Symptoms of multiple sclerosis (MS) are associated with significant and progressive functional disability and have a profound impact on patients’ quality of life (QoL). QoL and daily life activities are two areas that suffer major changes during the course of MS and there are currently no questionnaires specifically designed to evaluate these areas in MS patients.
PurposeTo evaluate QoL of MS patients using the PRIMUS questionnaire and determine the possible relationship between QoL, duration of disease, and disability measured on the EDSS.
Patients and methodsMulti-centre epidemiological and cross-sectional study including 261 patients with relapsing remitting MS (RRMS) or secondary progressive MS (SPMS) treated with interferon beta-1b for at least 6 months. The validated version of the PRIMUS questionnaire was used for patient reporting of changes in QoL and life activities.
ResultsMean age of patients was 41.7±10.3 years; 61.3% were women. Most had RRMS (83.9%). Mean time since MS diagnosis was 7.6±5.8 years, and longer in the SPMS group (11.2±7.4 vs 6.9±5.2, P<.0001). Mean EDSS score was 2.6±1.75 (5.1±1.3 in SPMS vs 2.1±1.4 in RRMS, P<.0001). Mean time since start of treatment was 5.5±3.8 years. The PRIMUS QoL component was higher in the RRMS group: 18.3±6.8 vs 9.9±7.1 (P<.0001); it also decreased with increases in both time since diagnosis (P<.01) and disability scores (from 18.8±6.6 in early stages [EDSS<3.5] to 8.4±6.3 in advanced stages [EDSS>5], P<.0001). The PRIMUS activity limitations component followed the same pattern: activity became more limited with increases in time since diagnosis (P<.0001) and overall disability (P<.0001).
ConclusionsQoL in MS patients varies according to the disease type, and it worsens progressively over time and with increasing disability. The PRIMUS questionnaire is a good tool for assessing QoL and activity in patients with MS.
Los síntomas de la esclerosis múltiple (EM) se asocian a un grado importante de discapacidad funcional progresiva y con un alto impacto en la calidad de vida (CV) de los pacientes. La CV y las actividades de la vida diaria son 2 áreas que presentan mayor modificación con el curso de la EM y, hasta hace poco, no se disponía de cuestionarios expresamente diseñados para cuantificarlos en pacientes con EM.
ObjetivoEvaluar la CV y la limitación de actividades del paciente con EM mediante cuestionario PRIMUS y discernir la posible relación entre CV, tiempo de evolución de la enfermedad y grado de discapacidad medida por EDSS.
Pacientes y métodosEstudio epidemiológico, transversal y multicéntrico, que incluyó a 261 pacientes con EM remitente recurrente (EMRR) o secundaria progresiva (EMSP) en tratamiento con interferón beta-1b durante al menos 6 meses. Se utilizó el cuestionario PRIMUS de calidad de vida validado para autoevaluar cambios en la CV y las actividades.
ResultadosLa edad media ± desviación estándar de los pacientes fue 41,7±10,3 años, siendo mujeres el 61,3%. La mayoría presentaba EMRR (83,9%) y el tiempo medio desde el diagnóstico fue de 7,6±5,8 años, resultando mayor en EMSP (6,9±5,2 vs. 11,2±7,4; p<0,0001). La discapacidad media según la EDSS fue 2,6±1,75 (EMSP 5,1±1,3 vs. EMRR 2,1±1,4; p<0,0001). El tiempo medio desde inicio del tratamiento fue 5,5±3,8 años. El componente de CV es superior en EMRR: 18,3±6,8 vs. 9,9±7,1 (p<0,0001), disminuyendo a medida que avanza el tiempo desde diagnóstico (p<0,01) y con la discapacidad del paciente (entre 18,8±6,6 en estadios iniciales [EDSS<3,5] hasta 8,4±6,3 en avanzados [EDSS>5], p<0,0001). El componente de actividades PRIMUS sigue el mismo patrón, aumentando la inactividad con el tiempo transcurrido desde diagnóstico p<0,0001) y con la discapacidad acumulada (p<0,0001).
ConclusionesLa CV del paciente con EM en tratamiento varía según tipología de la enfermedad y empeora progresivamente con la discapacidad y el tiempo. El cuestionario PRIMUS es una herramienta adecuada para la evaluación de la CV y la actividad en pacientes con EM.
