To review the physiology of the glutamate receptor subunits such as N-methyl-d-aspartate (NMDA).
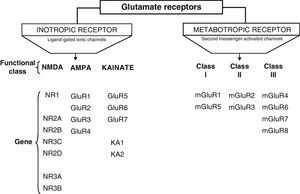
DevelopmentGlutamic acid (Glu) is the major excitatory neurotransmitter in the central nervous system (CNS) which interacts with two types classified as: metabotropic and ionotropic. Ionotropic receptors are classified according to the affinity of their specific agonists: NMDA, α-amino acid-3-hydroxy-5-methyl-4-isoxazole (AMPA) and kainic acid (KA). NMDA receptors (NDMAR) are macromolecular structures that are formed by different combinations of subunits, NMDAR1 (NR1), NMDAR2 (NR2) and NMDAR3 (NR3).
ConclusionsThe study of this receptor has been of great interest due to its role in synaptic plasticity, but mainly due to the permeability it has to Ca++ ion. This review examines the molecular composition of NMDA receptor and the variants of NR1 subunit edition in association with NR2 subunit dimer, the main form of this receptor. The composition, structure and function and their distinct expression patterns in both time and space, have shown the versatility and diversity of functionally different isoforms of the NR1 subunit and various pharmacological properties of the NR2 subunit.
Realizar una revisión de la fisiología de las subunidades del receptor a glutamato tipo N-metil-d-aspartato (NMDA).
DesarrolloEl acido glutámico (Glu) es el principal neurotransmisor excitador del sistema nervioso central la cual interactúa con dos tipos de receptores clasificados como: metabotrópicos y ionotrópicos. Los receptores ionotrópicos se dividen de acuerdo a la afinidad de sus agonistas específicos en: N-metil-d-aspartato (NMDA), ácido α-amino-3-hidroxi-5-metil-4-isoxazol (AMPA) y acido kaínico (KA). Los receptores NMDA son estructuras macromoleculares que se forman por combinaciones de diferentes subunidades: NMDAR1 (NR1), NMDAR2 (NR2) y (NR3).
ConclusionesEl estudio de este receptor ha sido de gran interés por la función que desempeña en la plasticidad sináptica, pero sobre todo por la permeabilidad que tiene para el ion Ca++. En esta revisión se analiza la composición molecular del receptor NMDA, así como las distintas variantes de edición de la subunidad NR1 que en asociación con la subunidad NR2 forman el principal dímero de este receptor. La composición, estructura y funcionalidad y sus distintos patrones de expresión tanto temporal y espacial, ha permitido conocer la versatilidad y la diversidad funcional tanto de las diferentes isoformas de la subunidad NR1, así como las distintas propiedades farmacológicas de la subunidad NR2.
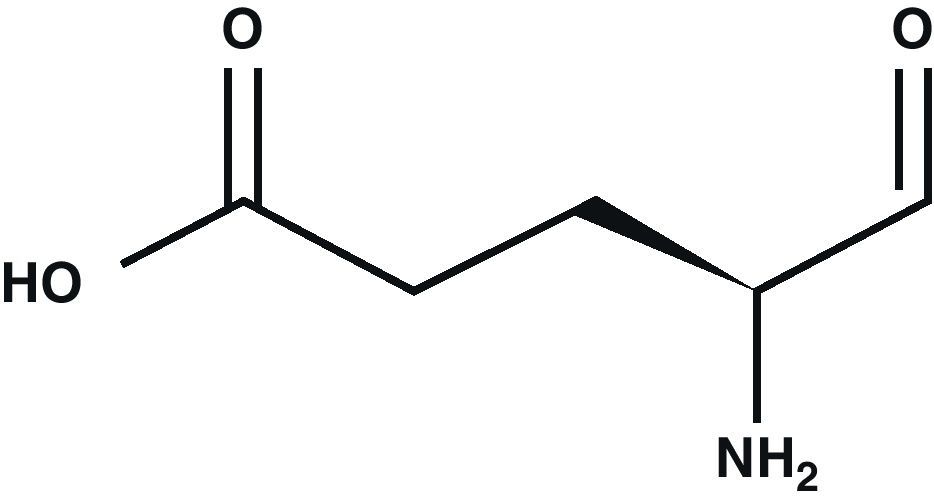
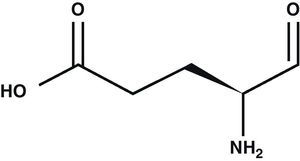
Glutamic acid (Glu) is the main excitatory neurotransmitter in the CNS1 (Fig. 1). Glu is a non-essential amino acid which does not cross the blood–brain barrier. It is synthesised in neuron mitochondria from glucose and several precursors. After being synthesised, Glu is released into the cytoplasm where it accumulates in synaptic vesicles through a process dependent on Mg2+/ATP.2 The propagation of the nerve impulse towards the axon terminal promotes the release of Glu from the synapse through a mechanism that depends on intracellular Ca2+ by means of exocytosis.3 Glu can then interact with its specific receptors.
