Erdheim-Chester disease (ECD) is a rare form of non-Langerhans cell histiocytosis characterised by multi-organ xanthogranulomatous infiltration of histiocytes (CD68+/CD1−). Two highly suggestive characteristics of this disease are sclerosis of the long bones and perirenal fat infiltration.1 The central nervous system (CNS) involvement occurs in 30% to 50% of cases and is the main predictor of a poor prognosis.1,2 High doses of interferon alfa have been proposed as first-line treatment in the case of CNS involvement.3,4 Alternative therapies are based on isolated cases reporting good clinical responses to such immunomodulatory drugs as imatinib5 (a potent tyrosine kinase inhibitor) and anakinra6 (a recombinant form of interleukin-1 receptor antagonist), or immunosuppressants such as cladribine.7 Recently, vemurafenib (a potent B-Raf enzyme inhibitor) has demonstrated efficacy on refractory patients with the BRAF V600E mutation.8
In this report we present 2 patients with ECD and neurological impairment, describe clinical and MRI findings, and characterise disease progression for each of the treatments employed.
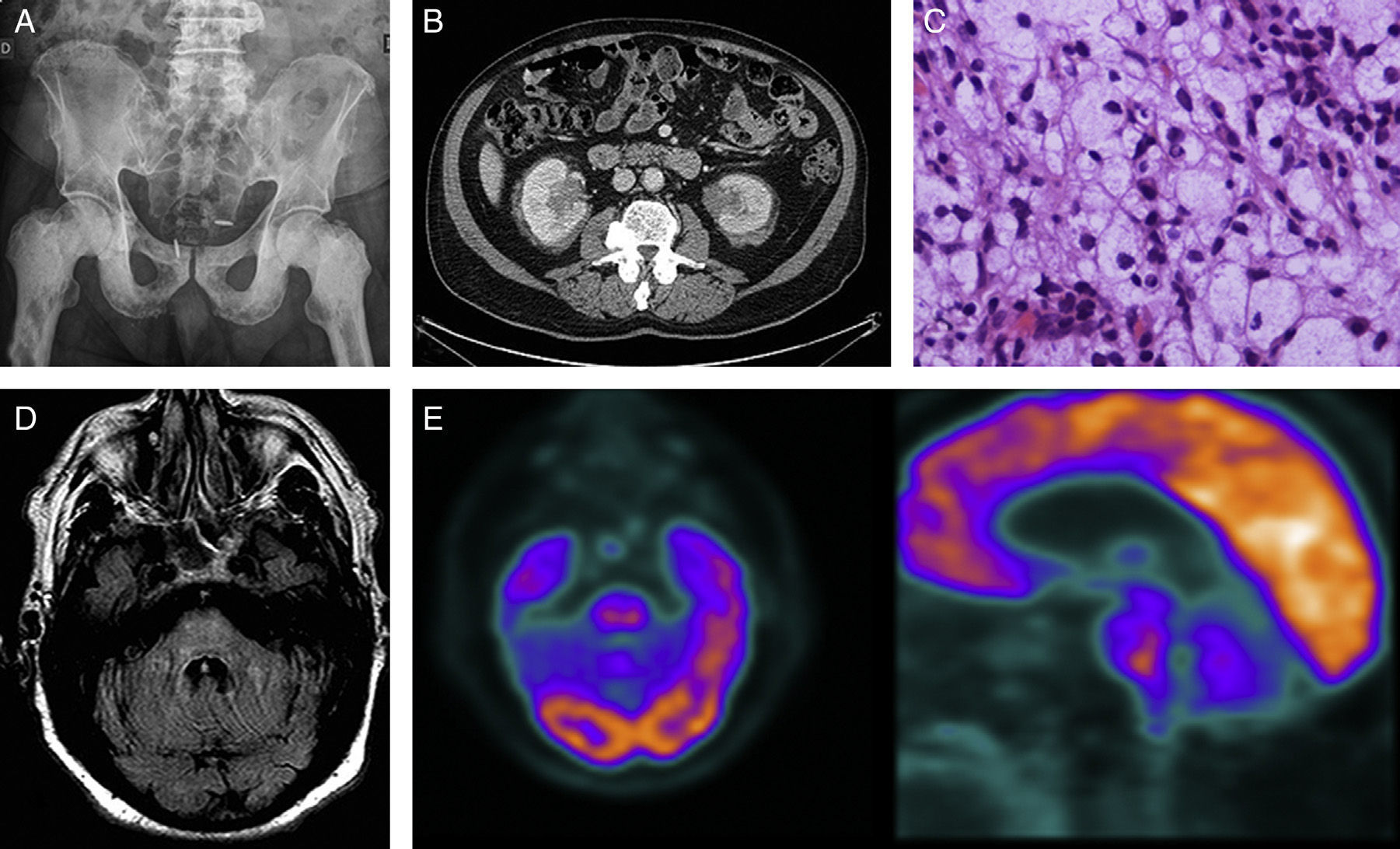
Case 1Our first patient was a 68-year-old man with a history of ischaemic heart disease and prostate cancer treated in 2008. He was admitted in June 2011 due to a 5-year history of osteosclerotic lesions; the initial biopsy was non-diagnostic (Fig. 1A). The patient presented progressive instability and dysarthria over the previous several months. The initial examination revealed pancerebellar syndrome; all other results were normal. Blood and CSF analysis results were within normal levels with the exception of an ESR of 56mm/h. A brain MRI scan showed a signal alteration in the pons that we interpreted as residual ischaemic gliotic changes. An abdominal CT scan disclosed bilateral perirenal mass infiltration (Fig. 1B); a biopsy showed a histiocytic infiltrate (Fig. 1C) with an immunohistochemical profile positive for CD68 and negative for CD1a and S100, findings that are compatible with