Multiple sclerosis (MS) is the most frequent cause of neurological disability unrelated to trauma in young adults. It affects nearly 400000 people in Europe, with associated social and economic costs ranging from 8.8 to 12.5 billion euros yearly on our continent.1 MS is an inflammatory, demyelinating disease of autoimmune origin that affects the central nervous system. It is characterised by repeated episodes of inflammation (exacerbation) followed by periods of complete or incomplete recovery (remission). If recovery from an exacerbation is incomplete, the destruction of the myelin sheath in concert with axonal damage will provoke many of the progressive and irreversible symptoms in MS.
There are 3 main types of MS: relapsing-remitting (RRMS), secondary progressive (SPMS), and primary progressive (PPMS). At disease onset, 85% of all patients present RRMS, but 50% of them will progress to SPMS within 10 years.2 Beta interferons, a disease-modifying treatment, are the current standard treatment for MS. These drugs have been shown to be effective and safe, although they do present tolerability and acceptance problems for patients over the medium term. These problems are essentially due to their method of administration. In addition, these treatment plans tend to affect the patient's quality of life (QoL).
MS symptoms entail significant levels of progressive and irreversible disability. Given that the disease is normally diagnosed in young patients, its physical and psychological repercussions are considerable, and it affects the QoL of both patients and their families. Most patients live more than half of their lives with this disease. Numerous studies have examined how MS affects QoL, and in fact, several general scales have been published and validated for measuring QoL in this patient population.3–7 Although most of these scales have been validated for use in MS patients, they were not designed for them specifically. One of the most widely used scales, considered by many to be the scale of reference in MS, is the Expanded Disability Status Scale (EDSS). The EDSS was specially designed to classify patients by disability level, but it does not evaluate QoL.
Self-evaluation scales are being used increasingly to analyse patient care and evaluate chronic health conditions. Some of these scales, such as the Patient Reported Outcome Indices for Multiple Sclerosis (PRIMUS), are used in MS to evaluate areas of QoL related to conditions specific to the disease, such as relapses.4,8 They are valuable for predicting outcomes9 and changes in associated socioeconomic costs. The PRIMUS self-assessments have been specifically designed to focus on QoL and activity limitations, which are 2 of the areas that undergo the most changes as MS progresses. Its Spanish-language version has been validated in Spain.11
Despite widespread demand for a specific scale evaluating these areas (QoL and activities) in patients with MS, most Spanish neurologists have not yet been made aware of the PRIMUS. This study aims to introduce Spanish MS specialists to the PRIMUS QoL and Activities scales. It also evaluates QoL in Spanish patients with MS and searches for links between QoL, progression time, and disability on the EDSS scale.
Patients and methodsThis cross-sectional multi-centre nationwide epidemiological study included a total of 261 patients with RRMS or SPMS who attended routine appointments with their neurologists and provided written informed consent to participate in this study. Patients were included in the study using consecutive sampling over a 7-month period. Selected patients had to have received treatment with interferon beta-1b during a minimum of 6 months prior to inclusion. The study protocol was submitted for independent evaluation by a clinical research ethics committee (CEIC in Spanish) belonging to Hospital Universitario Nuestra Señora de Candelaria in Santa Cruz de Tenerife, Spain.
Validated PRIMUS indices submitted by patients with MS were used to evaluate changes on the QoL and Activities scales. The QoL score on the PRIMUS was obtained from a measurement assessing the patient's needs. This approach maintains that a subject's QoL increases alongside his or her ability to meet basic needs. This PRIMUS scale is scored from 0 to 22. The PRIMUS Activities score was obtained from patients’ answers regarding the way MS affects their basic daily activities. It is measured on a scale of 0 to 38.
In a similar way, we recorded the last available EDSS score and its date under typical patient care conditions in normal clinical practice. We also listed values on the EuroQol scale measuring QoL in the general population. The scale examines 5 dimensions: mobility, personal care, activities of daily life, pain/discomfort, and anxiety/depression. Each dimension may have a score of 1 (no problems), 2 (indicating a few problems), or 3 (indicating many problems). Total scores on the EuroQol ranged from 5 to 15; higher values were related to a poorer state of health. Furthermore, the data that were recorded were accompanied by the patient's self-assessment of his or her state of health from 0 to 100 using a visual analogue scale (VAS).
In order to calculate potential relationships between the PRIMUS QoL scale, the QoL scale on the EuroQol questionnaire, and the VAS assessment of overall state of health, we calculated and then analysed Spearman rank correlation coefficients.