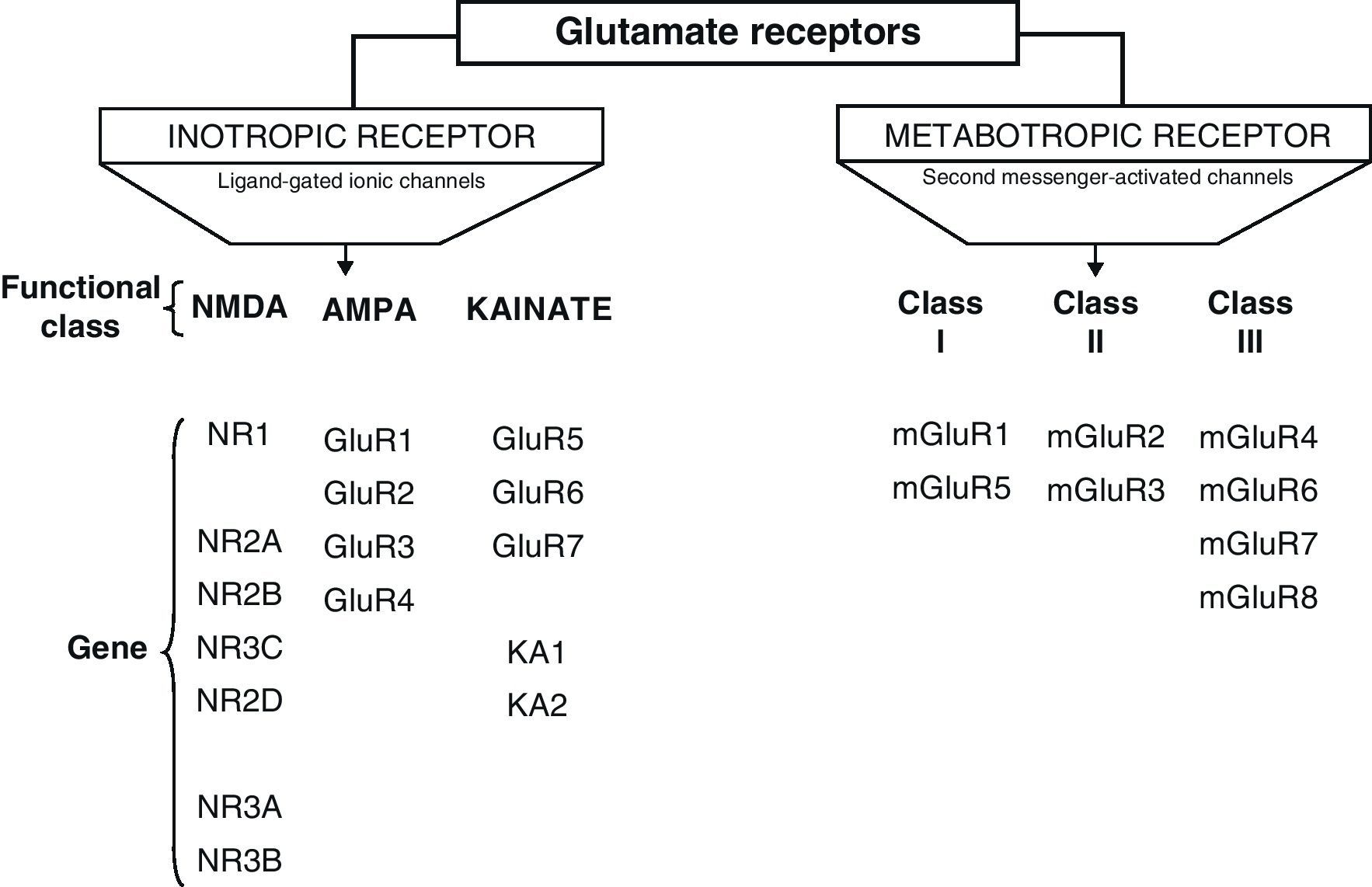
Glu receptors are classified as either metabotropic receptors (mGluRs), which promote the activation of second messengers via G-proteins, or ionotropic receptors. The latter are linked to an ion channel and their activation allows influx of many different ions, mainly Ca2+ and Na+, as well as the exit of K+ (Fig. 2).4,5
The structure of mGluRs consists of a protein chain that crosses the membrane 7 times. To date, 8 units named mGluR1 through mGluR8 have been cloned, and they are classified according to the following: (a) the homology of their amino acids (70% homology among members of the same class, and 45% homology between different classes); (b) in response to their agonists, and (c) the signal paths for second messengers.2
Ionotropic receptors are categorised according to whether their specific agonists have an affinity for N-methyl-d-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazole (AMPA), or kainic acid (KA).2 Ionotropic receptors are heteromers constituted by different subunits, which give the receptors different physiological and pharmacological properties (Fig. 2).
AMPA receptors are structured as combinations of GluR1-4 subunits which form an ion channel permeable to Na+. However, it has been shown that AMPA receptors whose structure does not include a GluR2 subunit are highly permeable to Ca2+. This is due to the presence of a residue of arginine (R), an amino acid present in position R586 in the TMII region of GluR2. In contrast, subunits GluR1, GluR3 and GluR4 present a glutamine (Q) residue at position Q582 of the GluR1 subunit protein.6
Kainate receptors are protein heteromers formed by combinations of the GluR5, GluR6, and GluR7 subunits, together with KA1 and KA2. The combination of KA2 and GluR5 forms a functional receptor that is permeable to Na2+.
