Platelet microparticles (PMPs) are small membrane fragments released from activated platelets in response to various stimuli. PMPs serve as biomarkers for several diseases and conditions and are useful tools for prognostic, diagnostic, and therapeutic purposes. The objective of our study was to compare the direct effects of ethylenediaminetetraacetic acid (EDTA) and sodium citrate anticoagulants on platelet structure and PMP vesiculation using transmission electron microscopy to visualize the morphologic changes in platelets. Micrographs revealed that platelets in the EDTA-anticoagulated tube manifested with significant morphologic changes and induced PMP vesiculation. On the other hand, the sodium citrate-anticoagulated tube showed a normal platelet structure and minor modifications in some cases, with poor indication of PMP vesiculation. In conclusion, EDTA induced platelet activation and PMP vesiculation and represents a major source of artifacts during the pre-analysis steps of PMP vesiculation.
Platelet microparticles (PMPs) are small fragments released from platelet cell membranes as a result of cell activation or apoptosis.1 They contribute to a majority (approximately 70–90%) of the total circulating microparticles.2,3 The diameter of PMPs ranges from 0.2 to 1μm; they expose the antigens that represent the parent cell and negatively charged phospholipid phosphatidylserine (PS) as a result of remodeling of platelet plasma membrane. Nowadays, PMPs are well-known as carriers of bioactive molecules that play a role in physiologic and pathologic conditions such as blood coagulation, cell activation, inflammation, and cancer.2,3 Scientific evidence reveals that PMPs have a high association with thrombi formation. For example, patients with deficiency in PMPs or Castaman's defect are at a very high risk of bleeding and tend to show an increased duration pertaining to the bleeding time, despite normal platelet count.4 Moreover, in Scott's syndrome, in which platelets are unable to release microparticles, patients are more prone to bleeding diathesis.5 In clinical practice, circulating PMPs that originate from activated human platelets are increased in various conditions such as prothrombotic disorders, cardiovascular diseases, inflammatory disorders, cancer, infectious diseases, and autoimmune diseases.5,6 In these clinical conditions, the PMP count can serve as a useful tool to identify patients at risk of thrombotic or vascular disorders and evaluate treatment response.7 Therefore, accurate measurement of PMPs is important.
The pre-analytical steps before PMP evaluation represent a potential source of variability and significant artifacts in PMPs preparation. One cause of this variability is the type of anticoagulant used during blood sample collection for PMP analysis. In the last decade, different types of anticoagulants were used for PMP preparation and isolation. It is imperative to consider how these anticoagulants may help reduce platelet activation during blood sample collection and preparation of plasma because platelet activation results in PMP formation and increased PMP count.8 According to Connor et al.,9 the concentrations of procoagulant phospholipid and Annexin V-positive PMPs in the sodium citrate-anticoagulated tube increased during the first 60min of sample collection compared with their concentrations in the EDTA tube. They concluded that, when blood samples are not directly processed, PMP preparation should be performed on EDTA-anticoagulated samples. Another study supported the fact that EDTA is a better anticoagulant than sodium citrate in terms of PMPs analysis.10 On the other hand, many scientists supported the idea that sodium citrate is more effective than EDTA for PMP analysis.11–15 However, no study has compared the direct effect of EDTA and sodium citrate16 and published data on related topics are few.17,18 Therefore, there is currently no recommendation for the ideal anticoagulant for PMP analysis. The objective of our study was to compare the direct effects of EDTA and sodium citrate on normal human platelets, including PMP vesiculation.
MethodsBlood sample collectionAfter obtaining ethical approval and informed consent, blood samples were drawn from eight healthy volunteers not taking any medications and with normal hematologic parameters, such as platelet count, hemoglobin, white blood cell count, and differential count. Blood samples were drawn simultaneously from all subjects. Samples were drawn from the antecubital vein without using a tourniquet and while the subject was lying in a supine position. A butterfly device (blood collection set, 21-G) was used to collect the blood samples. The initial 3ml of extracted blood was discarded to avoid artifacts. Blood samples were collected in 3.2% (0.109M) citrated plastic tube (Vacuette, 3.5ml; Greiner Bio-one, Frickenhausen, Germany) and EDTA tube (Venosafe, Terumo, Somerset, NJ, USA).
