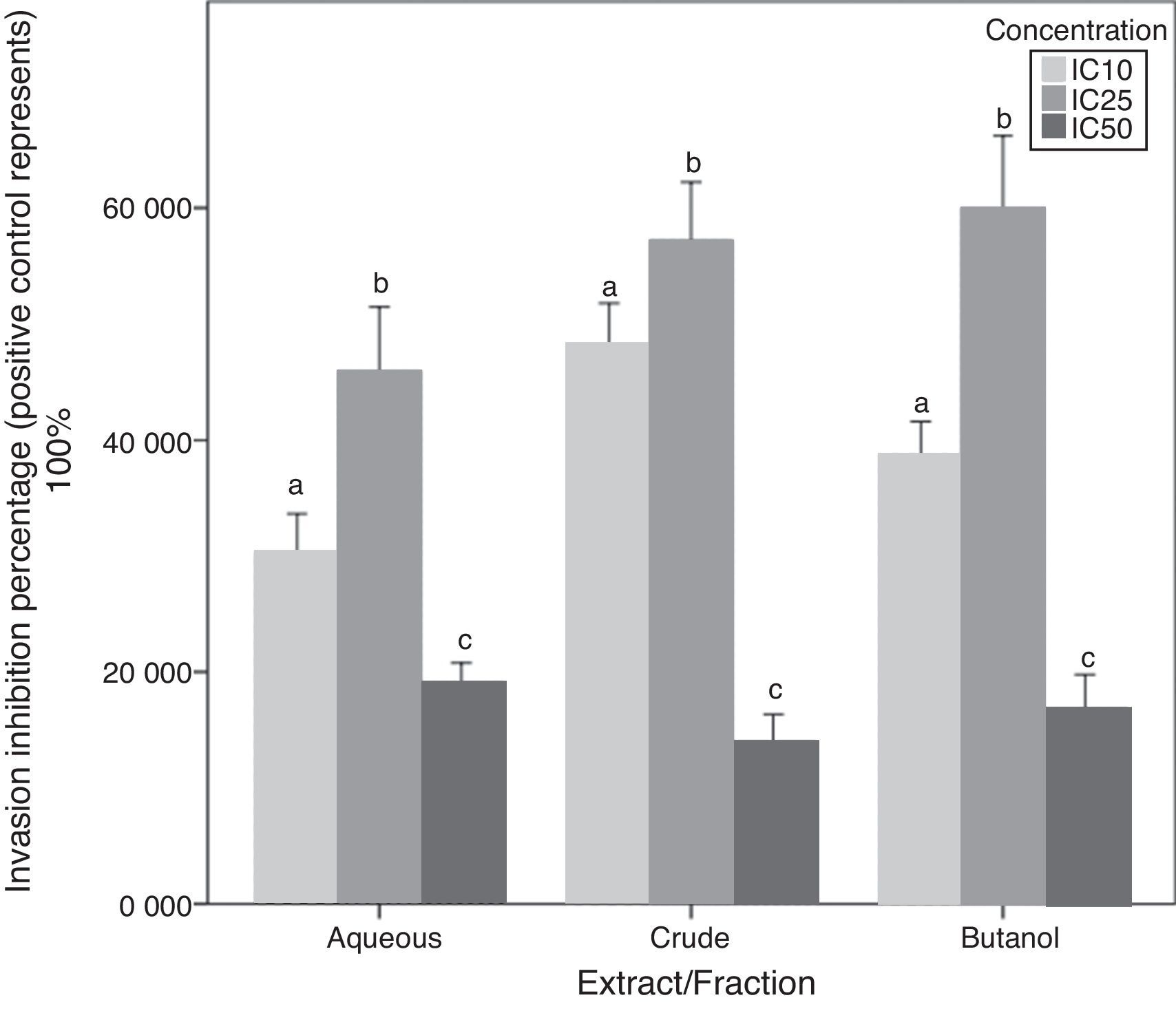
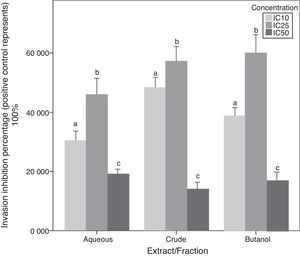
Chemoinvasion inhibition activityA-10 cell invasion inhibition activity for C. caudatus extract/fractions at three different concentrations is determined by this assay. Unlike migration assay, all fractions followed the same trend with increasing treatment concentrations. The IC25 dose showed significantly higher cell invasion inhibition than IC10 (p<0.05). However, when cells exposed to high doses at IC50, their invasion inhibition activity decreased. Similar to cell behavior in the chemotaxis assay, cell invasion inhibition maximum capacity was at the intermediate concentration (IC25) for the three extract/fractions (Fig. 6).