N-methyl-d-aspartate receptorsThe structure of NDMA Receptors (NDMAR) has not been completely explained, although it is hypothesised that they form tetrameric or pentameric structures.6 However, we do know that it is formed by combinations of different subunits: NMDAR1 (NR1), NMDAR2 (NR2) and NMDAR3 (NR3).2 The entire structure forms a Ca2+-permeable ion channel. NR1 is coded by a single gene, but its transcript may generate at least 8 different variants. In contrast, the NR2 subunits is found as 4 isoforms encoded by different genes: NR2A, NR2B, NR2C, and NR2D.7
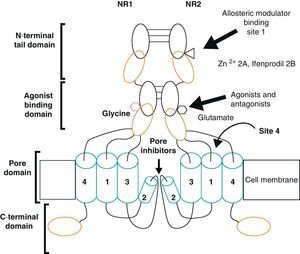
Activation of NMDAR requires simultaneous binding by 2 different agonists, Glu and glycine (Gly), which are therefore known as NMDAR co-agonists. In the CNS, the concentration of Gly in the extracellular medium is 1mM, which is sufficient for it to act as a co-agonist and for Glu to activate the receptor.2 Other important characteristics are its high permeability to Ca2+, its propensity for extracellular Mg2+ block and its voltage sensitivity, since when the cell membrane is depolarised, the binding site's affinity for Mg2+ decreases and the block is removed.8
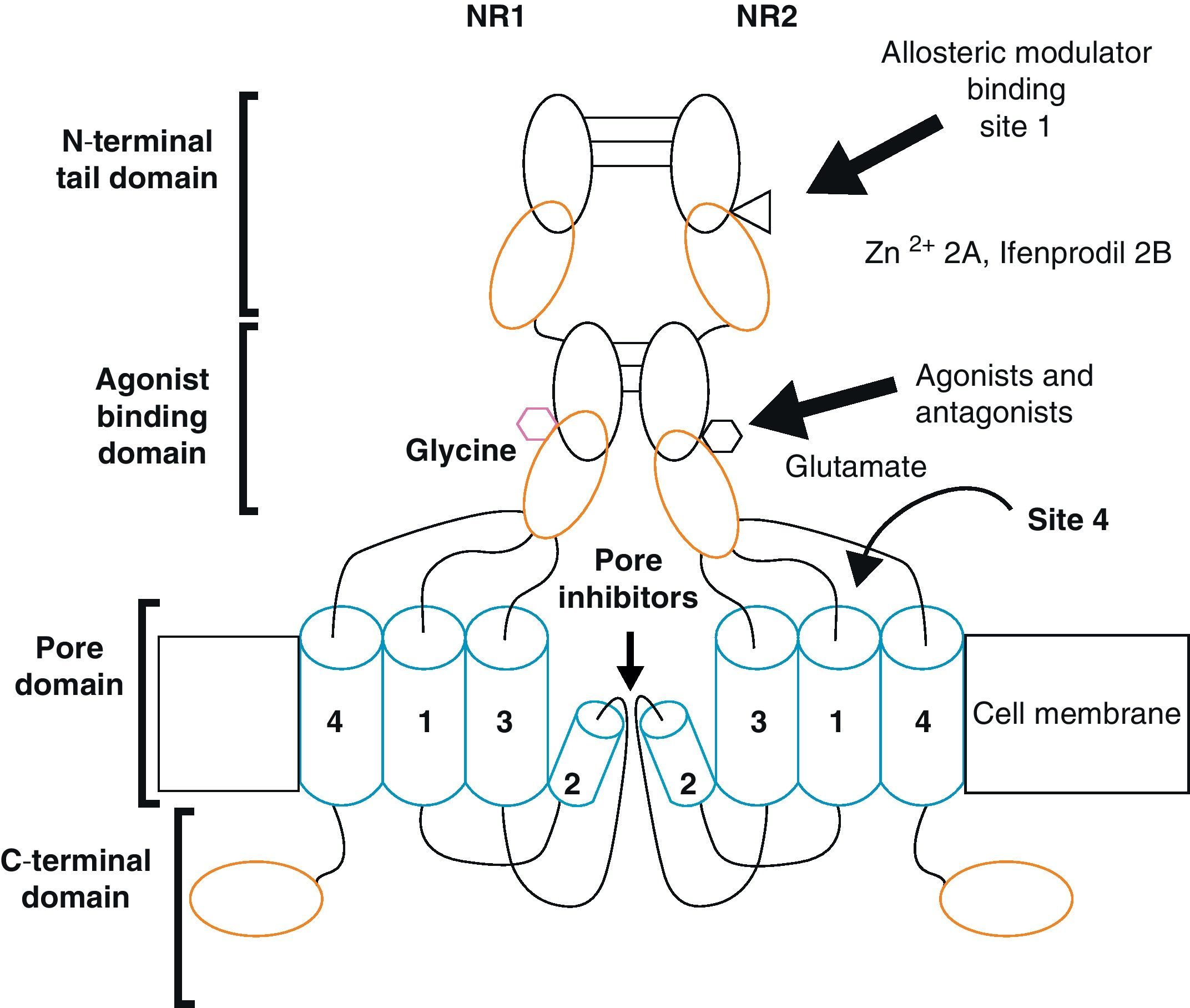
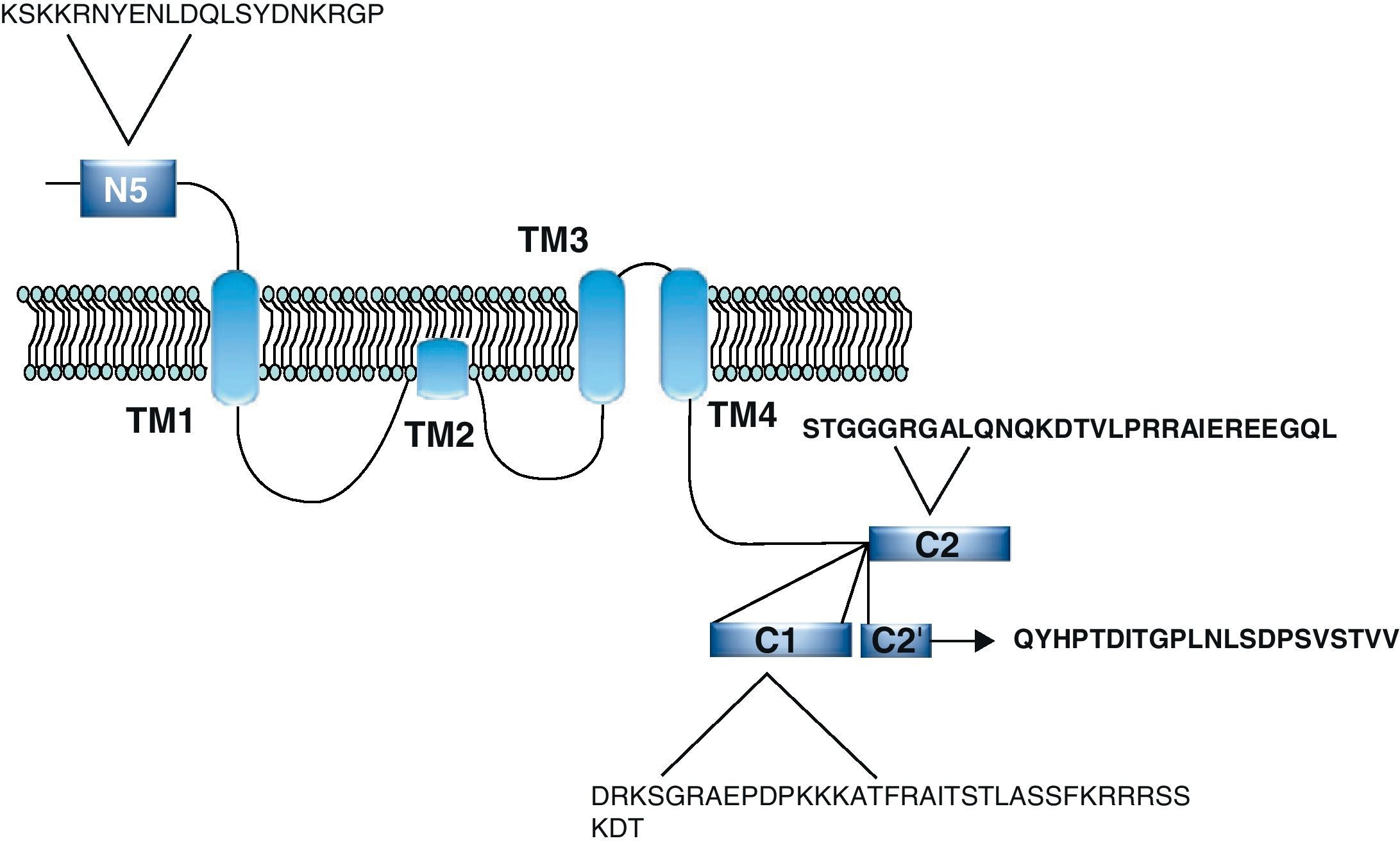
Functional NDMARs are generally formed by heterotetramers consisting of two dimers composed by NR1–NR2 subunits. Each of these NR1 subunits has a glycine binding site, and each of the NR2 subunits has a glutamate binding site, for a total of 2 glycine binding sites (S1) and 2 glutamate binding sites (S2) on each receptor.9–11 The NR1–NR2 dimer is therefore considered to be the basic functional organisation structure in each receptor. It contains various sites for the binding and recognition of different ligands, which may be either physiological or pharmacological. In this way, each ionotropic receptor subunit has a very similar molecular structure, divided into 4 functional domains. These consist of an amino-terminal extracellular domain (NTD); a ligand-binding domain (LBD); a transmembrane region formed by 