Ageing, which commonly underlies dementia, is usually associated with painful conditions. Nevertheless, utility analgesics are underused in dementia patients due to their difficulty communicating. Dementia lesions are also situated on the nociceptive pathways. For this reason, the pain experienced is different and distinctive for every lesion type.
DevelopmentThe lateral pain pathway (lateral thalamus and primary parietal cortex), which is in charge of primary pain perception, is preserved in dementia. Overall pain perception, including pain intensity and threshold, thus remains unmodified. The medial pain pathway includes the intralaminar thalamic nuclei, the pons (locus coeruleus: LC), the mesencephalon (periaqueductal grey: PG), the hypothalamus (paraventricular nuclei, mammillary bodies) and different areas of the parietal (primary, secondary, operculum), temporal (amygdala, hippocampus) and frontal (anterior cingulate cortex: ACC). Since these locations are affected by dementia lesions, pain features controlled by these areas will be compromised: the cognitive-evaluative and affective dimensions, pain memory, and autonomic responses. Alzheimer disease (AD) manifests with reduced anticipatory and avoidance responses and flattening of the autonomic responses. These alterations are essentially secondary to degenerative changes in the medial temporal lobe (pain memory) and ACC (cognitive and affective dimensions) areas. Vascular dementias feature a cortico-subcortical deafferentation secondary to white matter lesions, resulting in a state of hyperpathy and hyperalgesia. In frontotemporal dementias, there is a reduction in pain expression linked to lesions in the orbitofrontal and anterior temporal areas, which are responsible for the emotional component of pain. In Parkinson's disease, painful conditions are common. They are attributed to early damage to the LC, which reduces its antinociceptive activity. Finally, dementia patients expect nothing from analgesic treatments. This negates the placebo effect, which in addition to the drug's pharmacokinetic action is an inherent part of the analgesic response. The placebo response is related to activity in the ACC and PG, but because these areas are commonly affected by dementia, higher doses of analgesics will be necessary.
ConclusionsAssessing pain in dementia is complex, which is why scarcity of the analgesic treatment is underprescribed in dementias. Assessments must be specific and pain scales are useful for examining expressive, motor, emotional, functional, and social interaction capacities. For communicative patients, simple visual scales are helpful, whereas multidimensional scales are the most suitable for non-communicative patients. Pain may be responsible for progression and cognitive deterioration in dementia. Since this aetiology is treatable and reversible, doctors should not hesitate to start analgesic treatment. In order to minimise the risk of adverse events, treatment must be both intensive and also closely monitored.
El envejecimiento, consustancial con la demencia, se asocia comúnmente a patologías dolorosas. Sin embargo, por las dificultades de comunicación, el uso de analgésicos está reducido. Por otra parte, las lesiones de las demencias asientan en áreas comunes con las vías nociceptivas. Ello condiciona una modificación de la experiencia dolorosa, diferente para distintas lesiones.
DesarrolloDe las vías dolorosas, la lateral (núcleos talámicos laterales y cortex parietal primario) se encarga de la percepción primaria; está respetada en las demencias. De aquí que la percepción dolorosa, intensidad y umbrales estén preservados. Las vías dolorosas mediales, incluyen núcleos laminares talámicos, protuberancia (locus ceruleus: LC), mesencé-falo (sustancia gris periacaueductal: SGP), hipotálamo (núcleos paraventriculares, tubérculos mamilares) y zonas del cortex parietal (primario y secundario, opérculos), temporal (amigdala e hipocampo) y frontal (cortex cingular anterior: CCA). Coinciden con áreas de lesiones de las demencias. Por lo tanto, se verán afectados aspectos del dolor representados en estas zonas: cognitivo-evaluativo, emocional-vivencial, de memoria dolorosa y autonómico. En la enfermedad de Alzheimer (EA), hay un aplanamiento de respuestas autonómicas y una reducción de las de anticipación y evitación del dolor; se relaciona con los cambios degenerativos mediales temporales (memoria del dolor), y también con los afectivos y cognitivo-evaluativos, por afectación del CCA. En la demencia vascular, existe una desaferentización, por lesiones de sustancia blanca fronto-subcortical; la consecuencia, es un estado de hiperalgesia e hiperpatía. En la demencia frontotemporal, está reducida la expresividad del dolor, ligada al componenente afectivo-emocional, consecuencia de la lesión orbtofrontal y temporal anterior. La enfermedad de Parkinson, caracterizada por un exceso de síndromes dolorosos, se vincula con lesiones precoces del LC y atenuación de su acción antinociceptiva. Por otra parte, en el paciente demente no existe el componente de expectativa ante un tratamiento analgésico, es decir, no hay respuesta placebo que, sumada al efecto farmacocinético, se a¿nade a toda respuesta analgésica. Está ligada a activación preferente de CCA y SGP, asiento lesional común en demencias. La consecuencia, es que en la EA se requerirán dosis mayores de analgésicos.
ConclusionesLa evaluación del dolor en la demencia es compleja. Ello explica las carencias de analgesia. Ha de ser sistematizada. Las escalas la facilitan: en pacientes comunicativos, formas simples (verbales o visuales), en pacientes no comunicativos escalas multidimensionales. Examinan aspectos expresivos, motores, emocionales, funcionales y de interacción social. El dolor puede condicionar un deterioro o progresividad de la demencia. Es una causa tratable y reversible, por lo que no debe dudarse en iniciar tratamiento analgésico si se sospecha. Será intensivo y cuidadoso, para minimizar el alto riesgo de efectos secundarios.
Dementia and painful conditions are both linked to ageing. The number of patients with either type of condition increases almost exponentially with age. Ageing, described as a process of decline in multiple organs displaying reduced adaptive responses, has an impact on a wide variety of tissues. Affected osteoarticular tissue is accompanied by pain.1 Peripheral nervous tissue and its nociceptors are also affected, as is the central nervous system itself; this is particularly common in cases of dementia. Painful conditions appear almost systematically as the patient ages, while at the same time, changes in nervous structures may modify how pain is experienced and expressed. The purpose of this study is to shed light on the interaction between ageing and these conditions and describe specific traits of painful conditions appearing in either normal ageing or dementia.