Due to that cell incubation with treatments in chemoinvasion assay was for 24h, we had to prove that cell invasion reduction was not because of cell viability reduction. Chemoinvasion assay detects cells that already performed the invasion process by counting them on the lower surface of the polycarbonate membrane facing the lower chamber. It is obvious that more cells invaded toward the lower chamber in the highest concentration-containing inserts (IC50) compared to mild (IC10) and intermediate (IC25) concentrations-containing inserts. This proposes that cells exposed to IC50 had instantly invaded away from it in the first few hours of incubation and before its viability reduced by the treatment. The in vivo/in vitro discrepancies are well-established especially in terms of cell membrane permeation of drugs and phytochemicals.35 However, cells in culture are less suitable to express oxidative stress suppression by antioxidants due to overproduction of some molecules and signaling factors such as reduced NADP and pyruvate in vitro.36 Therefore, such work can reflect a partial image of the effects of phytochemicals on cell migration and invasion and further in vivo study is needed to confirm these results.
The difference between migration and invasion assays in cell behavior when treated with the aqueous fraction can be attributed to cell properties that make it able to respond to a chemotactic stimuli by migration or invasion. Cells ability to form contractile filaments and microtubules in order to migrate is different from properties needed for them to invade a basement membrane layer such as the activation of metalloproteinases enzymes.37 Besides, diverse treatments and oncogene transfections can possibly affect only one of these properties.38 For instance, Garbisa et al.39 found that epigallocatechin-3-gallate has reduced tumor cell invasion activity but not the migration one.
Cells in culture respond to H2O2 differently according to the cell line and H2O2-neutralizing substances produced by these cells such as the catalase enzyme.33 H2O2 enhances oxidative stress through the activation of transcription factors such as NF-κB and AP-1 and consequently increases expression of adhesion molecules VCAM, ICAM, and E-selectin.40 In this study, high polyphenolics concentration can be considered the key factor that potentiates cell adhesion on the ECM layer and subsequently a higher invasion rate. Moreover, it has been found that H2O2 directly increases the expression of c-Jun as part of its mechanism as an oxidant.41 The up-regulation of the c-Jun NH2-terminal kinase (JNK) signaling pathway activates the transcription factor c-Jun, and the latter increases MMP-2 production and consequently increases cell invasion and migration.42
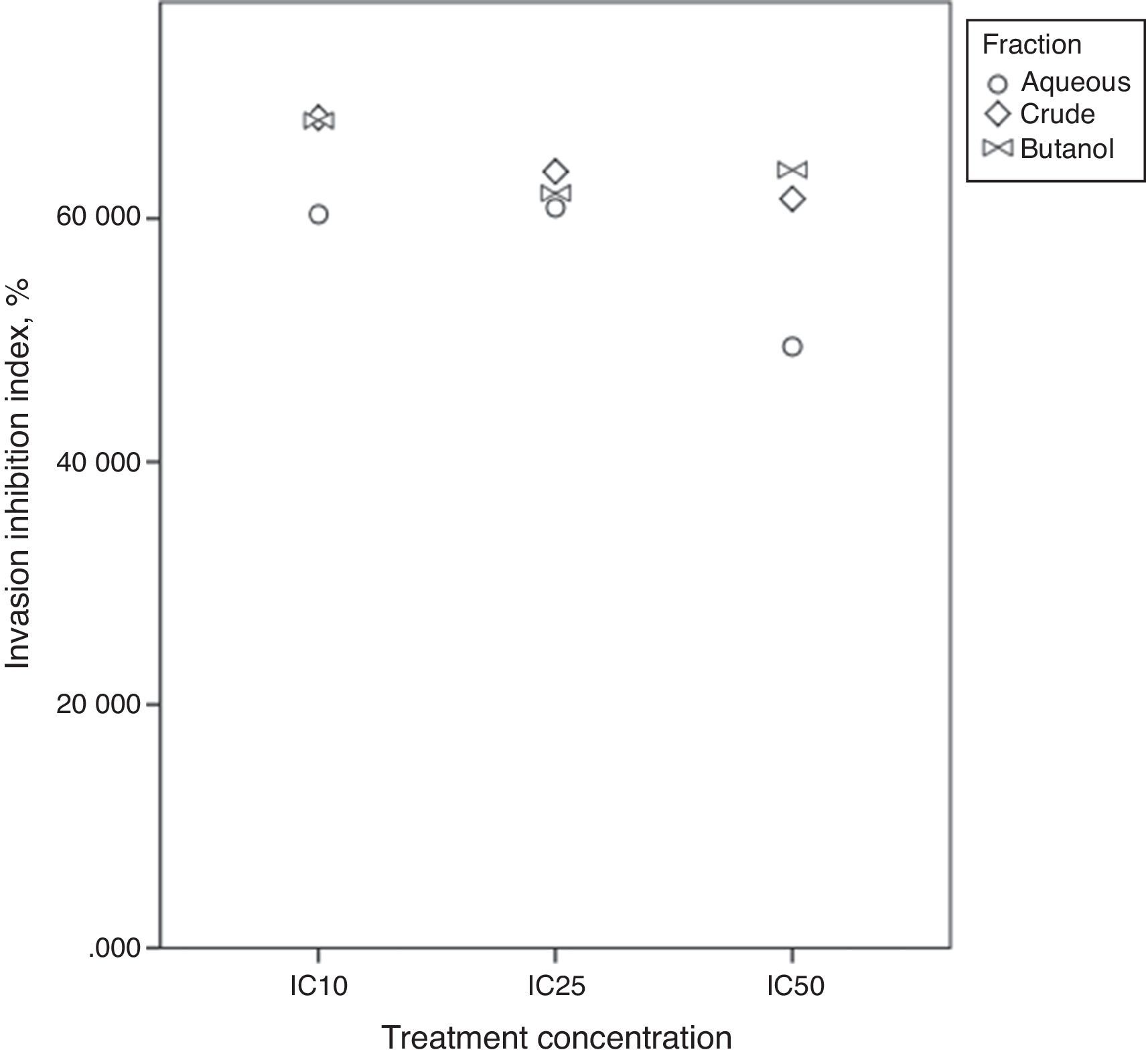