4 hydrophobic segments (M1 to M4), with M2 partially entering the membrane to form the ion channel; and a carboxyl tail domain (CTD) in the intracellular region (Fig. 3). In addition to natural glycine and glutamate binding sites in the NR1–NR2 dimer, the extracellular region of NR2 in particular contains binding sites for endogenous ligands such as polyamines, which are redox sites for protons and zinc. They may exert a regulatory effect on NMDA receptor activity by permitting increases or decreases in calcium flux through the receptor under physiological and/or pathological conditions. At the same time, exogenous ligands for steroids, ethanol, and ifenprodil, and a few synthetic molecules, act as experimental tools for the study of NMDA receptor properties and aid in the development of therapeutically useful antagonists.
Homomers of the NR2 subunit do not generate functional receptors, and are only considered as modulators. Homomers of NR1 subunits produce channels that are activated by Glu or NMDA in the presence of Gly, but they produce very low amplitude currents compared to receptors formed by NR1–NR2 combined.8
Studies carried out by Das in 1998 demonstrated the existence of 2 varieties of the NR3 subunit (a and b), which are coded by different genes. The NR3a variant is expressed throughout the CNS, but expression of the NR3b variant is restricted to motor neurons. Like the NR2 subunit, NR3 is a regulatory subunit and its presence decreases the ionic currents generated by activation of the NR1/NR2 heteromers. Later studies showed that co-expression of NR1/NR3b forms excitatory glycine receptors that are insensitive to Glu, NMDA and Mg2+ block. Based on this evidence, it has been hypothesised that these receptors may be involved in the activation of silent NMDA-alone synapses.12,13
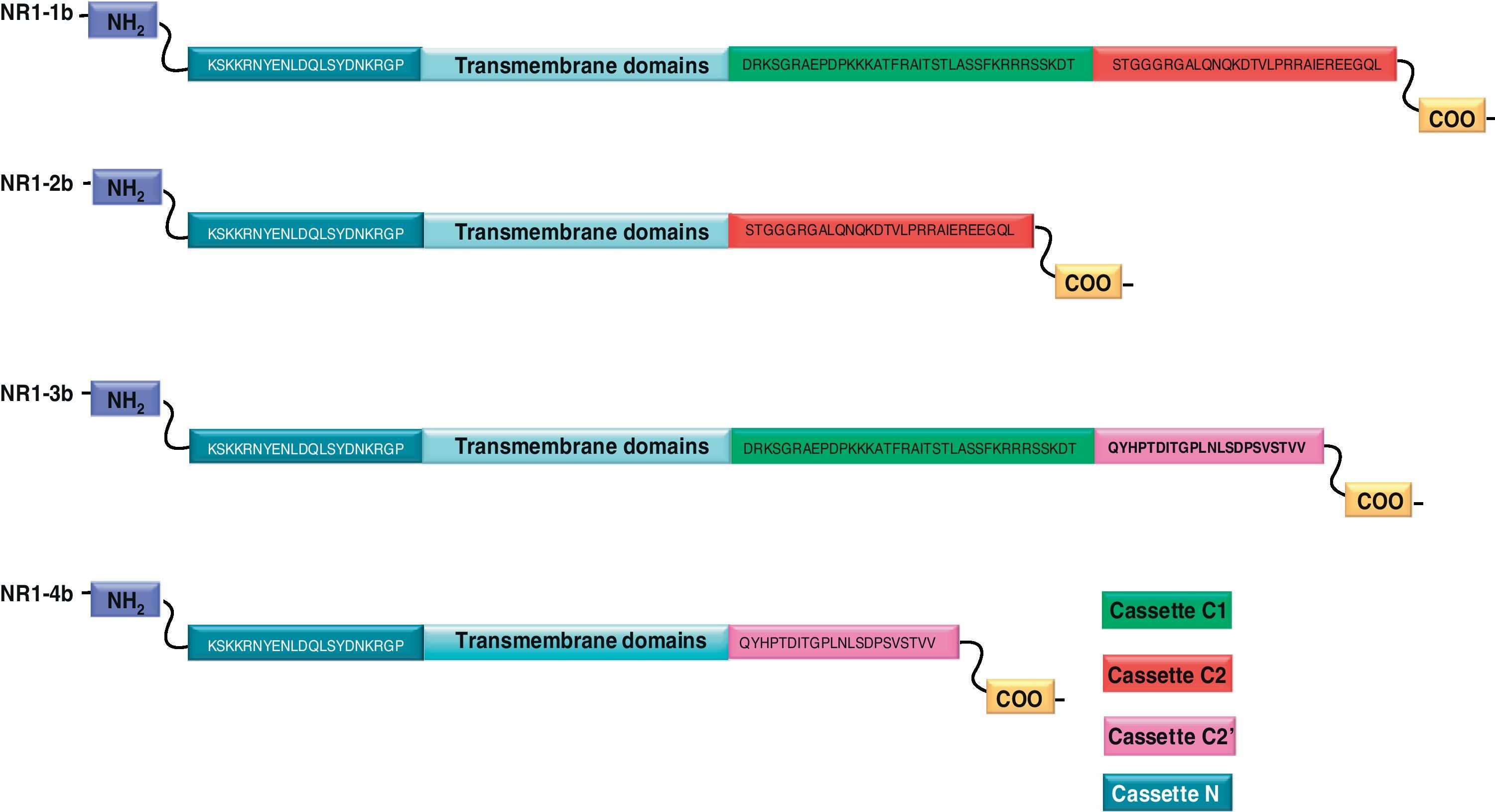
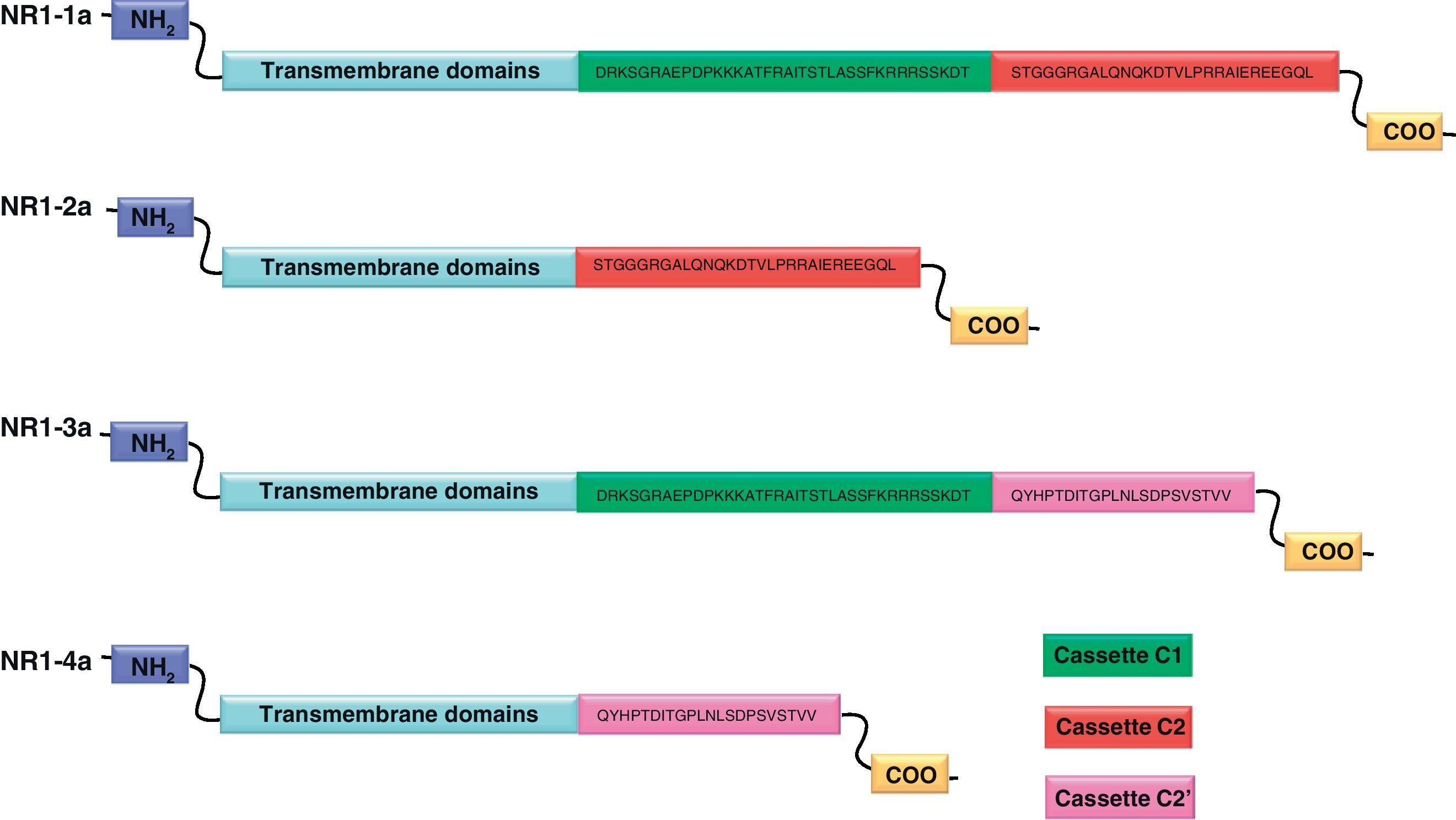
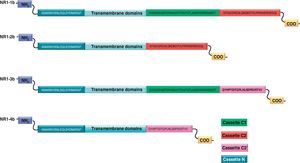
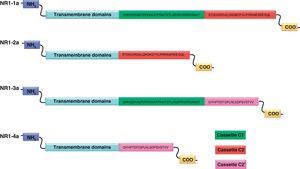