Doctors generally agree that analgesic consumption is low among dementia patients. It would be reasonable to posit that this lower use of analgesics might respond to a decreased rate of osteoarticular disease in patients with dementia.2 Nevertheless, this possibility has never been confirmed; the prevalence of osteoarticular disease is similar for age- and sex-matched patients with and without dementia.3,4 Lower consumption of analgesics is consistent for all drug groups: paracetamol, non-steroidal anti-inflammatory drugs (NSAIDs), and opiates.5 In fact, decreased cognitive capacity is the independent variable that best correlates with reduced use of analgesics in elderly patients with cancer and other painful diseases.6–9
If pain is in fact undertreated in dementia, we should investigate the potential causes of this tendency.10 First of all, we find a primordial, almost atavistic notion that pain is a part of ageing, and that growing old is accompanied by an increased burden of suffering that the patient's years and life experience will teach him to bear with the necessary stoicism. This idea is expressed in key works of philosophy and literature, both Spanish11 and international12; in addition, it coincides with the results from qualitative studies carried out to ascertain carers’ and institutionalised elderly patients’ opinions of the pain experienced by the latter group.13 This cultural or anthropological factor coexists with others – the fear of opiate dependence, for example – which undoubtedly contribute to the limited presence of pain treatment in dementia. And furthermore, the different degrees of sensory, sensitive, and especially cognitive impairment make it difficult to communicate with elderly patients. As a result, assessing their pain is a challenging task for the neurologist, who must first determine if pain exists, and then attempt to measure the level. On the other hand, anatomical changes, each corresponding to a different type of dementia, result in different degrees of impairment affecting pain signal transmitting or processing areas. As such, different dementia types may elicit distinct pain responses in the presence of the same painful disease.
DevelopmentIn the following sections, we will first describe the specific anatomical and clinical expressions of pain associated with the most common types of dementia, before touching on such factors as lack of expectations and the resulting absence of the placebo effect, which is common to all dementia types. Lastly, we propose guidelines for evaluating pain and using pain scales in dementia patients.
The link between pain in dementia and lesions specifically affecting the nociceptive pathwaysPain sensations are relayed by superficial and visceral nociceptors along fine-calibre, thinly myelinated axons that reach the dorsal horn of the spinal cord. From that point, they ascend along the lateral funiculus to reach the brain. The brain's anatomical structures forming the pain system are particularly intriguing, for 2 reasons. Pain is transformed into painful sensation in this system, which also houses the structural lesions typical in dementia, at locations that are often shared by central nociceptive pathways and regions.
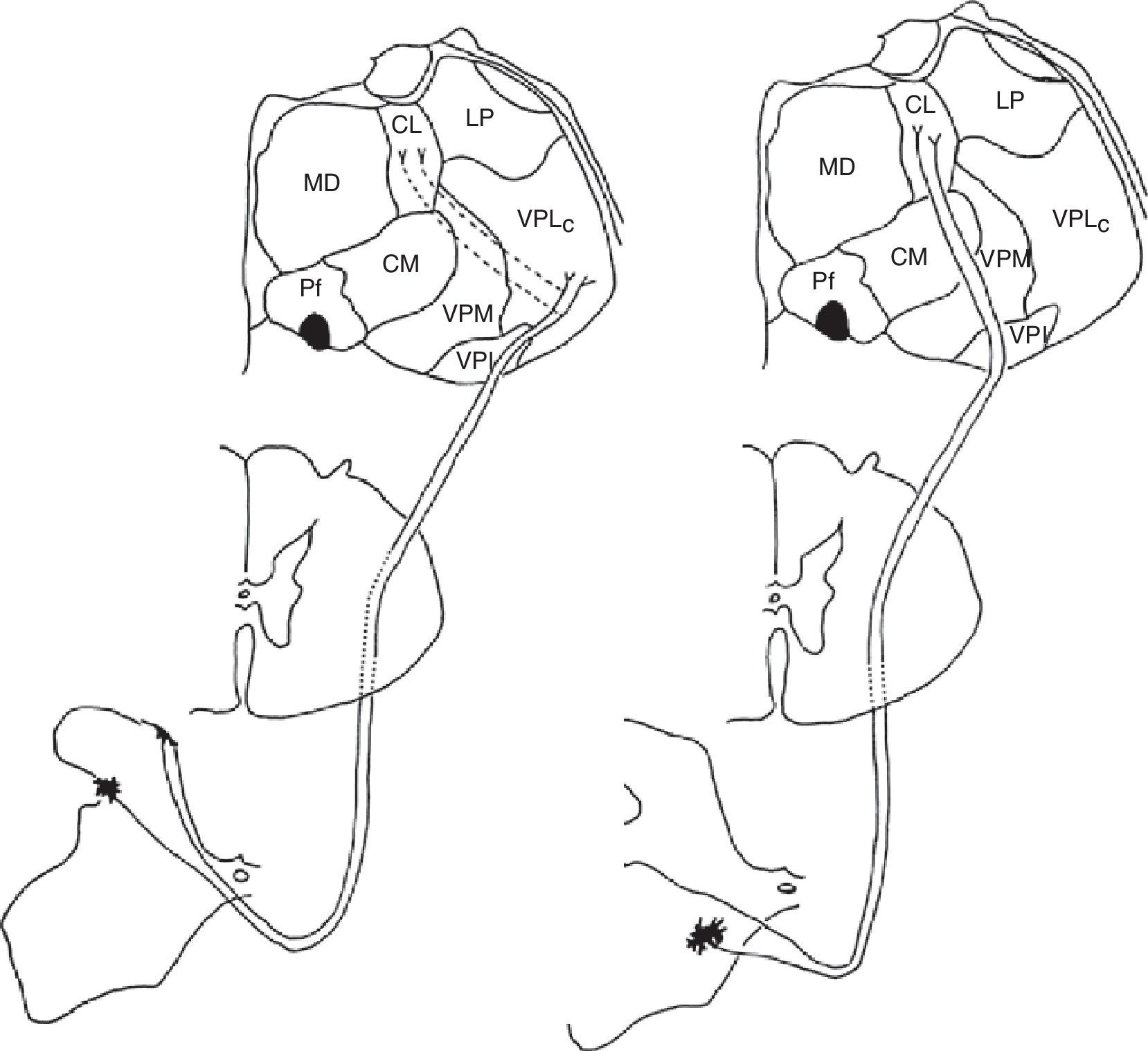
Medial and lateral nociceptive systemsFor practical reasons and to maintain clarity, we will mention only the most relevant localisations in the brainstem, thalamus, and cortex pertaining to the medial and lateral nociceptive systems (Figs. 1 and 2). The reader is invited to consult the excellent in-depth descriptions of these systems and other pain pathways provided in other works.14
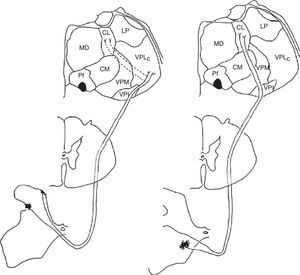
Diagram of the lateral (left) and medial (right) pain systems. The lateral system is represented by the lateral spinothalamic tract which has a sensory relay at the ventral posterolateral nucleus of the thalamus (left). The medial system relays sensory information by means of the medial spinothalamic tract, which ends at the midline and intralaminar thalamic nucleus. The thalamic centrolateral nucleus is shown in the figure (right). Thalamic connections between the two systems are scarce and shown on the left (dotted lines). Injury to these connections gives rise to thalamic pain syndromes.