ECD. The patient was treated with prednisone (1mg/kg/day) and interferon alfa (3-6million IU/day, subcutaneously, 3 times per week). After 6 months the treatment was discontinued due to progression of neurological symptoms, aggravation of the pancerebellar syndrome to the point that he was no longer able to walk, and development of pyramidal signs and behaviour disorders (Fig. 1D and E). Imatinib (400mg/d) and cladribine were ineffective. The patient, a carrier of the BRAF V600E mutation, started treatment with vemurafenib 2 months ago, with no clinical changes.
(A) Simple radiography of the pelvis: osteosclerosis on the diaphysis and epiphysis of both femurs and the pubic bone. (B) Abdominal CT scan: infiltrating perirenal mass showing increased uptake. (C) Haematoxylin–eosin stain: histiocytic infiltrate with abundant clear to foamy cytoplasm. (D) Brain MRI, FLAIR axial sequence at 9 months: signal alterations in the pons and spreading towards the middle cerebellar peduncles. (E) 18F-FDG PET-CT scan: ring-shaped uptake pattern in the pons showing hypometabolic activity at the centre and moderate peripheral activity.
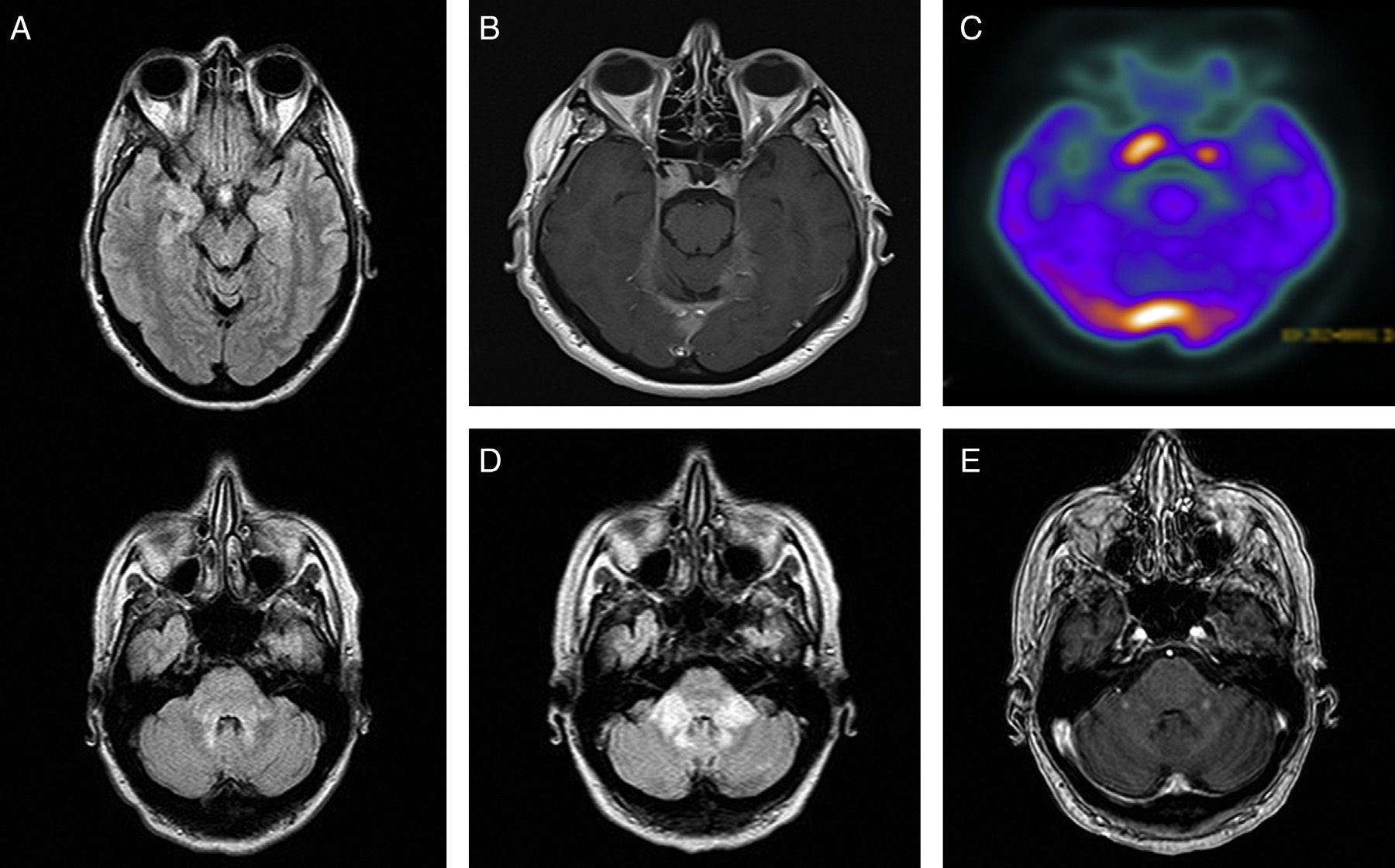
The second patient was a 50-year-old woman with a long history of depression. In December 2010, she was diagnosed with central diabetes insipidus. An MRI scan showed hypothalamus and pituitary gland enlargement, bilateral parasellar masses, and signal alterations at the level of the brainstem and uncus (Fig. 2A and B). The neurological examination showed mild dysarthria; all other findings were normal. The results of the blood and CSF analyses were within normal limits, except for an ESR of 82mm/h. An abdominal CT scan revealed perirenal and peri-aortic abdominal infiltration, and bone scintigraphy showed foci of increased uptake in the diaphysis of the tibias and fibulas. A bone biopsy confirmed the diagnosis of ECD. A brain 18F-FDG-PET/CT scan displayed increased metabolic activity in the brain regions which showed thickening and contrast enhancement on MRI (Fig. 2C). Treatment