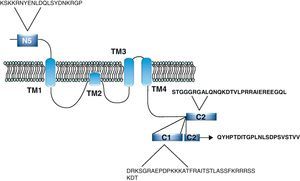
NR1 subunitThe mRNA of the NR1 subunit begins to be expressed in the brain of the rat embryo as early as day 14 of gestation, and its levels gradually increase until 3 weeks after birth.14 There are 8 processing variants for NR1 mRNA (NR1-1a/4a y NR1-1b/4b), which differ among each other according to the presence or absence of a sequence of 21 amino acids (N1: exon 5) in the N-terminal region (Fig. 4), and differential processing of exons 21 and 22 which provokes changes in sequences in the C-terminal region (units C1, C2 and C2′)15,16 (Figs. 5 and 6). The N1 region is important for the regulation of channel properties, since it alters its sensitivity to spermine, pH, and zinc.17 In isoforms containing the N1 exon, neither polyamines nor Zn+ increase stimulation by Glu, which may be due to it being a cation and its repulsion of the exon. The presence of the N1 exon is also linked to properties such as receptor affinity for agonists and their sensitivity to the antagonists APV ((2R)-amino-5-phosphonovaleric acid), CPP, 7-CK and MK-8 01. NMDAR sensitivity to pH is determined by the presence of exon 5. At physiological pH, receptors that include this variant are activated completely, while receptors that lack exon 5 are partially inhibited.18,19
On the other hand, exons in the C-terminal region play an important role in the regulation and location of NMDAR in the cell membrane. In this way, exon 21 codes for C1, with residues that are susceptible to PKC and PKA phosphorylation and active in positive regulation of NR1 in response to Glu20,21 and a target for interaction with calmodulin kinase, which could negatively modulate NMDAR activity.22,23 The C1 region also contains sites where neurofilament interaction takes place, and endoplasmic reticulum (ER) retention sequences. Respectively, these locations participate in positioning NMDARs and transporting them to the membrane.