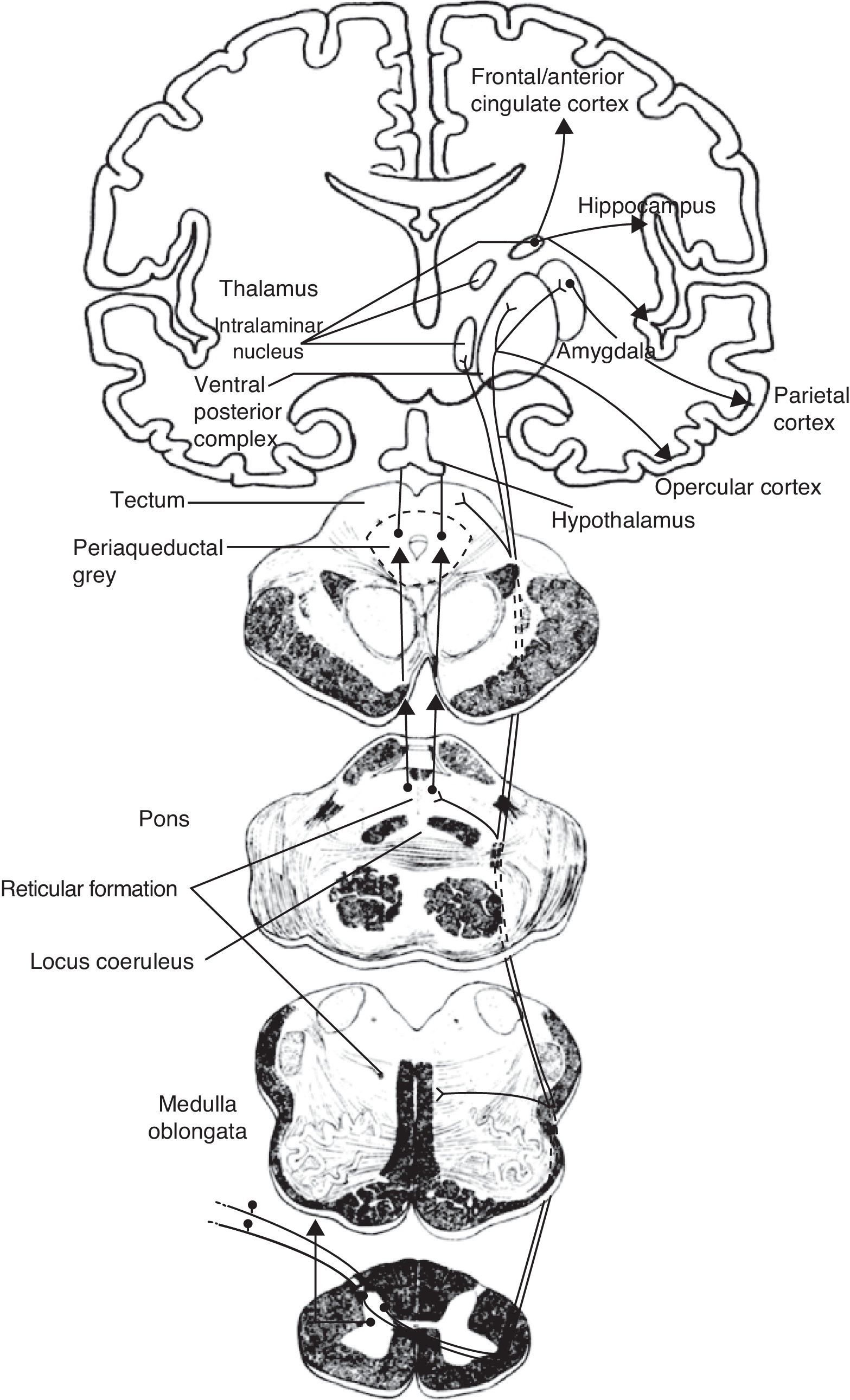
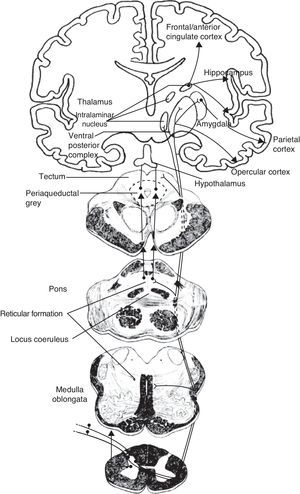
Schematic of the efferent pathways of the lateral pain system, which project from the ventral posterolateral nucleus of the thalamus to the primary parietal cortex; schematic of the efferent pathways of the medial pain system, which reach numerous cortical areas and the hypothalamus, according to the more complex aspects of pain perception that they transmit (see text).
The medial pain system is the most complex. It is related to the pain experience, that is, the emotional and cognitive aspects of pain. This system includes the spinothalamic tracts, which project directly to the intralaminar thalamic nuclei; the spinoreticular tract, which projects to the reticular formation of the pons (parabrachial nucleus and locus coeruleus [LC]); and the spinomesencephalic tract, which projects to the midbrain periaqueductal grey. At the same time, there are also connections between the midbrain and the pontine reticular formation, and between intralaminar and midline thalamic nuclei. The thalamic nuclei transmit information to sensitive perisylvian areas (insula, parietal operculum, secondary somatosensory cortex [S2]), and the anterior cingulate cortex (ACC). In turn, the nociceptive information travels directly from the parabrachial nuclei and LC of the pontine reticular formation to the amygdala, hippocampus, and hypothalamus (the paraventricular and tuberomammillary nuclei).
The lateral pain system, which is less complex than the medial pain system, is responsible for more simple facets of perception, specifically locating pain and measuring its intensity. Like the medial pain system, it also transmits this information by means of the spinothalamic tracts, although the lateral pain system transmits to the lateral thalamic nuclei. From this point, the most important projections stretch to the primary somatosensory cortex (S1) and to the S2, parietal operculum, and insula.
