The need for a diagnosis in the early stages of Parkinson's disease (PD) has led to increasingly more studies about early-stage signs and symptoms being published. A stereotyped and orderly progression of Lewy bodies in the brains of patients with PD1 has been reported, but this α-synuclein deposit is not necessarily correlated with neuronal dysfunction or death and, therefore, with the symptoms. Thus, the first symptoms of PD can be very varied.2 Longitudinal studies have shown that REM sleep behaviour disorder (RBD) can precede the diagnosis of a neurodegenerative disease (α-synucleinopathies), preferentially PD,3 in up to 45% of cases. It is known that autonomic failure is a late complication of PD and dementia with Lewy bodies (DLB), although it can sometimes constitute the presentation symptom.4,5 We recently described how a carrier of a mutation in the gene for α-synuclein (SNCA) presented dysautonomia as a sign of onset of her disease.6
We present the cases of 2 patients with idiopathic RBD and peripheral autonomic failure without signs or symptoms of neurodegenerative disease. We discuss the risk of developing synucleinopathies and the implications of an early diagnosis.
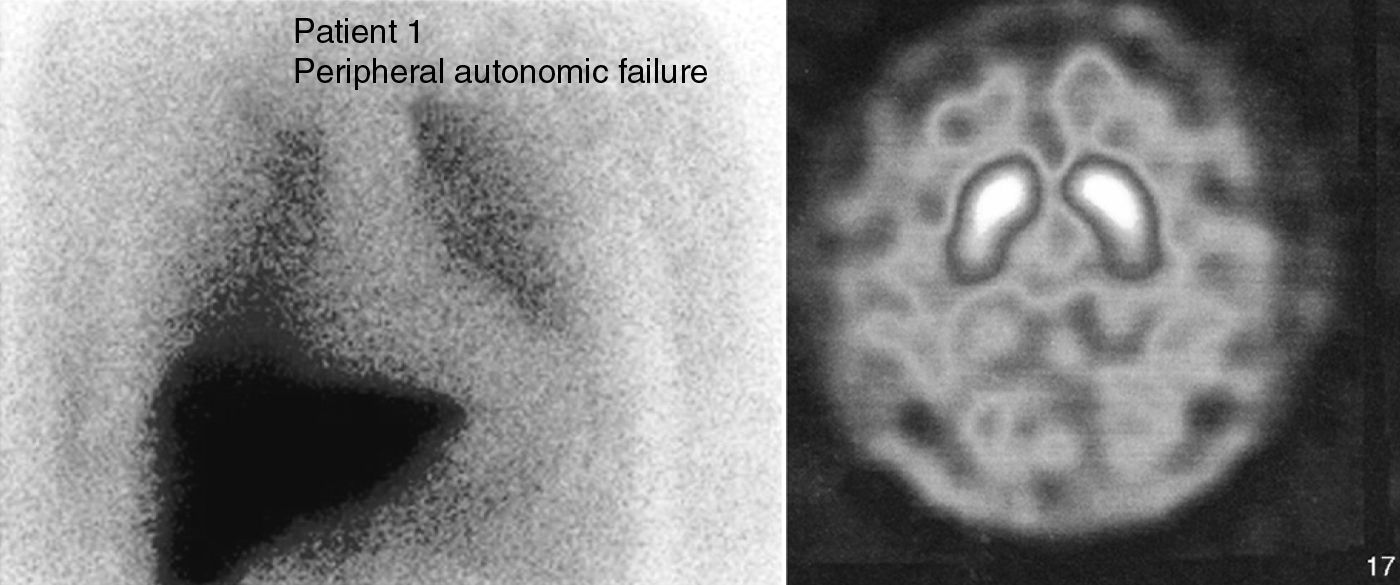
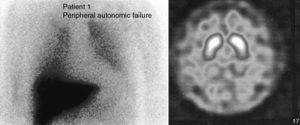
Case 1. This patient was a 66-year-old male with no family history of neurodegenerative diseases. In 2004, he attended consultation for a sleep disorder consisting of nightmares, restless sleep and falling from the bed, which the patient referred having suffered for years. A polysomnography recorded during REM sleep found increased phasic phenomena and a loss of the physiological atonia of this phase. We gave a diagnosis of RBD, which improved with clonazepam (0.5mg). Six months later he suffered cough syncope. A vegetative nervous system study showed a fixed heart rate unchanged by the Valsalva manoeuvre, administration of ephedrine or standing. There was also orthostatic hypotension (supine position 148/80mmHg, HR 53; standing 120/65mmHg, HR 53). He presented noradrenaline values while lying of 26pg/ml (VN: 100–750), which rose to 124pg/ml (VN: 200–1700) when standing. Olfactory deficit was not detected (11/12 items identified correctly in the Brief Smell Identification Test, BSIT). There was a marked uptake decrease in myocardial-MIBG SPECT I (Fig. 1). A brain CT (MRI could not be performed due to the presence of metal particles) was normal. A 123-I-FP-CIT SPECT found no striatal uptake defects (Fig. 1). This exploration was repeated 2 years later and showed normal values, although the striated/occipital lobe uptake ratios had decreased discretely.