Sample processingAll sodium citrate- and EDTA-anticoagulated blood samples were processed by the same operator, in the same way. Samples were centrifuged at 250×g for 15min at room temperature (RT). The supernatant, which contained the platelets, was then aspirated and transferred into a 1.5-ml polypropylene Eppendorf tube (USA Scientific, Ocala, FL, USA). To avoid disturbing the buffy coat layer, aspiration was stopped 1cm above the buffy coat. Then, the samples were centrifuged at 1000×g for 10min to produce platelet pellets. The supernatant was removed before adding the fixatives.
Sample preparation for transmission electron microscopyAfter obtaining the platelet pellets, the first important step was fixation by immediate addition of 2.5% of glutaraldehyde for 4–6h at 4°C. Next, the cell suspension was centrifuged and the supernatant (fixative) carefully removed. An appropriate quantity of animal serum was added to submerge the fixed platelets and to allow the serum to clot at RT. The clotted specimen was placed in a 3-mm tube and fixed in 2.5% glutaraldehyde for 1–2h at 4°C, followed by washing with 0.1M sodium cacodylate buffer three times for 10min each, with 5-min intervals. After fixation, the specimen was mixed in 1% osmium tetroxide for 2h at 4°C and washed again with 0.1M of sodium cacodylate buffer three times for 10min each. After the washing step, the specimen was passed through a series of increasing acetone concentrations, starting with 35% for 10min, followed by 50% for 10min, 75% for 10min, and 100% for 15min for three times each cycle to complete specimen dehydration. Next, infiltration was started by submerging the specimen in a series of acetone-resin mixtures at different concentrations, to remove the acetone.
After the infiltration was complete, the specimen was embedded in a beam capsule and filled with 100% resin. The beam capsule was incubated in the oven at 60°C for 24–48h for polymerization in preparation of specimen sectioning. An ultramicrotome with a glass knife was used to cut the block into 1-μm-thick sections, which were stained and left to stand for 2–4min before washing in distilled water and drying. After identifying the area of interest under the light microscope, the sections were processed under an