We therefore see that certain anatomical structures are shared by the medial and lateral pain systems, especially in the perisylvian areas.15 Nevertheless, connections between these systems are scarce and limited to the thalamus, between its medial and lateral relay nuclei, pertaining respectively to the medial and lateral pain systems. Despite being few in number, these connections are of considerable clinical interest because a disconnection at this level results in central post-stroke pain syndrome, a type of deafferentation pain.16
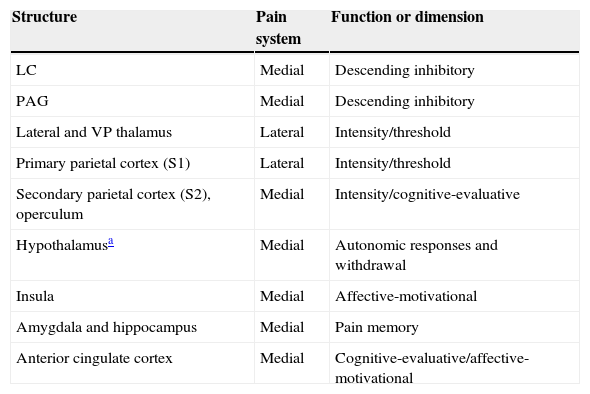
The many facets of pain are represented by the 2 anatomical systems described here (Table 1). As mentioned before, the medial pain system is more complex; it is responsible for the affective-motivational and cognitive-evaluative dimensions, for pain memory, and for autonomic-neuroendocrine responses. The lateral pain system engages in the more primitive sensory-discriminative dimensions of pain which are represented in the S1 cortex. The phylogeny and ontogeny of this structure pre-date those of the cortex involved in the emotional and cognitive responses of the medial pain system. Since these responses hold the most interest in the context of dementia, we will now describe them as well as their anatomical localisation.
Main anatomical structures and their function in cerebral pain pathways.
| Structure | Pain system | Function or dimension |
|---|---|---|
| LC | Medial | Descending inhibitory |
| PAG | Medial | Descending inhibitory |
| Lateral and VP thalamus | Lateral | Intensity/threshold |
| Primary parietal cortex (S1) | Lateral | Intensity/threshold |
| Secondary parietal cortex (S2), operculum | Medial | Intensity/cognitive-evaluative |
| Hypothalamusa | Medial | Autonomic responses and withdrawal |
| Insula | Medial | Affective-motivational |
| Amygdala and hippocampus | Medial | Pain memory |
| Anterior cingulate cortex | Medial | Cognitive-evaluative/affective-motivational |
LC, locus coeruleus; PAG, periaqueductal grey.
The lateral pain system manages this dimension by means of projections leading directly or sequentially from the lateral thalamic group to the S1 and S2 regions of the parietal cortex. Through this system, pain stimuli also reach the insula and the parietal operculum. The integrity of the latter structure determines whether the pain threshold will be normal, as well as if the individual will be able to identify nociceptive qualities related to pain location, intensity, and type.17,18 These anatomical systems are usually preserved in Alzheimer disease (AD), in contrast with structures pertaining to the medial pain system. As a result, pain perception, intensity, and threshold are typically spared in AD.15
Affective-motivational dimensionThis dimension involves 3 groups of structures: (1) the ventral posterior thalamic nuclei, which project efferent fibres to the parietal operculum and the insula; (2) the ACC, which receives afferent fibres from the spinothalamic tracts, the spinoreticular tract, and the spinomesencephalic tract (there are also connections between the insula and the ACC that reinforce motivational and affective traits in pain); and (3) the hypothalamus and prefrontal cortex, with the latter playing an additional role in pain anticipation and avoidance behaviour.19
Cognitive-evaluative dimensionThe pontine reticular formation, and specifically the LC, provides an initial cognitive approximation of pain by directing the individual's attention to it. This being the case, when other cognitive tasks or distractions are in progress, both the S2 and the ACC will respond less to painful stimuli.20 The ACC is the central structure in the cognitive-evaluative dimension of pain, and its function is based on the flow of information it receives from the parietal operculum and the insula.21
Pain memoryAs in other types of memory, the medial regions of the temporal lobe (hippocampus and amygdala) manage pain memory. They receive afferent signals from both the lateral and medial pain systems, fundamentally from S2 and the insula. By a more direct path, pain memory may reach the amygdala from the parabrachial nucleus in the pontine reticular formation.14 The ventral posterior thalamic nuclei also play a role in pain memory. Here, stimulation evokes and elicits specific painful sensations experienced at earlier times in a person's life.15
Lastly, the prefrontal cortex and the ACC assess the consequences of pain and prepare for neutralisation or defence responses by coordinating cognitive-evaluative and strictly sensory dimensions of the pain.21
Autonomic responsesPain has a significant autonomic component ranging from external signs (pallor, perspiration, piloerection) to cardiovascular responses (variations in heart rate or blood pressure), digestive alterations (vomiting or diarrhoea), or urinary changes (urgency or incontinence). This component is mediated by the mesencephalic periaqueductal grey,22 but most of all by the hypothalamus, which plays a central role in aversive behaviour and in autonomic and neuroendocrine responses to pain. The hypothalamus receives afferent signals from the pontine reticular formation, after which it establishes connections with the prefrontal cortex, amygdala, and hippocampus. Of particular interest here are the tuberomammillary nuclei (the only histaminergic nuclei in the brain) and the paraventricular nuclei. These structures produce vasopressin and oxytocin, co-localised with corticotropin-releasing hormone (CRH). All of these peptides exert an antinociceptive effect, and their expression is altered in ageing and in AD.23
Alzheimer diseaseThe changes in pain response in AD are determined by structural changes in the medial pain system and the sympathetic nervous system that houses it. As indicated in a previous section, the lateral pain system is essentially spared. This being the case, no studies have described lesions in the lateral thalamic nuclei, and both S1 and its connections to S2 also remain preserved until advanced stages of the disease.24,25
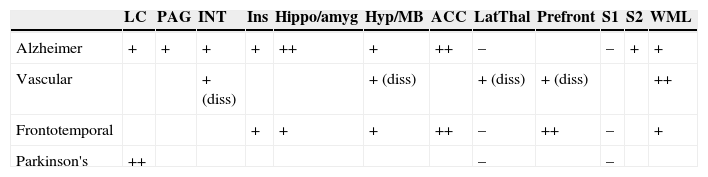
In contrast, the histopathological changes in AD can be found quite consistently at all levels of the medial pain system (Table 2):
- a.
Brainstem: both the pons (very early impairment of the LC26,27) and the midbrain (periaqueductal grey). Both of these structures engage in antinociceptive action.
- b.
Thalamus: here, they affect the medial and intralaminar nuclei.27
- c.
Cortex: affecting pain memory areas (amygdala and hippocampus), as well as in the frontal cortex, the ACC, and prefrontal cortex.28
- d.