Case 2. This patient was a 60-year-old woman who attended consultation due to repeated syncope and orthostatic intolerance, of 10 years’ evolution. She described other dysautonomic symptoms such as constipation and voiding problems: tenesmus and nocturia. She also presented a decrease in olfaction (7/12 in the BSIT). She reported vivid dreams and movement during sleep and the polysomnography found a loss of atonia during REM sleep. The dysautonomic study detected orthostatic hypotension on the tilting table (standing: 103/54mmHg, HR 66; supine position 66/34mmHg, HR 55) and nocturnal arterial hypertension in her blood pressure Holter results. Catecholamine study revealed a significant depletion of noradrenaline (supine position 17pg/ml; standing, 31pg/ml) with an increase of vasopressin during postural changes (supine position 3.9pg/ml, standing 5.1pg/ml). Both the Valsalva ratio (1.8) and the variability of heart rate with breathing were at the lower limit of normality. The reflex-sympathetic skin test was pathological in the lower limbs. The cranial MRI did not detect abnormalities that indicated the presence of multisystem atrophy (MSA). We performed a cardiac-MIBG SPECT with a myocardial/mediastinum ratio at 4h of 1.8 (VN>1.7), while the 123-I-FP-CIT SPECT was normal.
The 2 patients described met the criteria for the diagnosis of RBD. The study results of both patients pointed towards a primary autonomic failure with sympathetic and parasympathetic postganglionic involvement.7 This entity includes 3 neurodegenerative diseases: pure autonomic failure (PAF), Parkinson's disease (PD) and multisystem atrophy (MSA).8 Autonomic dysfunction in MSA is due to the degeneration of the preganglionic neurons, with α-synuclein cytoplasmic inclusions being found in neurons and glial cells of the brain stem and spine.9 The sympathetic ganglia and postganglionic terminals are not affected. In contrast, the involvement of the vegetative nervous system in PD and PAF is predominantly postganglionic. Lewy bodies and neurites are found in the sympathetic ganglia and myenteric plexuses10 and, to a lesser extent, in the intermediolateral horns.11
There have been reports of reduced cardiac MIBG uptake in patients with RBD12 without other additional symptoms, unlike the cases described above, with no clinical signs of dysautonomia. This shows that hypoperfusion in cardiac-MIBG SPECT may be an early finding and precede the onset of motor manifestations. Olfaction deficit, with the same characteristics as in PD patients, has also been reported in this group of patients.13
The hypothesis that neurodegeneration in PD and DLB may begin in the postganglionic autonomic neurons10 has important therapeutic and diagnostic implications, given that the study of the vegetative nervous system can be a useful tool in identifying patients in premotor stages, prior to neuronal degeneration of the substantia nigra. Recently, synuclein inclusions have been detected in the nerve fibres of cutaneous sweat glands in patients with pure autonomic failure.14 The administration of drugs with neuroprotective effects, such as rasagiline, could be indicated in this group of patients. However, we believe that this should be preceded, firstly, by prospective studies that define the values of different biomarkers in these disorders15 and, secondly, by long-term clinical trials with these indications (RBD and pure autonomic failure).
Please cite this article as: Tijero B, et al. Trastorno de conducta del sueño REM y fallo autonómico ¿puro? A propósito de 2 casos. Neurología. 2011;27:55–7.