During exon 22 processing, the variable use of an acceptor site makes it possible for 1 of 2 different units, C2 or C2′, to be expressed. In C2′, amino acids in the C-terminal tail make up a domain which binds to PDZ proteins (postsynaptic density-95/Discs Large/Zonula Occludens-1-binding Motif). This enables NMDAR to form clusters on the cell surface.24,25 Through interaction with PDZ proteins, these domains can also mask ER retention signals present in C1, which facilitates assembly of NMDARs and their transport to the cell membrane. In some varieties of C2′, the additional loss of unit C1, and the resulting elimination of ER retention sequences, contribute to an increase in these NR1 variants reaching the membrane.26 C2 and C2′ exons are involved in the transport, insertion and maintenance of the NR1 subunit in the synaptic membrane. Exon C2′ in rat NR1 contains a PDZ domain interaction sequence by which the NMDA receptor interacts with postsynaptic density proteins. However, NR1 in birds and fish lacks these domains. In birds, the C2 exon contains an N-myristoylation site that may provide an alternate anchoring system in the membrane, and both C2 and C2′ contain 2 consensus sequences for PKC phosphorylation. These traits suggest that the mechanisms controlling the number of NDMAR in the synapse, and their variation under physiological and pathological conditions, differ considerably among species.
On the other hand, experiments have shown that processing on the C2/C2′ site is regulated by synaptic activity. As a result, there is a direct relationship between level of activity, processing, and subunit traffic to the membrane during modification of excitatory synapses. Studies carried out by Kreutz in 1998 showed changes in the expression of NR1 isoforms under pathological conditions. In an experimental model with controlled optic nerve crush, NR1b isoforms were expressed preferentially, which significantly increased the possibility of survival among affected cells.27,28 It is plain to see that traffic and assembly of NMDAR subunits are precisely regulated processes. These activities are critical to expression of subunits in the cell membrane, especially in the case of NR1.
Subunit NR2Compared to NR1, expression patterns for NR2 are different in terms of both time and space. They are restricted to specific nuclei defined within the CNS, which change over the course of its development.29 This subunit determines the functional diversity of the NMDA receptor, as well as the receptor's sensitivity, conductance, deactivation kinetics, and sensitisation kinetics,30,31 which have a direct influence on the duration of excitatory postsynaptic currents.32
In general, the intracellular C-terminal domain in NR2 units is considerably more extensive than in NR1 subunits. Analysis of transgenic mice expressing truncated forms of NR2 demonstrated that the C-terminal tail is fundamental to the function and location of these subunits within the synaptic membrane.33 Near TM4, we find regions that are active in clathrin-dependent internalisation, and subsequent degradation, of NR2A and NR2B subunits. These regions are similar to those found in NR1 and in NR2C and NR2D subunits. A second internalisation domain has been found in the distal C-terminal region of the NR2B subunit, and it is linked to internalisation and subsequent recycling, rather than degradation, of that protein. NR2B also contains ER retention sequences similar to those in NR1, and these sequences may mask each other when both the subunits are found in the ER.34 NR2 subunits also contain PDZ domain interaction sequences in C-terminal tail amino acids.35,36 In addition to facilitating NMDAR clustering on the cell surface, these interaction sequences can contribute to subunit stability by masking internalisation domains, as also occurs with NR1.
Lastly, the function of NR2 subunits is subject to regulation through phosphorylation,20 as occurs with NR1. In this way, cAMP-dependent protein kinase (PKA) and protein kinase C (PKC) phosphorylate serine and threonine residues in these subunits,37,38 which results in positive modulation of the receptor's activity. In addition, these subunits are substrates of other kinases, such as Src and Fyn, which phosphorylate tyrosine residues39,40 and mediate NR2 susceptibility to calpain proteolysis.