Hypothalamus: especially in histaminergic neurons of the mammillary bodies, which exert an analgesic effect. Paraventricular nuclei do not display structural changes, and therefore CRH-producing neurons may be hyperactive in AD and elicit a rise in plasma cortisol.29
Localisation of lesions affecting pain pathways in the main dementia types.
| LC | PAG | INT | Ins | Hippo/amyg | Hyp/MB | ACC | LatThal | Prefront | S1 | S2 | WML | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alzheimer | + | + | + | + | ++ | + | ++ | – | – | + | + | |
| Vascular | + (diss) | + (diss) | + (diss) | + (diss) | ++ | |||||||
| Frontotemporal | + | + | + | ++ | – | ++ | – | + | ||||
| Parkinson's | ++ | – | – |
LC, locus coeruleus; PAG, periaqueductal grey; INT, intralaminar nuclei of the thalamus; Hippo/amyg, hippocampus and amygdala; Hyp/MB, hypothalamus, mammillary bodies; ACC, anterior cingulate cortex; LatThal, lateral nuclear group of thalamus; S1, primary (perceptual) somatosensory cortex; S2, secondary (associative) somatosensory cortex; WML, white matter lesions; diss, lesion by dissociation. Spaces left blank indicate lack of studies or conflicting information.
According to this histopathological pattern, the pain threshold is preserved in AD, which is consistent with an intact S1. This tendency has been demonstrated in studies using painful electrical stimulation.5,15
Autonomic responses to low-intensity stimuli are blunted such that potent pain stimuli must be used in patients with AD to elicit responses equivalent to those exhibited by control subjects.30 More recent studies have shown that these responses are inversely correlated with the degree of cognitive impairment: a lower score on the mini-mental state examination (MMSE) corresponds to a lower heart rate in response to electrical pain delivered to the wrist.31,32 This phenomenon occurs in the context of intact responses to touch and pain. This means that even if autonomic responses are abnormal, normal pain and touch thresholds will be spared in patients with AD.
Whereas lesion to the tuberomammillary nuclei of the hypothalamus is responsible for blunted autonomic responses, degenerative changes in cortical structures and brainstem nuclei are responsible for impairment of all other dimensions of pain. Impairment in any of these dimensions has to do with damage to the medial pain system. Specifically, plaques, tangles, and deposits on the amygdala and hippocampus explain the decrease in pain memory. Likewise, we can predict that lesions to the LC, S2, and ACC will affect the cognitive-evaluative dimension of pain, or that atrophy of the ACC and the prefrontal cortex will decrease the affective-motivational response to pain such that the patient will no longer anticipate pain properly or display aversive motor responses to pain. Nevertheless, this decrease in the most complex components of the pain response may be compensated by other factors. The first is hyperactivity of the hypothalamic-pituitary-adrenal axis, consecutive to the paraventricular release of histamine described previously and to the subsequent rise in CRH. The second is the presence of vascular lesions in the white matter. These lesions are responsible for cortical-subcortical disconnection and an increase in pain responses similar to that in hyperpathia, a typical finding in vascular dementia (see below).
Thus, there may be a theoretical basis for altered pain transmission and processing, although the studies aimed at confirming this basis are scarce. In addition to previously cited studies, which demonstrated threshold preservation together with increased pain tolerance in AD, we find 2 other types of evidence: neurophysiological and functional neuroimaging findings (fMRI).
Direct neurophysiological studies were carried out by recording event-related and evoked potentials secondary to painful stimuli. The amplitude of the potentials was not affected, but the latency to peak amplitude was longer in patients with AD.34 These results suggest that the pain threshold, or its perceived intensity, remains intact, whereas transmission and processing of the nociceptive information may be delayed.
Studies employing fMRI are even more revealing. In contrast to what was expected, functional activity in both the lateral and the medial pain systems was preserved. Evoked pain was associated with the same level of activity in patients with moderate AD (MMSE: 19) and in control subjects. The study examined S1 and S2, the insula, and the ACC. This study also revealed increases in amplitude and latency in areas involved in affective and cognitive pain processing; this is probably linked to heightened attention in the presence of pain.35 This finding might indicate disordered cognitive processing of the perceived pain information, a dissociation that might be explained by an altered response of the dorsolateral frontal cortex.
Lastly, it has been shown that perception of acute pain remains relatively intact. Perception of chronic pain, in contrast, is specifically altered.36 In this type of cases, which predominate in institutionalised patients with advanced dementia,37 anticipation, motor responses, and aversive responses are all affected.21 This is consistent with the finding of altered activation of the dorsolateral prefrontal cortex in advanced AD.
Vascular dementiaOnly a few studies have been designed to examine vascular lesions and pain specifically.38 In vascular dementia, in contrast with AD and the dementia found in Parkinson's disease (PD), the LC remains intact.39 Another study revealed the disconnection between the hippocampus and hypothalamus which may cause hyperactivity of the hypothalamic-pituitary-adrenal axis, with a resulting increase in CRH40; this finding is also present in AD.
The affective dimension of pain is the most altered in vascular dementia. It is attributed to an increase in pain, or rather in perceived pain, due to cortical-subcortical disconnection specifically between the intralaminar nuclei of the thalamus and S2,33 and also between the parietoinsular system and the ACC.15 The result of both types of white matter lesions is deafferentation pain. It appears in infarct sites at this location beginning in the sixth month after onset. It can also appear in vascular dementia.
Headache is another chronic pain syndrome affecting patients with cerebral infarct or vascular dementia, but one that has hardly been studied.41 Headache may be related to the cause of the infarct (vasculitis, arterial dissection), to treatment (antiplatelet drugs, anti-inflammatory agents), to complications (systemic or CNS infections, metabolic disorders), or to new infarcts, especially larger ones or those affecting the posterior fossa. Nevertheless, a significant number of patients may continue to experience headache months or even years after a stroke. Experts are unsure as to whether a prior history of primary headaches, or of emotional factors associated with this entity and its sequelae, may be related to post-stroke headache. One clinical study examining this entity found that headaches were correlated to white matter lesions, but not to the number, volume, or direct symptoms of infarcts.42
Hyperpathia was demonstrated when patients with vascular dementia scored significantly higher than controls on pain scales.43
Since no lesions of the lateral pain system have been described, there are no data to suggest a decreased pain threshold, a finding that could compensate for the deafferentation-related hyperpathia typical of these patients.
Frontotemporal dementiaIn frontotemporal dementia (FTD), frontal and temporal lateral atrophy are more marked than in AD. Positron emission tomography and regional blood flow studies have been able to identify the circuits that are more affected in FTD than in AD. In FTD, the major blood flow reductions affect the prefrontal cortex, the orbital prefrontal cortex, and the ACC.44 The amygdala and hippocampus remain relatively intact. The most severely affected areas of the temporal lobe in FTD are in the anterior and perisylvian regions. Along with the frontal region, they are related to the motivational and affective dimensions of pain.45 The result of impairment in this dimension is diminished expression of pain. This being the case, the profile also resembles that in AD, and both AD and FTD differ from vascular dementia. The crucial finding in FTD is a decrease in the cognitive-evaluative pain dimension following a frontal lesion.