ResultsPopulation characteristicsWe recruited a total of 319 patients with MS. In this group, 261 (81.8%) met all of the study's selection criteria and were able to fill out the PRIMUS scales correctly (Activities and QoL). The main reasons for exclusion were as follows: not pertaining to the group of patients diagnosed with RRMS or SPMS and treated with interferon beta-1b during at least the preceding 6 months (13.8%); and not answering the questionnaire for the PRIMUS QoL scale properly (19.0%).
Mean age±standard deviation for the included subjects was 41.7±10.3 years; female patients made up 61.3% of the included population. Most patients presented RRMS (83.9%) and mean time elapsed since detection of the first sign of disease was calculated at 9.7±7.7 years. The mean time since diagnosis of MS was 7.6±5.8 years; this period was longer in patients with SPMS than in patients with RRMS (6.9±5.2 vs 11.2±7.4, P<.0001). Mean disability according to the EDSS was 2.6±1.75; disability level was nearly 4 times higher in patients with SPMS than in patients with RRMS (5.1±1.3 vs 2.1±1.4; P<.0001).
Description of treatment for MSThe mean time elapsed following the official onset of treatment with disease-modifying drugs was 5.5±3.8 years. Interferon beta-1b was the most frequent treatment among Spanish patients with MS (87.9%), followed by glatiramer acetate (6.4%) and natalizumab (3.0%). In addition, interferon beta-1b was also the most common initial treatment for MS (used in 95.4% of these cases).
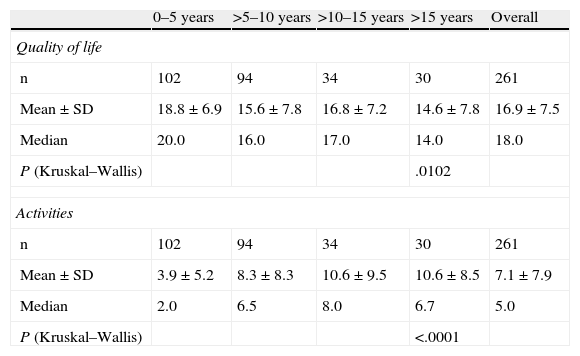