Electrophysiological characteristics of the N-methyl-d-aspartate receptorNearly every available study has used the NR1a subunit since it is co-expressed with one or several NR2 subunits. The reason for needing 2 types of subunits to be co-expressed lies in earlier studies which found that expression of NR2 units alone did not generate functional receptors. They also found that expression of homomeric NR1 channels results in channels that are activated by Glu or NMDA in the presence of glycine, and do experience voltage-dependent magnesium block, and yet have very low-amplitude current with respect to neuronal receptors. On the other hand, when NR1 and NR2 subunits are co-expressed, we observe an increase in current amplitude similar to that seen in native receptors. In addition, sensitivity to l-glutamate, desensitisation, and deactivation kinetics are properties that are also influenced by the NR2 subunit, and they affect the activation threshold, modulation and duration of postsynaptic excitatory currents measured by the NMDA receptor.41
Voltage-dependent magnesium block is more pronounced for negative potentials in NR1a/NR2A and NR1a/NR2B receptors (2.4 and 2.1μM), than in NR1a/NR2C and NR1a/NR2D receptors (14.2 and 10.2μM). For this reason, different NMDA receptor subtypes are activated at different membrane potential ranges.29,42,43 The closed time constant for NR1a/NR2A heterodimers is very brief, roughly 3 or 4 times shorter than that of NR2B or NR2C heterodimers and up to 40 times shorter than that of NR2D heterodimers.29 Furthermore, these receptors are distinctive in that they form channels with a higher conductance than those formed by the NR1a/NR2Cand NR1a/NR2D subunits.44
The expression of NR1a and NR2B subunits is correlated with the distribution of NDMAR with a high affinity for agonists.45,46 In rats, the NR2B subunit is predominantly expressed in the forebrain, medial striate cortex and cerebellum; it practically disappears from this last structure in adults.29,47–49 Functional studies show that the receptors that include this subunit have a greater affinity for Gly co-agonists than receptors with NR1a/NR2A.45
Transfection studies in heterologous systems show that NR1a/NR2B heterodimers have greater permeability and Ca2+ influx, and are activated at lower Glu concentrations than NR1a/NR2A heterodimers. Insertion of the NR1a subunit with the NR2A and NR2B subunit forms receptors that are highly sensitive to magnesium block, and only activate under depolarised conditions.41
Studies in mouse cerebral granule cells for the NR2C subunit showed an increase in excitatory postsynaptic currents, demonstrating the low probability of NR1/NR2C receptors opening.50 The amplitude of evoked currents in these knockout mice is higher than in native mice and the currents decay at a faster rate.51
On the other hand, heterodimers with NR2D subunits have a higher affinity for Glu, are less sensitive to magnesium block, and have a very slow closing time compared to NR1a/NR1A, NR1a/NR2B and NR1a/NR2C heterodimers. This subunit therefore plays a very important role in excitotoxic neuronal damage.52 Additionally, this activity may result in a slow, steady influx of Ca2+ to the a cell that actively expresses this subunit, which coordinates pre- and post-synaptic activity and permits a degree of temporal flexibility for the formation of specific synapses during the development stage, during which the most expression of the NR2D subunit takes place.29
ExcitotoxicityNeuronal death caused by excessive liberation of Glu and overactivation of Glu receptors is known as excitotoxicity.53 This phenomenon is associated with a number of pathological states of the CNS, which include epilepsy,54 hypoxia/ischaemia, and trauma. It is also thought to be involved in Huntington, Alzheimer and Parkinson diseases.55,56
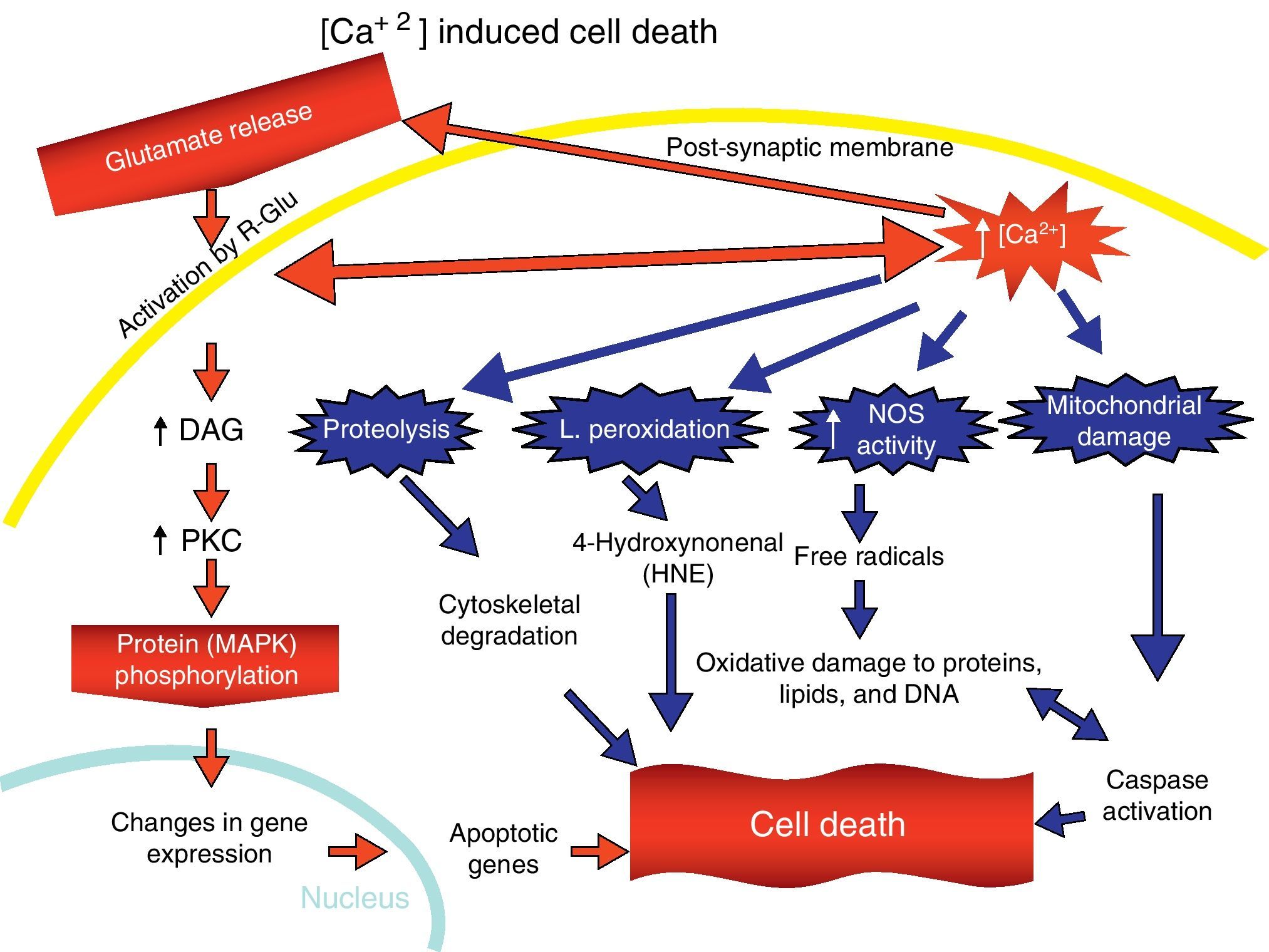
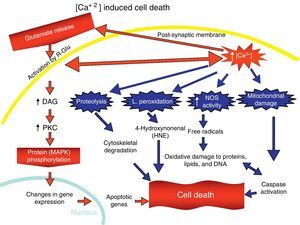
Overactivation of Glu receptors, mainly NDMAR, is one of the processes involved in the neurodegeneration and cell death that are present in a number of diseases.57 This produces an increase in intracellular Ca2+, thereby promoting lipid peroxidation (LP) of the cell membrane, ER and mitochondria.58 This LP is due to the production of nitric oxide (NO) and superoxide radicals (O2−), which form peroxynitrites. There is also production of 4-hydroxynonenal (HNE), which alters the activity of membrane transporters and ionic channels when lipids in the membranes are peroxydated.59 LP also damages ATPase Na+/K+, glucose transporters and Glu as part of the excitotoxic process and disturbs ionic homeostasis in the ER and mitochondria, which compromises the supply of ATP.58,59
The increase in intracellular Ca2+ concentration elicits the activation of intracellular signal pathways related to cellular apoptosis, such as the activation of different Ca2+-dependent enzymes (proteases, nucleases, and phospholipases).58 In addition, excitotoxicity is correlated with MAPK activation (Fig. 7).60
Pathological implications of the N-methyl-d-aspartate receptorThe modulation of excitatory neurotransmission mediated by Glu receptors, mainly NMDA-type receptors, is known to have important implications for the origin of cell injury and death. This is observed in an array of different conditions including cerebrovascular accident, hypoxia, ischaemia, epilepsy and others, and in chronic neurodegenerative diseases such as Alzheimer, Parkinson, Huntington, and amyotrophic lateral sclerosis (ALS). All of these entities share common pathological characteristics, including gradual and selective neuronal loss, mainly due to overactivation of Glu receptors. Although the neuronal groups that are primarily affected vary according to the disease, and causes of neuronal death are unknown, overactivation of Glu receptors generally leads to an increase in Ca2+ concentration in the cytoplasm and the generation of reactive forms of oxygen. These factors appear to play a very important role in neurodegeneration and progressive neuronal death mechanisms in diseases such as Alzheimer and Huntington, for example.