The LC remains preserved, and there have been no descriptions of lesions in the lateral pain system or hypothalamus.46,47
In the only clinical study in which pain response was compared between patients with FTD, AD, and vascular dementia, hand withdrawal from hot stimuli (hot water) was faster and more pronounced in the vascular dementia group. This finding was expected because of associated hyperpathia. Cognitive-evaluative and affective impairment in FTD would explain why these individuals showed a slower response.48
Parkinson's diseasePain is a common manifestation in PD. It generally signals the onset of ‘off’ periods or an end-of-dose effect. Patients with PD also experience more frequent and intense pain of other origins. They are described as having a primary central pain syndrome that is not attributable to any prior aetiologies.33,49
The predominance of pain syndromes is explained by early lesions to brainstem nuclei with antinociceptive actions, especially the LC and subcoeruleus areas.50
Pain and treatment expectationsPain is a subjective experience that is greatly influenced by exogenous factors that cause it, but also by endogenous emotional and cognitive factors. These include anxiety, concentration, prior pain experiences, and expectations. Expectations are of prime importance because they determine presence or absence of the placebo effect for pain. This effect is one of the most potent modulating cognitive factors known to act on pain perception.51
The placebo effect is witnessed when administration of an ‘inert’ substance produces an effect because the subject believes that the substance is acting on the target symptom. This effect is particularly evident in emotional disorders, movement disorders, and pain. When placebo analgesia is used, multiple psychological factors interact: expectations, Pavlovian conditioning, and decreased anxiety. ‘Expectations’ refers to the emotional and physiological response that the subject believes will follow treatment. The verbal information provided may alter expectations.52 Conditioning is a type of learned behaviour that activates mechanisms able to evoke a response to a placebo once the subject associates them with active treatment. One example of conditioning is the placebo analgesia response in an experiment in which pain from ischaemia provoked in one arm was treated with a morphine-based drug; on the second day, patients who had responded to the morphine-based drug received an antibiotic instead. This population displayed a significant analgesic response to the antibiotic.53
Today, we know that the placebo effect is not an exclusively psychological phenomenon depending on an event that affects the subject's attention, afferent pathways, or pain perception. Well-defined neurobiological circuits also intervene in this phenomenon.54 They were discovered using functional neuroimaging techniques, thanks to which we now know which cell groups are involved in placebo analgesia. These groups are frontal and prefrontal structures, especially those in the most rostral part of the ACC, and others in the periaqueductal grey. These groups all contribute to the response to topical or systemic placebos, and even to sham acupuncture. The result is an increase in functional connectivity of the ACC with subcortical structures that are essential for transmitting nociceptive information via descending pathways.55
In addition to the ACC, other cortical structures become activated during placebo analgesia. These areas, which are usually functional during pain experiments, are the thalamus, insula, amygdala, and parietal cortex. However, the same areas show decreased activity during the placebo response, meaning that placebo analgesia depends on active inhibition of the nociceptive input.56 The prefrontal cortex is another crucial structure in anticipating the placebo effect; specifically, the activation of its dorsolateral areas correlates with the magnitude of the placebo analgesic effect. This is considered a necessary structure for generating, maintaining, and integrating different mental constructs and expectations, including placebo response.57
Pioneer studies performed 4 decades ago managed to show that placebo analgesia could be inhibited by naloxone.58 This finding indicated that at least part of the effect depends on the endogenous opioid system. Further support for this hypothesis was provided by recent studies using PET and opioid radioligands, which demonstrated activation of μ-opioid receptors in subcortical and cortical areas in the context of placebo analgesia.59 Nevertheless, we know that the opioid system is not the only neurotransmitter system involved in the placebo effect. Also contributing are the dopaminergic, serotonergic, and cannabinoid systems, probably to different extents in distinct pain syndromes and in other placebo responses.53,54
After listing the anatomical structures involved in the placebo response to pain, we see that they correspond to specific pain areas. In the same way, the regions affected in dementia form part of those that mediate the placebo effect, so the discovery of an attenuated placebo effect is not surprising in dementia cases. The only study to analyse this topic obtained this exact result: in a group of patients with mild to moderate AD, the placebo effect for topical anaesthetic was significantly reduced. The result was directly correlated with the score on a battery of frontal lobe tests, but not with the degree of impairment indicated by the MMSE.60 This finding highlights the role of the prefrontal cortex in the placebo analgesic response: lesion or disconnection at this site reduces that cortex's ability to communicate with areas such as the ACC that are related to expectations and the placebo effect. This would prevent the inactivation of painful cortical areas (insula, amygdala, S1 and S2) and neutralise the opioid effect on subcortical areas that arises in the placebo response.
The absence of the placebo effect found in AD has not been studied in other dementia types. Frontal lobe impairment, or lesions provoking cortico-subcortical dissociation, are more extensive in FTD and vascular dementia. Absence of a placebo analgesic effect is thus also to be expected in these entities.15 Unfortunately, there are still no published studies supporting this premise.
If a prefrontal lesion is accompanied by an attenuated placebo effect and reduced expectations for analgesia, then we can predict limited effectiveness of analgesics. The analgesic effect results from the true pharmacokinetic effect itself, which is added to the effect produced by expectations. The 2 components can be studied separately in analgesia and placebo experiments.61 Therefore, diminished analgesia is indivisible from the lack of the placebo effect. The result in AD patients, and very likely in those with other types of dementia affecting the prefrontal lobe, is that higher doses of analgesics are needed to achieve the desired result. It can also be predicted that environmental factors inherent to administering analgesia would be added to the direct pharmacological placebo effect. Since these environmental factors exert less influence on dementia patients, the patients’ expectations for analgesia will also be lower.
Assessing pain in dementiaSince pain is a complex sensation and experience possessing multiple dimensions, quantifying or otherwise measuring pain objectively is an intricate task. Its intensity, quality, location, emotional burden, and functional repercussions are all difficult to gauge. Nevertheless, obtaining such measurements is a critical step in assessing the patient's progress or response to treatment, and important for comparisons between different patients in studies or clinical trials. To this end, researchers have designed pain scales with varying degrees of complexity that evaluate different aspects of pain.62
The comprehension problems and communication disorders inherent to dementia make this evaluation especially difficult, and these challenges grow as patients enter more advanced stages. The simplest visual scales, most of which are one-dimensional, are valid for use with communicative patients. However, for those less inclined to communicate, doctors need scales that use more indirect signs for evaluating pain, such as expressive motor or autonomic signs.