ultramicrotome with a diamond knife and were further cut into 0.8-μm-thick sections. Then, sections with silver color were selected, fished with a grid, and dried with paper for the staining process. The sections were stained with uranyl acetate for 15min and washed with double distilled water. Finally, while still on the grid, sections were stained with lead for 10min and washed with double distilled water before viewing under the transmission electron microscope (TEM, H7100FA, ×50–×600,000 magnification, 100kV acceleration voltage, Hitachi, Tokyo, Japan).
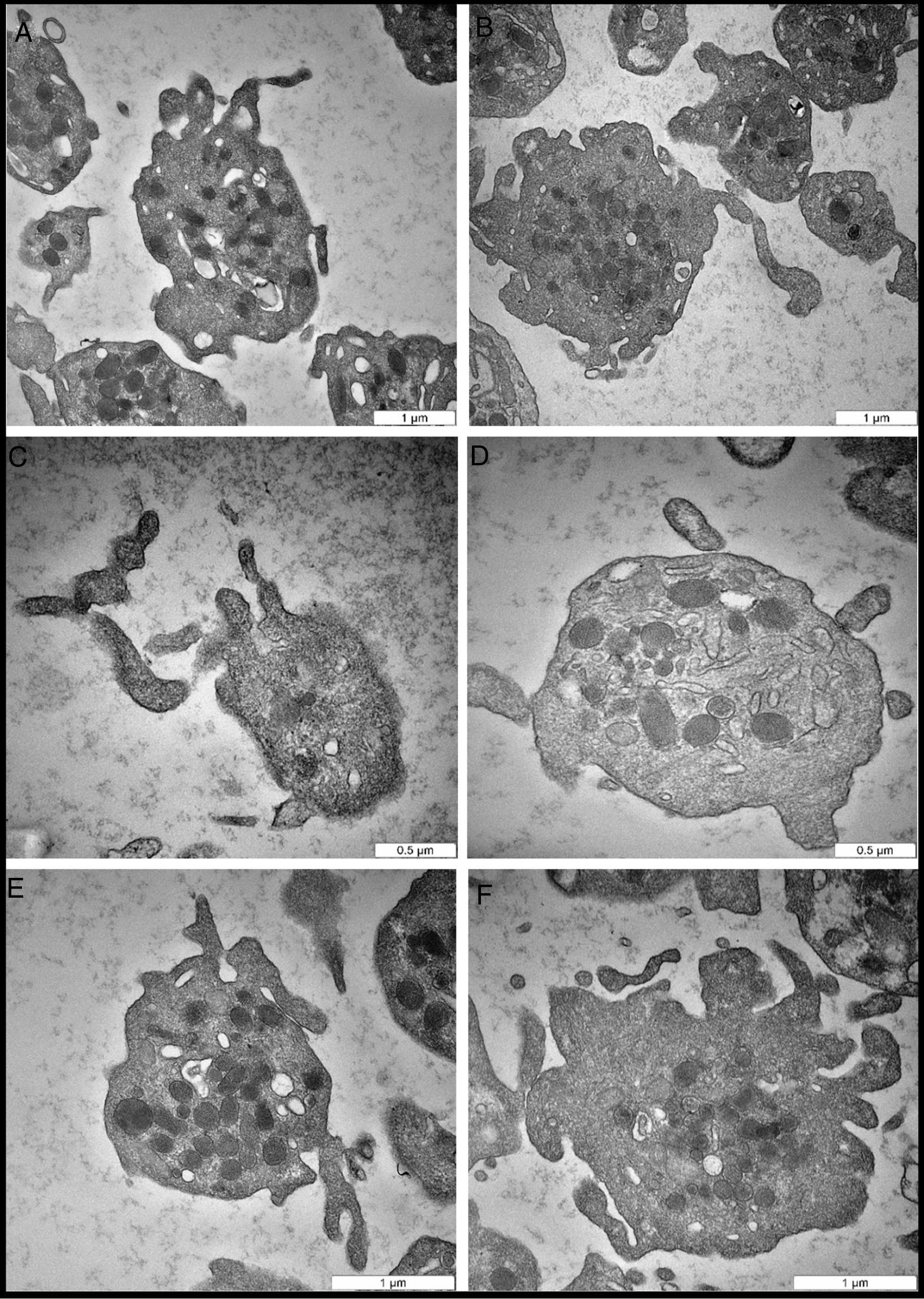
ResultsIn this study, 16–20 platelets were analyzed per subjects, giving an average of 285 platelets of the total 8 donors. The TEM, micrographs of the EDTA-anticoagulated samples revealed dramatic morphologic changes in platelet structure, including the release of PMPs. Platelets from the same healthy donor and same technical preparation showed different morphologic responses to EDTA. Differences in platelet response translated into different morphologic features and response to PMP formation. As clearly shown in Fig. 1A, the cell membrane changed and started to form projections. The open canalicular system (OCS) began to shrink slightly and unfold the invagination to expand the cell membrane area. In Fig. 1B, there was condensation of alpha granules preparing to fuse with each other in the center of the cell to produce platelet secretions.
Representative transmission electron microscopy micrographs of EDTA-induced changes. (A) Activated platelets with significant morphologic changes in the cell membrane, including elongation of projections. (B) Large activated platelet in the center shows potential morphologic changes in the alpha granule bodies, which are preparing to condense and fuse with each other. (C) Extended platelet projections and initiation of PMP vesiculation. (D) Intact activated platelet with cell membrane fusion and PMP formation. Note the dense bodies, and projection of the cell membrane. (E) The cell membrane projections, and condensation of alpha organelles. (F) Platelet in the advanced stage of activation, in which the cell membrane is damaged. PMPs, and other cell organelles, such as dense bodies, and alpha granules, are released.