with interferon alfa (3 million IU/day, 3 times per week, subcutaneously) was discontinued after 6 months due to intolerance and systemic disease progression. Anakinra and cladribine were ineffective and the patient developed cerebellar and pyramidal syndrome to the point of being bedridden; she died 2 years after diagnosis (Fig. 2D and E).
(A) Brain MRI, FLAIR axial sequence: hyperintensities in the middle cerebellar peduncles, dorsal pons, mesencephalic periaqueductal grey, and uncus. (B) Brain MRI, T1-weighted axial sequence with gadolinium: contrast-enhanced bilateral parasellar lesions, larger on the right side, and in the torcula and bilateral tentorium cerebelli. (C) 18F-FDG PET/CT: increased uptake in the area of the pituitary gland and the torcula. (D) Brain MRI, FLAIR axial sequence at 18 months: signal alterations at the level of the middle cerebellar peduncles. (E) Brain MRI, T1-weighted axial sequence with gadolinium: nodular contrast enhancement at the level of the middle cerebellar peduncles.
Progressive cerebellar syndrome and pyramidal syndrome are the main neurological manifestations of ECD. Diffuse hyperintensities extending through the pons and middle cerebellar peduncles are characteristic of the disease, although several other neuroradiological patterns have been described.9,10 The correct diagnosis is difficult to make if the systemic disease is unknown. PET/CT with 18F-FDG is a useful tool in both the diagnosis and the evaluation of response to treatment.11 However, as observed in these 2 cases, not all brain lesions demonstrate the same behaviour. One possible explanation for these findings is the existence of a neurodegenerative pattern in addition to the mass infiltration, which could explain the progression of neurological impairment as well as the lack of response to treatment.12,13
Please cite this article as: Rouco I, Arostegui J, Cánovas A, González del Tánago J, Fernández I, Zarranz JJ. Manifestaciones neurológicas en la enfermedad de Erdheim-Chester: a propósito de 2 casos. Neurología. 2016;31:426–428.