The assessment must be rounded out with information provided by carers. In this area, we know that patients and carers agree that pain exists, but they are unable to measure it.15,33,60
Assessing communicative patientsDescriptive verbal scales, visual analogue scales (VAS), and numeric scales are useful in patients who communicate. Pain can be quantified by assigning scores to verbal descriptors (choosing from among 5 descriptors, from ‘no pain’ to ‘worst pain imaginable’), numeric values (from 0 to 10, indicated in digits or graphed along a horizontal line like a visual analogue thermometer, as with the VAS), or in more elaborate ways, such as BS-21, the 21-point scale of boxes numbered from 0 to 100 in increasing intervals of 5 from left to right,63 or facial descriptors (the series of 7 faces drawn to express increasing intensity of pain beginning with an initial neutral face: FPS, the faces pain scale64). The last type is the most appropriate for patients with dementia; nevertheless, good correlation between all scales has been determined for patients in intermediate stages with an MMSE between 10 and 19. In fact, pain scores were shown to be very similar across all scales, which demonstrates that their psychometric qualities are superimposable; the only difference found by one study was preference by race: white patients preferred descriptive verbal scales, whereas black patients preferred the FPS.65 Numeric scales were also evaluated in all groups and found to be just as valid as other types.
All scales are therefore one-dimensional and able to measure pain intensity. Even so, some patients may have difficulty understanding what the scales mean, or representing their pain level using a paper and pencil. For these reasons, experts recommend assessing the patient's cognitive capacity, using the clock-drawing test at the least.
Other dimensions of pain are too subtle for these scales and will require more complex and time-consuming methods.
Assessing non-communicative patientsFor patients with more advanced dementia, we must focus our attention on indirect signs which, when viewed as a whole, provide a perspective on the presence of pain. These assessments are performed using more complex scales that quantify 3 facets of pain: intensity and location, affective components, and autonomic responses. Numerous scales are available, but the ones with the highest levels of interobserver reliability, validity, and consistency, and therefore the most widely accepted and recommended by expert groups,10,66 are the PACSLAC67 and Doloplus-2 scales.68 The first is divided into 4 subscales, which collect the following data: 1 – facial expressions; 2 – activity/body movement; 3 – social/personality mood; 4 – other: physiological and autonomic changes (sleeping, eating, yawning, etc.). Doloplus-2 is a scale that groups 3 response subtypes: somatic, psychomotor, and psychosocial responses.
Signs used by both scales are indirect and consist of the following: 1. Changes in facial expression, with a generally rigid face, distorted expression, squinting or, in contrast, increased eyelid movement; 2. Verbalisations or vocalisations: moans, cries, calls, or inappropriate reactive behaviour; 3. Body movements, whether protective or as unrest expressed as repetitive movements, pacing, or complex stereotypic movements; 4. Changes in activity patterns or routines that affect sleep, eating, or typical movement habits in their environment; 5. Changes in interpersonal relationships: patients become irritable, aggressive, uncommunicative, isolated, and display obstructive behaviour; 6. Changes in mental state, which eventually adopt the form of confusional syndromes or dementia progression.
The neurologist is particularly interested in the first and last items, which address facial expressions and changes in mental state. Facial expression is a trait that is examined by all scales adapted for patients who have dementia or do not communicate. Doctors can use coded example expressions providing an accurate view of the degree of pain, or at least accurate enough to be included in a validated scale with good interobserver agreement. These expressions have been evaluated in different clinical situations69,70 ranging from prosthetic joint placement to treatment of pressure ulcers. The most extensive review of this topic found a high level of agreement for confirming presence or absence of pain, but not for quantifying it.71 This limitation is probably attributable to the fact that facial expression may be affected by underlying or concurrent diseases that change or limit patients’ facial expressiveness. PD and other parkinsonian syndromes, and FTD in which lesions are predominant in the right temporal lobe, provide examples of situations in which lack of facial expression may not equate to lack of pain.5,10,15,64,65
In the same way, pain, whether acute or chronic, is a cause of cognitive impairment in patients with prior dementia or communication disorders. It is easy to understand how pain would limit attention to surroundings; it is also associated with emotional changes that can limit cognitive capacity. As such, some pain syndromes may be expressed in a way that resembles progressive dementia. They may be mistaken for confusional syndromes. While these syndromes are reversible when pain is acute, we remain unaware of the prognosis for patients with chronic pain. This highlights the importance of correct diagnosis and treatment of these patients: pain can be treated, which can slow decline and disease progression.
Practical diagnostic orientation for dementia patients with painIf pain is undertreated in the elderly, especially in those with communication difficulties and dementia, it is mainly due to inadequate assessments, primarily of the presence of pain, but also of the pain level and repercussions. We must fully understand this problem if we are to implement an assessment and management strategy that will provide the right diagnosis and treatment for symptoms.
This assessment requires an overarching approach (Table 3) that includes the following: 1. directed medical history and physical examination; 2. correct measurement of pain; 3. functional and emotional evaluation; 4. particularities of neuropathic pain. We will now examine these 4 points one by one.
Recommendations for evaluating pain in patients with dementia.
| 1 | History | Painful conditions, past medication use |
| 2 | General examination | Impaired motor function, inflammatory signs, pain in spine and load-bearing joints |
| 3 | Neurological examination | Common causes of neuropathic pain: herpes zoster, trigeminal neuralgia, post-stroke pain syndrome, painful polyneuropathy |
| 4 | Intensity-1 | Communicative patients: simple analogue scales (VAS, FPS) |
| 5 | Intensity-2 | Non-communicative patients: PACSLAC and Doloplus-2 scales |
| 6 | Functional repercussions | Motor behaviour, habits, relationships, activity |
| 7 | Emotional impact | Assess presence of anxiety and depression (recommend GDS) |
| 8 | Autonomic repercussions | Appetite, sleep |
| 9 | Analgesic response | Begin empirical analgesic treatment and check response |
| 10 | Follow-up | Personalise treatment and follow-up on the listed variables |
The medical history must include any painful conditions and medication use. Prior painful conditions produce a habitual response to pain in the form of a motor, behavioural, or autonomic pattern that tends to recur, and which can often be identified by a dementia patient's family members. History of medication use is also important: long-term analgesic treatments may lead to chronification of painful conditions, as occurs in primary headaches. These disorders are expressed differently in dementia patients. In the same way, analgesic drug treatments must be adapted to the clinical situation of these patients, who frequently have multiple diseases and are polymedicated, thereby increasing the risk of interactions and side effects.