Changes in QoLThe pertinent sections of the PRIMUS questionnaire were analysed according to the time elapsed following initial diagnosis of MS. Table 1 shows that the mean QoL score (on a scale of 0–22) for the Spanish population with MS was 16.9±7.5, while the mean Activities score was 7.1±7.9. Both patient QoL and level of activity decreased as time increased following diagnosis of the disease. In the specific case of the QoL scale, on which higher scores are associated with better QoL, scores ranged from a mean of 18.8±6.9 in early years following diagnosis to a minimum mean of 14.6±7.8 when more than 15 years had passed since the subject had initially been diagnosed with MS (P<.05).
PRIMUS outcome measures according to time elapsed since MS diagnosis.
| 0–5 years | >5–10 years | >10–15 years | >15 years | Overall | |
| Quality of life | |||||
| n | 102 | 94 | 34 | 30 | 261 |
| Mean±SD | 18.8±6.9 | 15.6±7.8 | 16.8±7.2 | 14.6±7.8 | 16.9±7.5 |
| Median | 20.0 | 16.0 | 17.0 | 14.0 | 18.0 |
| P (Kruskal–Wallis) | .0102 | ||||
| Activities | |||||
| n | 102 | 94 | 34 | 30 | 261 |
| Mean±SD | 3.9±5.2 | 8.3±8.3 | 10.6±9.5 | 10.6±8.5 | 7.1±7.9 |
| Median | 2.0 | 6.5 | 8.0 | 6.7 | 5.0 |
| P (Kruskal–Wallis) | <.0001 | ||||
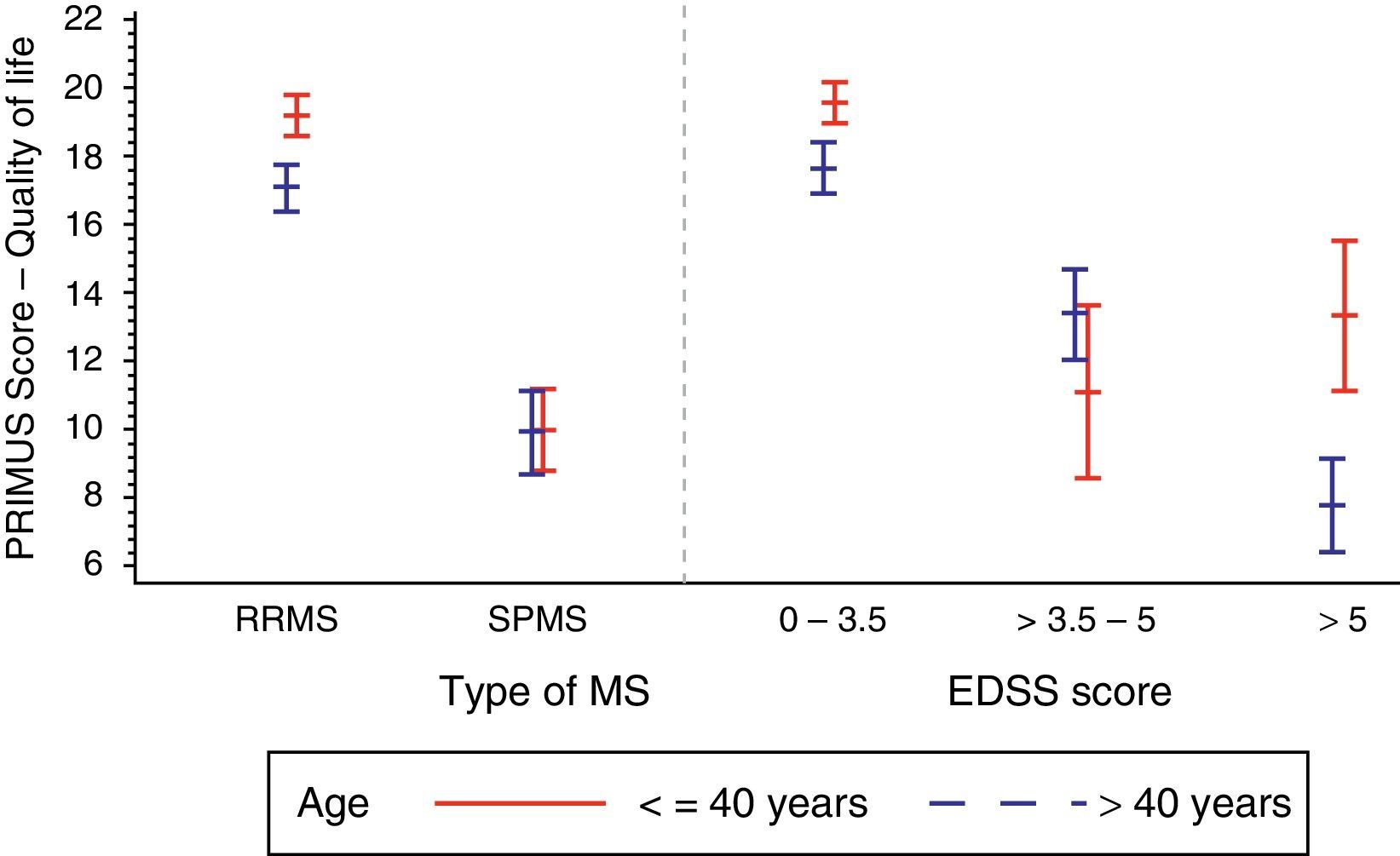
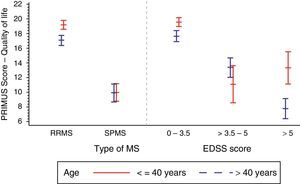
Furthermore, we observe an apparent age effect on the QoL component of PRIMUS. The age effect was more marked in RRMS than in SPMS: 18.3±6.8 vs 9.9±7.1 (P<.0001), with the greatest age effect observed in patients younger than 40 with RRMS (Fig. 1).
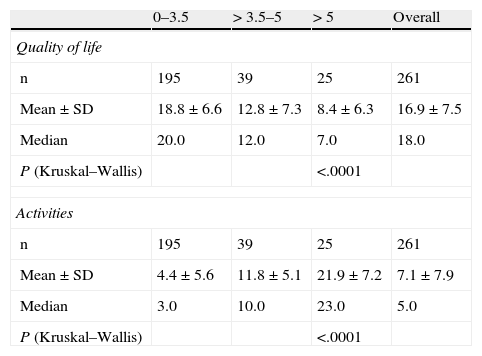
Table 2 shows that the PRIMUS QoL score decreased as the patient's disability level progressed (from 18.8±6.6 in initial stages of the disease [EDSS<3.5] to 8.4±6.3 for the most advanced disability levels [EDSS>5], P<.0001 (Fig. 1).