Alzheimer diseaseThe physiopathology of Alzheimer disease (AD) is highly complex and has a direct influence on a number of systems, both metabolic and neurotransmission. Its effects on these systems include alterations in amyloid precursor protein (APP) metabolism and cholinergic, adrenergic, serotinergic, dopaminergic and glutamatergic changes in neurotransmission systems.61 A number of studies show that the first events to occur in the course of AD are alterations in glutamatergic synapses. There is a significant correlation between these alterations and the degree of cognitive decline in this disease.62 Evidence from both clinical and molecular experiments has shown that NMDA-type Glu receptors are dysfunctional during early stages of the disease. In vitro studies performed by Shankar in 2007 showed that βA oligomers suppress long-term potentiation in NDMAR.63 In addition to playing an important part in the regulation of synaptic activity, NDMARs also participate in processing the APP, which affects the release of βA peptide. Formation of amyloid-β promotes the endocytosis of NDMAR in cortical neurons. In AD, this allows these neurons to express fewer NDMARs, which elicits rapid, persistent depression of NMDA-evoked currents in cortical neurons. Amyloid-β-dependent endocytosis of NDMAR requires participation by the α-7 nicotinic receptor, protein phosphatase 2B (PP2B) and the tyrosine phosphatase STEP (striatal-enriched protein tyrosine phosphatase). Dephosphorylation of the NR2B subunit (NMDA 2B receptor) of the NMDA receptor is correlated with endocytosis of the receptor. These data indicate the presence of a new mechanism by which β-amyloid may contribute to AD neuropathology: originating synaptic dysfunction by inhibiting the functional location of NMDA-type Glu receptors on the synaptic level.64
Huntington diseaseHuntington disease is an autosomal dominant neurodegenerative disorder caused by a specific mutation in the gene coding for the huntingtin protein. Studies carried out by Coyle in 1976 showed that injecting kainic acid into the striate cortex produced lesions similar to those found in tissue samples from patients with Huntington disease.65 Based on the above, it is hypothesised that there is an excitotoxicity mechanism arising from the overactivation of NDMAR, which leads to increased Ca2+ and Na+ influx. The excitotoxicity in this disease could also be explained by a decrease in Glu uptake by glial cells, or the recently described mechanism involving the co-participation of NMDA and NO receptors, with the latter acting as a free radical.66 Experiments with knockout mice support the hypothesis that states that increased sensitivity in the NMDA receptor mediates excitotoxic phenomena, specifically in the combination of subunits in the subtype including NR1A and NR2B, which will be responsible for the neurons’ selective vulnerability to neurodegeneration.67 Post-mortem analysis of the brains of subjects with Huntington disease supports the idea that excitotoxicity plays a part in the selective degeneration of striatal neurons in these patients. Striate nuclei in subjects with Huntington disease display decreased levels of expression for both mRNA and proteins in the subunits of the NMDA-type Glu receptor. This decrease is associated with the degree of neuronal degeneration. However, NDMAR are expressed in interneurons and striatal projection neurons,68 as well as in neurons in the hippocampus and cerebellum.69 The latter areas are not affected in Huntington disease, and therefore the expression of NDMAR alone does not explain the selective affectation of striatal projection neurons, or why some areas of the brain would be affected why others are not. The data suggest that the differentiated make-up of NDMAR in these areas of the brain may explain the selective degeneration that occurs in very specific zones in cases of Huntington disease. It is important to recall that susceptibility to damage depends on the composition of the subunits in the NMDA-type Glu receptor, and on its expression patterns in both space and time. We know that sensitivity to magnesium block is greater at negative potentials in receptors with NR1a/NR2a and NR1A/NR2B combinations (2.4 and 2.1μM), compared to receptors containing the NR1a/NR2C and NR1a/NR2D subunit combinations (14.2 and 10.2μM). At the same time, the latter group has a faster closing time. As a result, decreased expression of NR2A and NR2B subunits may be associated with these neurons’ susceptibility to degeneration due to excitotoxic neuronal damage.29,42,43 This theory is reinforced by the fact that the mutated Huntington gene favours excitotoxic death mediated by NDMAR containing the NR2B subunit.70–72 These receptors are expressed in all of the striatal projection neurons,73 which are the most affected by Huntington disease.
Based on the above, we can conclude that advances in molecular biology research into NDMAR have been significant. We may consider these receptors to be molecular targets for the development of more effective treatments involving the design of new highly potent, highly selective agonists and antagonists for glutamatergic neurotransmission in order to reduce the neuroexcitotoxicity observed in these neurodegenerative diseases and the conditions mentioned previously.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Flores-Soto ME, et al. Estructura y función de las subunidades del receptor a glutamato tipo NMDA. Neurología. 2012;27:301–10.