Stroke is currently the main cause of permanent disability in adults. The impairments are a combination of sensory, motor, cognitive and emotional changes that result in restrictions on the ability to perform basic activities of daily living (BADL). Postural control is affected and causes problems with static and dynamic balance, thus increasing the risk of falls and secondary injuries. The purpose of this review was to compile the literature to date, and assess the impact of ankle-foot orthosis (AFO) on postural control and gait in individuals who have suffered a stroke.
DevelopmentThe review included randomised and controlled trials that examined the effects of AFO in stroke patients between 18 and 80 years old, with acute or chronic evolution. No search limits on the date of the studies were included, and the search lasted until April 2011. The following databases were used: Pubmed, Trip Database, Cochrane library, Embase, ISI Web Knowledge, CINHAL and PEDro. Intervention succeeded in improving some gait parameters, such as speed and cadence. However it is not clear if there was improvement in the symmetry, postural sway or balance.
ConclusionsBecause of the limitations of this systematic review, due to the clinical diversity of the studies and the methodological limitations, these results should be considered with caution.
El accidente cerebrovascular (ictus) actualmente es la primera causa de discapacidad permanente en la edad adulta por sus consecuentes secuelas, quedando una combinación de deficiencias sensoriales, motoras, cognitivas y emocionales que conducen a restricciones en su capacidad para realizar actividades básicas de la vida diaria (ABVD). El control postural se afecta y da lugar a problemas en el equilibrio estático y dinámico que incrementan el riesgo de caídas. El objetivo de la revisión consiste en realizar una revisión sistemática que permita valorar los efectos de las ortesis de tobillo (OTP) en el control postural y en la marcha, en sujetos que han presentado un ictus.
DesarrolloSe incluyeron ensayos controlados y aleatorizados que analizasen los efectos de las ortesis de tobillo en pacientes con ictus de entre 18 y 80 años, con evolución aguda o crónica. La búsqueda de ensayos no tuvo limitación en fecha de inicio y se extendió hasta marzo de 2011. Se emplearon las bases de datos Pubmed, Trip Database, Cochrane Library Plus, Embase, ISI Web knowledge, CINHAL y PEDro.
ConclusionesLa intervención logró mejorar algunos parámetros de la marcha como velocidad y cadencia. Sin embargo, no está clara la mejora en la simetría del peso, el balanceo postural o el equilibrio. Las limitaciones de esta revisión sistemática, debidas a la gran diversidad clínica de los estudios que incluye y las limitaciones metodológicas de estos, justifican una lectura precavida de los resultados.
Cerebrovascular accident (stroke) is currently the leading cause of permanent disability in adults because of sequelae suffered by stroke patients.1,2 Stroke survivors exhibit a combination of sensory, motor, cognitive, and emotional deficits restricting their ability to perform basic activities of daily living (ADLs).2
Postural control refers to maintaining the position of the body in such a way as to achieve both stability and spatial orientation. Postural control requires complex interaction between the musculoskeletal and neurological systems. It involves muscle properties, range of motion, flexibility, biomechanical relationships between bodily regions, motor processes, sensory perception processes, and higher processing levels ranging from sensation to action with anticipatory and adaptive aspects.3
Postural control is impaired in patients with stroke, and this creates problems with static and dynamic balance4 which mainly manifest when external disturbances are present. Numerous studies have examined deficiencies in dynamic balance in patients with hemiparesis. Pay et al.5 found patients unable to successfully transfer weight in the frontal plane during the transition to single-limb stance. Di Fabio and Badke6 reported that instability was mainly found along the frontal plane in stroke patients. These problems with postural control may have severe consequences for the patient's physical function and psychosocial well-being, such as restrictions on activities, social isolation, or fear of falling, all of which may increase risk of falling and secondary injury.7 Furthermore, balance disorders and truncal ataxia during the rehabilitation period also constitute a prognostic factor for functional recovery post-stroke.8
Gait disorders are due to motor control deficits that are secondary to muscle tone disorder, ataxia, agnosia, perceptual difficulties, and others. In general, walking patterns in these patients are characterised by lower metabolic efficiency. Deficient postural control explains the metabolic expenditure in these subjects. In fact, lack of intermuscular coordination by the central nervous system explains the presence of unstable gait, asymmetric gait pattern and weight distribution, and increased risk of falling. Normally, these patients walk at slower speeds with shorter step and stride lengths and longer double stance times at the expense of single stance times. At the kinematic level, abnormalities vary enormously from one patient to another: flexure of the hip or knee during the stance phase, hyperextension of the knee during the stance phase, incorrect hip and knee flexion during the swing phase, excessive contralateral pelvic drop, contralateral trunk lean, pes equinovarus, or excessive hip abduction during the swing phase, and others.9 Therefore, restoring gait is a very important objective for neurological rehabilitation. In fact, patients identify restoring gait as the first and most important objective of post-stroke rehabilitation.10–12
One common finding in the gait of stroke patients is varus deformity of the foot, which is frequently caused by spasticity of the posterior tibial muscle. If spasticity is not severe (1–2 on the Ashworth scale), use of an ankle-foot orthosis (AFO) may provide sufficient support.13 Few published studies have evaluated the effect of AFOs on patients with chronic hemiplegia. Currently available clinical evidence consists of just a few articles with small sample sizes and poor methodological quality.14
AFOs are braces used to provide anterior-posterior and medial-lateral stability to the ankle joint, while simultaneously modifying the movements of the subastragalar joint.15 AFOs are used to correct gait anomalies by restricting the degree of plantar flexion and inversion and thereby eliminating ‘drop foot’. AFO use results in increased medial-lateral ankle stability, which promotes a more normal walking pattern.16 The different types of orthoses on the market may be dynamic or rigid, anterior or posterior, standard or made to measure, and constructed from different materials. All of these braces exert an effect on static and dynamic balance, walking speed, cadence, step length, and dynamic base.17 Prescribing AFOs to stroke patients may reduce the risk of falling and associated morbidity by increasing stability in the affected foot, which improves the gait pattern. This will play a major role in a stroke patient's functional recovery.18
There is currently only one systematic review, conducted in 2003, that examines the effects of AFO use in stroke patients.19 This review includes articles up to 2001 and has several methodological limitations.