One of the most prominent morphologic indicators of platelet activation, an irreversible process, is the presence of cell membrane projections that start to extend (Fig. 1C). Interestingly, one of the distinct morphologic features observed were PMP vesiculation and the intact cell membrane with minor changes (Fig. 1D). This feature was more likely due to chronic activation of platelets, in which platelets release microparticles into the circulation without adhesion or aggregation; however, this process is far from being completely understood.
The micrograph in Fig. 1E illustrates the most frequent changes during platelet activation when induced by EDTA. In this process, the cell membrane extended to form cell projections, the OCS disappeared to give the platelet more surface area, and alpha granules condensed in the center of the platelet. The number of alpha granules in EDTA samples was 21.6±9.3 per cell and 7.8±3.8 per cell in sodium citrate samples. Importantly, there was statistically significant mean difference in alpha granules number between EDTA and sodium citrate samples, t=3.36, P=0.007. Finally, the most advanced dramatic changes in platelet morphology are shown in Fig. 1F. The cell membrane was damaged and started to break down into small fragments. The OCS has completely disappeared, the dense bodies released secretions, and the alpha granules condensed; eventually, all these changes led to cell death, the most advanced stage of platelet activation.
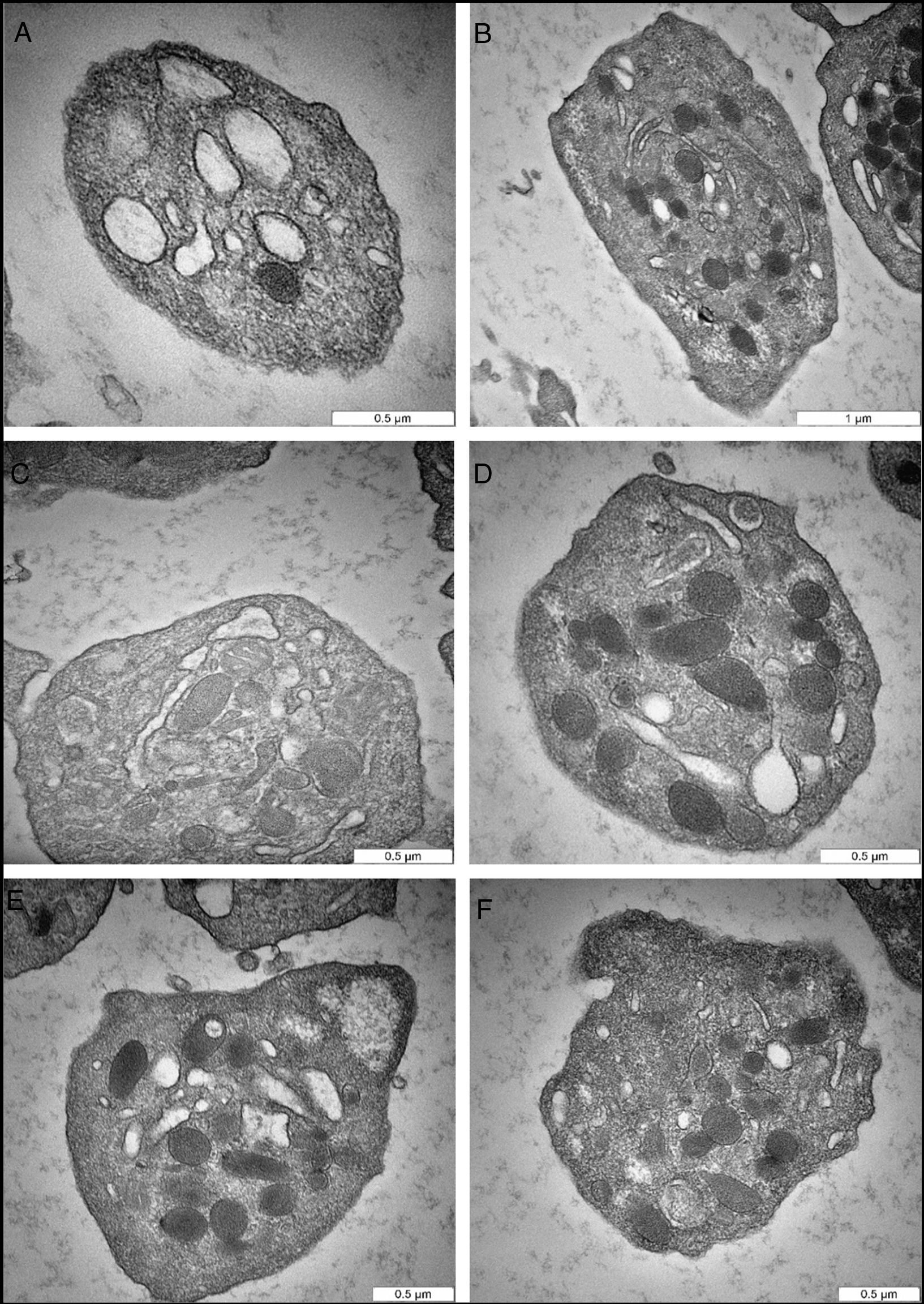
The sodium citrate-anticoagulated samples showed different response in terms of morphologic modulation and PMP vesiculation. Specifically, the micrographs did not show notable changes in platelet structure and there was a poor indication of PMP formation. In Fig. 2A and B, the platelets have smooth, regular, disk or spherical shape; the plasma membrane is relatively smooth without any extended