Invasion inhibition indexIn vivo, cell migration and invasion are inseparable, therefore, cell ratio that has the ability to invade the ECM layer to the general cell mobility should be determined. In order to do so, we employed the concept of “the invasive index”.37 This study targets atherosclerosis reduction power of extract/fractions derived from C. caudatus, where we measure their inhibition to smooth muscle cells migration and invasion in vitro. Therefore, we employed the following formula to calculate the invasion inhibition index.
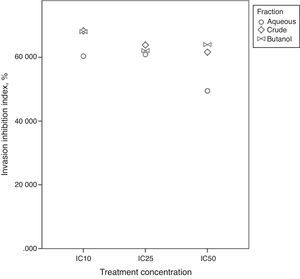
Fig. 7 shows close index values for butanol and crude fractions, while aqueous fraction followed a different trend. This reflects the similarity between crude and butanol fractions constituents and activities. IC25 was the concentration that all three fractions were in close index values. Unexpectedly, Bu.f and CEE at the lowest concentration at (IC10) showed the highest ratio of cell invasion to the total cell migration inhibition, 68.05%, and 68.28% respectively. This indicates that when a closer-to-in vivo system was applied, a lower treatment concentration had achieved a higher cell invasion to migration inhibition ratio and possibly, a more beneficial effect in atherosclerosis reduction.
ConclusionThe present study was designed to determine the antioxidant and cell migration and invasion inhibitory in vitro activities of C. caudatus extracts and derived fractions on vascular smooth muscle cells. In addition to that, it provides a comparison between effects of these extract/fractions as well as the correlation between their phytochemical contents and antioxidant activities. Despite the fact that this study did not screen phytochemicals that are available in each fraction, it offered an insight into potential effects of C. caudatus as an atheroprotective herb that is also used in many aspects of folk medicine in tropical areas.
Firstly, this study has revealed a cost effective extraction method by using aqueous-butanol fractionation system, which piles up high contents of saponins and phenolics in the butanol fraction. Secondly, since Bu.f comprises more phenolics, saponins, and steroidal saponins contents, it showed the strongest activities in all antioxidant assays, besides, it did decrease cell viability in MTT assay more efficiently than other fractions. Thirdly, high concentrations of all fractions demonstrated pro-oxidant effects characterized by increasing migration and invasion rate of the VSMCs, while the lowest concentration of the butanol and crude fractions were better in invasion inhibition index. At the same time, intermediate concentration (IC25) for the three fractions showed the highest cell invasion inhibition. Finally, Cosmos caudatus presents a promising potential for developing new leads that can be used in atherosclerosis suppression.
Conflicts of interestThe authors declare no conflicts of interest.
This research was supported by Universiti Putra Malaysia, Sports Academy, Selangor, Malaysia (Grant number: 5450765-P27799).