We therefore believe it necessary to complete another systematic review to measure the effects of AFOs on postural control and walking among subjects who have suffered a stroke. Our reasons are twofold: firstly, AFO use is frequently prescribed as a treatment measure for stroke rehabilitation, and both device designs and materials have improved in recent years. Secondly, there are currently no evidence-based guidelines to help doctors decide how to prescribe this treatment measure.
Materials and methodsStudy typesThe review included randomised controlled trials (RCTs) that analysed the effects of AFOs in stroke patients aged 18 to 80 with either acute or chronic hemiplegia. Studies in which other types of therapy were administered concomitantly with AFO were excluded.
Type of outcome measuresMain outcome measuresPostural control and balanceBerg Balance Scale (BBS),20 Tinetti scale,21 computerised dynamic posturography instrument systems (CDP),22,23 Postural Stability Test (PST);24 Functional Reach Test.25 Risk of falling: Timed Up and Go test (TUG),26 Fall Risk Test,27 Falls Efficacy Scale-International (FES-I).28
Motor control and gaitThree-dimensional movement analysis: data on spatio-temporal parameters, articular kinematics, joint moment and joint power, ground reaction force and muscle activation; independent walking speed over a short distance (5–10m);29 walking endurance: 6-minute walking test.30
Secondary outcome measuresLower limb motricityMotricity Index of the Affected Leg,31 Fugl-Meyer,32,33 RiverMead Motricity Index,34,35 Functional Ambulation Category (FAC);36 Brunnstrom Lower Limb Motor Stage,37 Ashburn Walking,38 Stair Test,38 STREAM: stroke rehabilitation assessment of movement measures.38
Satisfaction, quality of life, basic ADLChedoke–McMaster Stroke Impairment Inventory,39,40 SF-36 questionnaire.41–43
Cognitive stateMini-Mental State Examination (MMSE)44,45 and Utrecht Communication Observation (UCO).46
Muscle toneSearch strategy for identifying studiesThe article search process did not limit studies by starting date and it continued till March of 2011. The search was not restricted to full-text studies only. There were no restrictions on the language of the study. Sources of information employed are listed below: Pubmed (Medline), Trip Database, Cochrane Library Plus, Embase, ISI Web knowledge, CINHAL, and the Physiotherapy Evidence Database (PEDro). To this end, we applied the following search protocol: (1) stroke, (2) balance, (3) 1 and 2, (4) afo, (5) 1 and 4, (6) 1, 2 and 4, (7) ankle foot orthosis, (8) 1, 2 and 7, (9) gait, (10) 1, 4 and 9, (11) 1, 7 and 9.
Articles located using this method were divided into ‘chronic hemiplegia’ and ‘acute hemiplegia’ patient groups. For each selected study, we recorded the author, year of publication, study design, study population (number of patients and time since stroke onset), evaluation measures, and conclusions.