As stated previously, pain itself can cause these patients’ condition to deteriorate. An elderly dementia patient in pain will show behavioural changes, manifesting as withdrawal, impulsiveness, or aggression; at the same time, he or she will display altered emotional expression and vegetative and autonomic responses affecting appetite, sleep, sphincter control, and intestinal rhythm. These changes would logically appear alongside decreases in their prior manipulation, spatial navigation, or planning and executive abilities. If doctors do not regard pain as a potential source of these syndromes, they will search for different aetiologies or consider only primary impairment due to dementia. As a general rule, we should recall that the fundamental causes of acute pain are trauma and inflammation, and that the most common sources of chronic pain are musculoskeletal disease and neuropathic pain.
Red flags that must first be ruled out in elderly dementia patients with pain are as follows.
- •
Herpes zoster, which can result in pain syndromes prior to herpes eruption, but especially post-herpetic neuralgia that becomes chronic in absence of skin lesions.
- •
Temporal arteritis, which causes headache, limb pain or rigidity, and proximal weakness of the limbs.
- •
Traumatic lesions, which may not be noticed and give rise to chronic pain: rib and limb fractures and head trauma, which can cause chronic subdural haematoma.
- •
Night-time or resting bone pain in elderly patients with spondylodiscitis, which requires a high level of clinical suspicion to be diagnosed correctly, points to a cancerous, inflammatory, or infectious origin, even in absence of fever.
- •
Acute ischaemia of the limbs, which often affects this patient group and is fundamentally caused by arteriosclerosis or underlying atrial fibrillation, whether diagnosed or not.
The physical and neurological examination should contemplate all of these possibilities. Likewise, doctors must not neglect to search for local inflammation or trauma or impaired motor function of the limbs, in addition to performing a neurological exam that closely scrutinises areas showing hyperaesthesia or muscle weakness, especially limb weakness in a radicular pattern.
Before proceeding to the next step, the second in our system for pain measurement, doctors should be aware that administering analgesics is indicated if there is a possibility that pain is causing decline in dementia patients. If cognitive decline follows onset of a painful condition, it may be reversible and administering analgesics can improve the patient's mental state.72
On the other hand, we should attempt to quantify pain. This can be achieved using different types of more or less complex scales that are adapted for communicative or uncommunicative patients, as described previously. In general, patients may directly complain of pain even in advanced stages of dementia. The most simple and straightforward scales providing an accurate record of pain intensity should therefore be applied to all patients with dementia. Graphic and analogue verbal scales (VAS, pain thermometer, FPS) are very brief and informative for both diagnosis and follow-up. For more advanced stages of dementia, scales collect indirect information that indicates pain, including gestures, function, behaviour, and autonomic responses. We should be aware that brief scales demonstrate less sensitivity for pain detection in favour of a faster application time and a low rate of false positives for pain. In contrast, more complex scales have a greater capacity for detecting pain in dementia patients, but they take longer to apply and are less specific. These tendencies hold for all types of diagnostic tests. In advanced dementia and in the sphere of neurological practice, the most recommended pain scales (PACSLAC and Doloplus-2) are especially useful in hospitalised patients with painful conditions, including hip fractures and pressure ulcers.
The next part of assessment covers functional and emotional aspects. ‘Functionality’ refers to the impact of pain on areas such as social interaction or activities of daily living, and on behaviour, sleep, and appetite, which are all likely to worsen in the presence of acute or chronic pain. Emotional repercussions arise in the same context, since pain is very often associated with anxious or depressive tendencies, or even frank depression, which may be a dominant feature or else masked by other manifestations. Refractory pain requires specific treatment which may directly lessen the sensation of pain by increasing the patient's ability to adapt to and cope with the situation. If a patient with dementia is still able to communicate, we recommend administering a simple and brief depression scale, such as the Geriatric Depression Scale (GDS).73
Lastly, the process of assessing neuropathic pain and performing physical examinations has a few peculiarities in this group of patients. We must recall that many elderly people display muscular atrophy, especially distal atrophy, or decreased deep distal sensitivity in the legs. As with decreased joint extension, the above are regarded as physiological changes. Being aware of these traits makes it easier to optimise the neurological examination. Doctors must search for direct sensory changes manifesting as hypoaesthesia, or its opposite, hyperalgesia, especially in the form of hyperpathia or allodynia. Tests such as quantitative sensory testing allow us to measure these changes.74 In clinical practice, the most common causes of neuropathic pain in elderly patients75,76 are as follows.
- •
Peripheral neuropathic pain: post-herpetic neuralgia, trigeminal neuralgia, and other axonal polyneuropathies, especially those secondary to diabetes or toxicity (chemotherapy).
- •
Central neuropathic pain: post-stroke pain syndromes, especially thalamic syndromes. These deferred-onset syndromes present with intact deep tissue sensitivity and may cause proximal spread of referred pain, especially in the shoulder (post-stroke shoulder pain77). Infiltrations should therefore be avoided.
Pain is a very common manifestation in dementia that is often neither diagnosed nor treated. This situation arises because lesions specific to dementia affect the same areas of the brain that manage central pain pathways. Specifically, the prefrontal cortex, ACC, perisylvian areas, hippocampus, and hypothalamus are the areas responsible for the cognitive-evaluative, emotional, memory, and autonomic dimensions that make up the pain experience. Degenerative deposits and tangles occurring in AD, FTD, and PD modify the processing and expression of the pain sensation by destroying their respective anatomic localisations. It is striking that this phenomenon would occur while pain perception and pain threshold remain intact, since the lateral spinothalamic tract and its connections are preserved.
On the other hand, the placebo effect, which depends on the patient's expectations, but also anatomically on the activation of the ACC, is also attenuated in dementia cases. For this reason, the action of analgesic drugs, which includes a purely pharmacokinetic effect and the additional effect caused by expectations, is always less pronounced in these patients, who may therefore require higher doses than those typically prescribed.
Doctors need more sensitive tools for diagnosing pain in the clinical practice setting. Simple visual scales are useful in communicative patients. In those who are not, observing indirect signs and using specific scales (PACSLAC and Doloplus-2) is useful for detecting and measuring pain. Since pain alone can be sufficient to explain a deteriorating cognitive state, starting analgesic treatment if pain is suspected to be the cause of unexplained dementia progression is entirely justified. Red-flag situations that can cause pain, whether neuropathic (post-herpetic) or non-neuropathic (temporal arteritis, broken hip or ribs, acute limb ischaemia), should be detected and subjected to a directed examination.
Conflict of interestThe author has no conflicts of interest to declare.
Please cite this article as: González LCÁ. El neurólogo frente al dolor en la demencia. Neurología. 2015;30:574–585.