PRIMUS outcome measures compared to the EDSS scale.
| 0–3.5 | > 3.5–5 | > 5 | Overall | |
| Quality of life | ||||
| n | 195 | 39 | 25 | 261 |
| Mean±SD | 18.8±6.6 | 12.8±7.3 | 8.4±6.3 | 16.9±7.5 |
| Median | 20.0 | 12.0 | 7.0 | 18.0 |
| P (Kruskal–Wallis) | <.0001 | |||
| Activities | ||||
| n | 195 | 39 | 25 | 261 |
| Mean±SD | 4.4±5.6 | 11.8±5.1 | 21.9±7.2 | 7.1±7.9 |
| Median | 3.0 | 10.0 | 23.0 | 5.0 |
| P (Kruskal–Wallis) | <.0001 | |||
Low scores on the Activities scale are associated with lower degrees of activity limitation. Table 1 shows that the score obtained on the PRIMUS activity scale increases progressively over time after MS is diagnosed (from 3.9±5.2 for patients diagnosed less than 5 years previously to 10.6±8.5 in patients diagnosed more than 15 years previously; P<.0001). This is indicative of a progressive and significant decrease in patient function related to the duration of the disease.
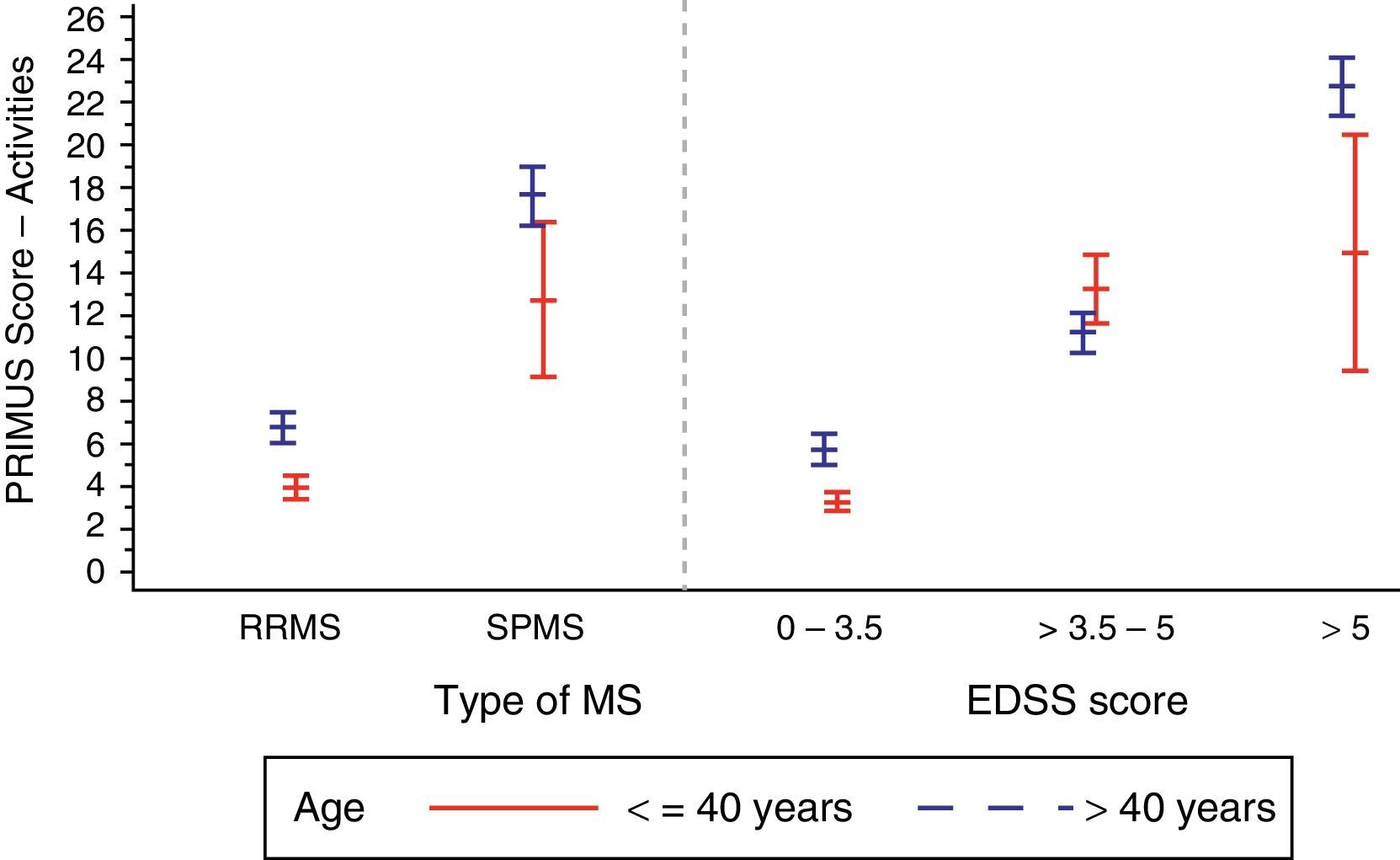
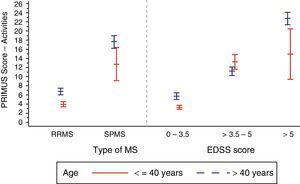
In the analysis of the effect of the EDSS score associated with the disability level (Table 2), we observed a tendency similar to that for disease duration: values ranged from 4.4±5.6 in patients with EDSS scores below 3.5 to 21.9±7.2 in patients with EDSS scores greater than 5 (P<.0001). We also observed higher PRIMUS Activities scores in patients older than 40 as the EDSS scores increased. This trend reflects a rise in difficulty completing activities of daily living as disability level and age increase (Fig. 2). In patients younger than 40, this increase only occurred where EDSS scores were between 0 and 5.