projections. The alpha granules (α granules) and dense bodies (δ bodies) are the defining morphologic features of platelets, and should not be confused with mitochondria, which have similar density and size (Fig. 2C). Fig. 2D shows α granules in oval to round shapes ranging in size from 200 to 500nm in diameter. The granules remain distinct from each other, indicating the organized substructure of the cytoplasm. The δ bodies, which are surrounded by a clear area, are fewer in number than the α granules, appear sharply rounded, and some have a long extension tail (Fig. 2D). In addition to α granules and δ bodies, another remarkable morphologic feature of platelets is the OCS (Fig. 2B and C), which is intertwined with the compact tubular system (DTS) and continues with the cell membrane to form a unique membrane complex. Interestingly, the OCS can be seen clearly and distinctly (Fig. 2B). Finally, in Fig. 2E and F, the platelets manifested some minor changes in the cell membrane, but the cell organelles remain intact. The α granules are distinct and completely separated from each other with no indication of PMP formation.
Representative transmission electron microscopy micrographs of citrate-induced changes. (A) Cross-sectional plane of normal platelet shows smooth regular cell membrane. The open canalicular system, and alpha granules are shown. (B) A platelet cut through the longitudinal plane shows the structure containing alpha granules, and open canalicular system. (C) Normal platelet cut through the cross-sectional plane. The open canalicular system, mitochondria, and alpha granules are shown. (D) Normal platelet reveals intact cell membrane and some cell organelles. The platelet structure shows alpha granules, dense bodies, and filaments extended dense bodies. (E) Normal platelet with glycogen deposit. (F) Platelet in the early stage of activation, as indicated by cell membrane changes.