Patients with RRMS generally had lower scores on the PRIMUS Activity scale compared to patients with SPMS, which is indicative of fewer physical limitations being present in the first stage of the disease (5.2±6.3 vs 17.1±8.4; P<.0001).
The EuroQol scale and the general state of health self-assessment (visual analogue scale)The EuroQol scale lets us examine differences in how activities of daily life change over time (Table 3). For example, in the area of ‘mobility’, we observe that 51.0% of all patients had trouble walking; that percentage contained 90.5% of the patients with SPMS and 43.4% of the patients with RRMS (P<.0001)
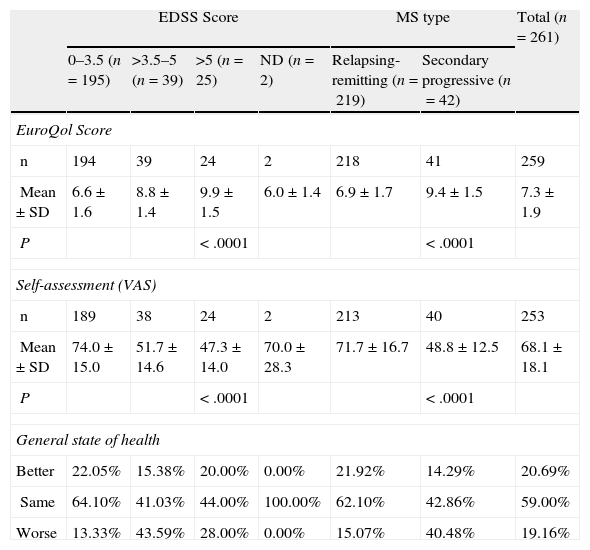
PRIMUS outcome measures compared to the EDSS scale.
| EDSS Score | MS type | Total (n=261) | |||||
| 0–3.5 (n=195) | >3.5–5 (n=39) | >5 (n=25) | ND (n=2) | Relapsing-remitting (n=219) | Secondary progressive (n=42) | ||
| EuroQol Score | |||||||
| n | 194 | 39 | 24 | 2 | 218 | 41 | 259 |
| Mean±SD | 6.6±1.6 | 8.8±1.4 | 9.9±1.5 | 6.0±1.4 | 6.9±1.7 | 9.4±1.5 | 7.3±1.9 |
| P | < .0001 | < .0001 | |||||
| Self-assessment (VAS) | |||||||
| n | 189 | 38 | 24 | 2 | 213 | 40 | 253 |
| Mean±SD | 74.0±15.0 | 51.7±14.6 | 47.3±14.0 | 70.0±28.3 | 71.7±16.7 | 48.8±12.5 | 68.1±18.1 |
| P | < .0001 | < .0001 | |||||
| General state of health | |||||||
| Better | 22.05% | 15.38% | 20.00% | 0.00% | 21.92% | 14.29% | 20.69% |
| Same | 64.10% | 41.03% | 44.00% | 100.00% | 62.10% | 42.86% | 59.00% |
| Worse | 13.33% | 43.59% | 28.00% | 0.00% | 15.07% | 40.48% | 19.16% |
Another area showing significant differences was related to ‘personal care activities’; while 73.2% reported no problems whatsoever, a stark difference was observed in this area when patients were listed by disease type. Difficulty with daily hygiene tasks was reported by 57.1% of the patients with SPMS vs 28.6% who reported no problems related to personal care. In contrast, 81.7% of patients with RRMS indicated that they had no difficulties with personal care, and only 15.5% reported problems with daily hygiene tasks (P<.0001).
Patients with SPMS had higher overall scores on the EuroQol scale (indicating a poorer QoL) compared to patients with RRMS (9.4±1.5 vs 6.9±1.7 (P<.0001). Similarly, the self-assessment for general state of health (VAS) found significant differences between the 2 disease types (71.7±16.7 in RRMS vs 48.8±12.5 in SPMS; P<.0001).
Note that time since diagnosis has a significant effect on the scales examined here: the score on the EuroQol questionnaire gradually increases (P<.0003) and the VAS score decreases (P<.0002) as time elapses following the initial diagnosis of MS. The EDSS stage is another factor that contributes substantially to lower daily activity scores and poorer self-assessments of the general state of health (EuroQol P<.0001 and VAS P<.0001).
Relationship between scalesThe association between the PRIMUS QoL scale and the EuroQol questionnaire was strong but negative (Spearman correlation coefficient: −0.7869). This indicates that high EuroQol scores suggest greater deterioration of the patient's condition, a trend which in contrast is evoked by low scores on the PRIMUS QoL scale. In turn, the correlation between the PRIMUS QoL scale and the VAS scale score was strong and positive (Spearman correlation coefficient: 0.7272), indicating that high scores on both scales show an overall decrease in the patient's QoL.
A strong positive correlation was determined for the PRIMUS Activities score and the EuroQol questionnaire (Spearman correlation: 0.8179). There was a high but negative correlation between the PRIMUS Activities score and the VAS self-assessment (Spearman correlation coefficient: −0.7571).
DiscussionChronic diseases like MS present a host of major challenges when it comes to correctly evaluating self-reported assessments of disease progression and course. These challenges essentially result from the lack of scales specifically designed for this purpose.10 At the same time, the rapid changes in immunomodulatory regimens for MS over the last few years have made effective monitoring of symptoms extremely important in a disease that traditionally led to a rapid decrease in the patient's QoL, in addition to placing a heavy burden on the family and society.12
The development of self-assessment scales that are specifically designed to evaluate the course of MS in patients treated with disease-modifying drugs should allow us to follow the actual course of the disease and determine needs for symptom control in new patients. Among these recently created tools, we find the PRIMUS indices which effectively quantify patients’ symptoms, QoL, and activity limitations.10,13
Our task was to complete a cross-sectional multi-centre observational study in order to evaluate QoL and activity level in Spanish patients with MS using the PRIMUS questionnaire. Our secondary objective was to measure the validity of the PRIMUS for monitoring disease progression in Spanish MS patients. This objective was met by analysing the precise relationship between the PRIMUS QoL scale, the EuroQol questionnaire evaluating QoL, and the visual analogue scale used to measure patient-perceived general state of health.
Key results are that MS-associated disability and the time elapsed since MS was diagnosed both have a large impact on deterioration of the patient's QoL as measured using the PRIMUS indices. This finding coincides with results obtained in other European studies.14–16 It is also in line with recently gathered evidence supporting early onset of treatment, which hampers disease progression in addition to containing the social and personal costs associated with the patient's increasing disability and worsening state of health.17–20
Returning to the previous observation, QoL as measured by the PRIMUS questionnaire is also affected by disease type, since it is clearly lower in patients with SPMS than in patients with RRMS. This tendency largely reflects the fact that advanced stages of MS tend towards the SPMS type; therefore, SPMS is associated with longer elapsed times after diagnosis and higher overall levels of disability.21
In addition to evaluating the PRIMUS QoL scale, this study examined changes in the Activities scale and how they vary with respect to the main clinical factors in Spanish patients with MS. As with QoL, limitations on the patient's activities increase as the degree of disability (EDSS score) increases, which occurs over time after diagnosis. These results are similar to those from previous studies that used the PRIMUS scale,22 but this is the first time they have been specifically confirmed for the Spanish population with MS.