This is the first study that used TEM to compare the direct effect of EDTA and sodium citrate on platelets and PMP vesiculation. During the pre-analytical processing of the samples from healthy donors, the types of anticoagulant (sodium Citrate and EDTA) employed in PMP preparation were identified as major factors that strongly affected PMP analysis. Given that platelets can be easily activated during the pre-analysis steps, avoidance of ex vivo PMP generation is needed for optimal measurement of circulating PMPs. In this study, we followed the common practices in hemostasis laboratories in order to mitigate the impact of artifacts. Blood sample collection was performed using 21-G needle, a diameter large enough to prevent in vitro hemolysis.19,20 The first 3ml of blood was removed to avoid tissue damage caused by venipuncture.21,22
Technically, the most commonly used anticoagulant in hemostasis laboratories is sodium citrate, which acts by chelation of free calcium to prevent ex vivo coagulation and platelet degranulation.23–25 Because calcium ion is a key molecule in remodeling of membrane phospholipids, sodium citrate was believed to hinder, at least partially, the process of PMP vesiculation.26 Despite this, reactivity of platelets may vary among different anticoagulant tubes. Our findings confirmed that citrated tube produced less artifactual PMPs, compared with EDTA tube. As demonstrated, EDTA had a significant impact on normal platelet morphology and induced PMP vesiculation. It is known that EDTA induces P-selectin-dependent platelet activation and results in platelet aggregation.27 Further, EDTA-anticoagulated tube contains a very high concentration of potassium, which may have unknown effects on PMP vesiculation.28
Connor et al. have approved that the number of A-V-positive PMPs and the concentration of phospholipids in sodium citrate further increased after more than 60min from blood sample collection, compared with the contents in EDTA tube. They recommended that, when blood samples cannot be immediately processed, PMP measurement should be performed in the EDTA-anticoagulated tube. After a 3-h waiting period from blood sample collection, the PMP number increased two-fold when plasma was placed in citrated anticoagulated tube.
Even in normal platelets, ultrastructural variations are usually expected under TEM. For instance, there is heterogeneity in normal platelet size, specifically among adults. Normal platelets have a diameter of 2–3μm and are smooth, regular, and disk-shaped or spherical. The plasma membrane of platelets is relatively smooth compared to that of other circulating blood cells. On the other hand, activated platelets have an irregular cell membrane that corresponds with the number of cytoplasmic organelles in the middle of the cell as a result of remodeling of the cytoskeleton.
The α granules and δ bodies are the defining morphologic features of platelets. The α granules measure 200–500nm in diameter and number 1–25 per cell. The δ bodies are electron-dense, are usually surrounded by a clear area, and are fewer in number than α granules. Based on our TEM observation, δ bodies appeared sharply rounded, but some had a long tail extension. In addition to α granules and δ bodies, another remarkable morphologic feature of platelets is an OCS, which is intertwined with the DTS and continues with the cell membrane to form a unique membrane complex. Interestingly, the OCS can be seen clearly and distinctly in a normal platelet. In an activated platelet, the OCS may shrink and sometimes disappear totally during the activation process.
Many anticoagulants are used to collect blood samples for PMP preparation. It is crucial to bear in mind how these anticoagulants might help prevent platelet activation during blood sample collection and plasma preparation, because platelet activation will lead to release of α granules and δ bodies, and eventually, to PMP vesiculation.8 Sodium citrate is the most widely used anticoagulant for PMP analysis.29 Another anticoagulant that has been used is EDTA,30,31 a strong chelator of calcium ions.
In previous studies, different laboratory techniques have been used to assess the effects of anticoagulants on platelet activation and PMP formation. The techniques include flow cytometry for PMP count, thrombin generation test, and procoagulant phospholipid-dependent clotting assay. However, in this study, we used a different laboratory approach: TEM. We assessed the direct impact of sodium citrate and EDTA anticoagulants by direct visualization of platelets under the TEM. In this study, we assessed the platelets within 1h of blood sample collection; therefore, we could not evaluate the effect of time on the sample. Also, we did not include other coagulation tests aside from the TEM. According to our results, sodium citrate is the more appropriate anticoagulant for PMP analysis.
In conclusion, based on available data from the literature and our findings, EDTA has a significant impact on platelets and induced PMP vesiculation. It may be considered one of the sources of artifacts that could affect PMP analysis.
Authors’ contributionBahaa Hadi Almhanawi designed and conducted the study; Bahariah Khalid supervised the study and review the manuscript; Tengku Azmi Ibrahim TEM consultation and reviewed the manuscript; Eusni Rahayu Mohd Tohit reviewed the manuscript.
Conflicts of interestThe authors declare no conflicts of interest.
The authors wish to thank the University Putra Malaysia and Institute of Biomedical Science (IBS), University Putra Malaysia for the financial support.