Similarly, this is the first time that sociodemographic data from Spanish patients with MS (age, etc.) have been correlated to the PRIMUS QoL and Activities scales. These associations had only been confirmed in earlier studies using older scales, such as the Musi-QoL,23,24 EuroQol,25 FAMS,26 and the SF-36 questionnaire.6 In light of the above, PRIMUS fills the niche for a new scale measuring impact on QoL and activities and designed specifically for use in patients with MS who are treated with new disease-modifying drugs.
Our study has also enabled us to determine that other scales employed previously, such as EuroQol or the self-assessment using the visual analogue scale, are impacted by the same clinical parameters: time since MS diagnosis, the EDSS stage for disability, and the type of MS. In turn, these 3 parameters determine the degree of deterioration of a patient's state of health.
The new treatments available and earlier treatment onset mean that more time will pass before the patient's disability progresses. Likewise, having access to objective tools that accurately measure changes in these patients’ degree of disability and QoL is crucial to a better understanding of MS pathophysiology.27,28
Previously published studies have listed both QoL and functioning in MS patients as key factors for measuring treatment efficacy.29 Until now, QoL questionnaires used for MS were either generic or had been adapted for use with MS. The PRIMUS developed specifically for MS, and its disability and QoL scales in particular, provide clinical and functional information that is more useful for interpreting changes in QoL and how they may be linked with the courses of treatment prescribed.13 The validity of the PRIMUS indices has been demonstrated by studies that have validated them for MS and by the validations of numerous translated and adapted versions.10,13 Our results from a Spanish population show that PRIMUS correlates well with other types of scales used in MS, whether for QoL (EuroQol) and its components, or for patients’ self-assessments using a visual analogue scale.
Limitations of this study include its cross-sectional design which does not enable us to detect seasonal variations or variations associated with more recent courses of treatment. Even so, certain positive traits support its conclusions, such as the geographic homogeneity and large size of the patient sample included here.
Viewed as a whole, the results from our study confirm that Spanish MS patients currently treated with interferon beta-1b and who are evaluated using the PRIMUS questionnaire show QoL ratings that vary according to disease type and worsen progressively with increases in disability level and in time elapsed since diagnosis. Lastly, we have provided structured arguments showing why PRIMUS indices are a good tool for evaluating QoL components and activities of daily life in patients with MS.
FundingThis study received financial support from Novartis Farmacéutica, S.A.
Conflicts of interestSheila Mora is employed by Novartis Farmacéutica and Novartis S.A. Miguel Ángel Hernández has no conflicts of interest to declare.
We would like to thank all of the researchers in the SLIMS study working group for their active participation.
We also thank Emili González-Pérez (Scientific Department at Trial Form Support, Spain) for his assistance with the manuscript.
Lorena García (Hospital Clínico de Zaragoza); Gerard Soriano, Marina Bufanda, Francisco Lacruz and Teresa Ayuso (Hospital de Navarra); María José Sedano (Hospital Marqués de Valdecilla); Domingo Pérez (Hospital El Bierzo); Sergio Merino (C. Asistencial Palencia); Miguel Ángel Llaneza (C. Arquitecto Marcide); José Ramón Lorenzo (Hospital Povisa); Elvira Muntéis (Hospital del Mar); Miguel Marco (Hospital de Sabadell); Cristina Montero (F. Salut Empordà); Ana Belén Caminero (Hospital Nuestra Señora de Sonsoles); Inmaculada García (Hospital de Fuenlabrada); Inmaculada Pérez and María Rosa García (Hospital Virgen de la Salud); Octavio Sánchez (Hospital Nuestra Señora del Prado); Virginia Meca (Hospital de la Princesa); Antonio Yusta (Hospital Universitario Guadalajara); Josefina Martínez (Hospital Torrecárdenas); Francisco Padilla (Hospital Virgen de la Victoria); Francisco Barrero (Hospital San Cecilio); Esther Cancho (A. de Especialidades Don Benito); Laura Lacruz (Hospital Francesc de Borja); Antonio Belenguer (Hospital General de Castellón); José Meca and Rocío Hernández (Hospital Virgen de Arrixaca); José Antonio Pérez (Hospital Universitario Santa Maria del Rosell); Cristina Croissier (Hospital Universitario de Canarias); Claudia Villar (Hospital Virgen de la Candelaria); Laura Gubieras (Hospital Moisés Broggi); Antonio Cano (Hospital de Mataró).
Please cite this article as: Hernández MA, Mora S. Evaluación de la calidad de vida mediante cuestionario PRIMUS en población española de pacientes con esclerosis múltiple. Neurología. 2013;28:340–7.
Appendix A lists the researchers included in the SLIMS study working group.