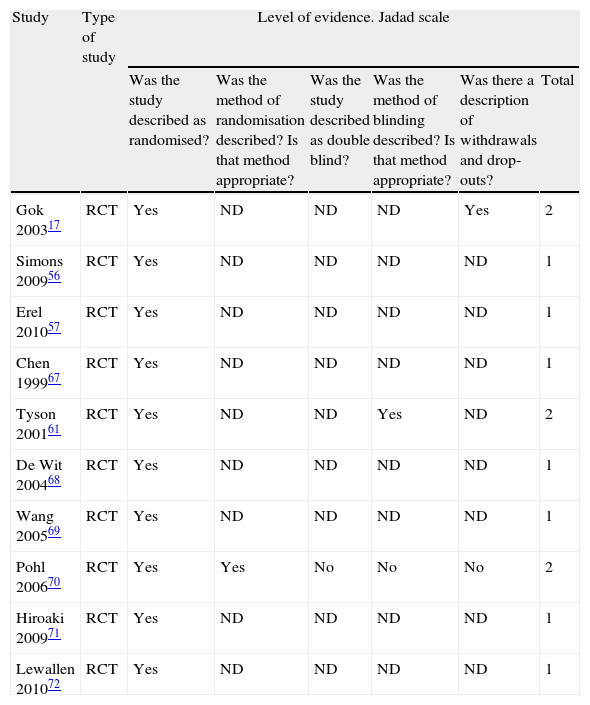
Methodological qualityFor each article, methodological quality was evaluated using the Jadad scale51 which comprises 5 closed-ended questions. This scale is one of the oldest and most frequently-used instruments for measuring the quality of clinical studies.52 The Jadad scale has not been thoroughly tested to gauge its reliability, and the studies published to date describe discrepant results. For example, the study by Clark et al. showed that the scale demonstrates good inter-examiner reliability,53 whereas a different study found low inter-examiner reliability.54
The highest possible total score for the studies examined by this review is 3 out of 5, since participants and physiotherapists cannot be double blinded for this intervention. None of the studies achieved the maximum score. We sent e-mails to 13 authors38,55–66 to request clarifications of the study design or information not included in the study in order to complete quality evaluations. Six authors provided the requested data.56–61 Only published data were used for the rest of the studies.
ResultsDescription of studiesFifty-seven studies were identified by March 2011. We selected 23 studies related to the topic of the review, 13 were later excluded. Data were gathered from the remaining 10 studies,17,56–72 (Fig. 1) which together included 289 patients. Of this total, 201 were men and 88 were women. There were 122 chronic hemiplegia patients and 50 acute hemiplegia patients; time from stroke was not indicated in 97 patients. Of the 289 patients, 129 had left-sided hemiplegia, 148 had right-sided hemiplegia, and data were not given for 12 patients. Several studies included patients with ischaemic or haemorrhagic strokes.56,57,67,68,71,72 The sample in another study was constituted by patients with stroke and head trauma,70 while the sample in 3 studies consisted of patients with hemiparesis of unspecified aetiology.20,61,69
Articles included in the review defined the following inclusion criteria for all participants: patients aged 18 or older who had suffered a stroke, with sufficient cognitive and communication ability to understand the task and no progressive neurological deficits; absence of lower limb musculoskeletal abnormalities affecting gait; no pain while walking; Ashworth scale for spasticity <3; no history of falls in the preceding 3 months; and passive dorsiflexion of 90° or more with the knee extended. Additional inclusion criteria were no skin or other lesions contraindicating AFO, no vision problems, ability to walk more than 500 steps, or 8 to 15 metres, in and out of the room with or without assistance; score of 3 to 5 on the FAC scale; ability to remain standing unaided for 20 to 90 seconds with and without AFO. Exclusion criteria were as follows: patients with a history of more than one stroke; patients with other deficits affecting ability to walk unaided; patients with aphasia or hemispatial neglect; those taking medication for other disorders affecting balance; knee contracture; and increased muscle tone affecting range of movement of the ankle, knee, or hip. Prior use of an AFO was an inclusion criterion in some studies57,69,71 and an exclusion criterion for another.58
Some of the studies included patients who used walking sticks17,67,69,71,72 while another did not70 and 3 studies did not provide this information.56,57,61
Studies did not indicate whether participants received concomitant therapy or drug treatment as a part of the rehabilitation programme.
All studies were approved by the pertinent ethics committee and all patients signed their informed consent.
Methodological qualityThis systematic review found that all examined studies had poor methodological quality since none had a score of 3 (Table 1). However, it should be noted that the Jadad scale only measures random selection, blinding, and patients removed or lost to follow-up to evaluate the quality of methods employed in primary research. Only 2 of these 3 items, as we mentioned above, can be applied to physiotherapy interventions since they do not permit blinding of therapists and patients under some conditions. In consequence, this item does not contribute to the scale's ability to determine RCT quality for physiotherapy. Similarly, the Jadad scale provides no items evaluating specifics of the treatment protocol, adherence to or completion of treatment. All these factors are important in physiotherapy. As such, the Jadad scale does not completely measure methodological quality for physiotherapy trials, but we should recall that the scale does provide the best evidence of RCT validity.73
Jadad scale.
| Study | Type of study | Level of evidence. Jadad scale | |||||
| Was the study described as randomised? | Was the method of randomisation described? Is that method appropriate? | Was the study described as double blind? | Was the method of blinding described? Is that method appropriate? | Was there a description of withdrawals and drop-outs? | Total | ||
| Gok 200317 | RCT | Yes | ND | ND | ND | Yes | 2 |
| Simons 200956 | RCT | Yes | ND | ND | ND | ND | 1 |
| Erel 201057 | RCT | Yes | ND | ND | ND | ND | 1 |
| Chen 199967 | RCT | Yes | ND | ND | ND | ND | 1 |
| Tyson 200161 | RCT | Yes | ND | ND | Yes | ND | 2 |
| De Wit 200468 | RCT | Yes | ND | ND | ND | ND | 1 |
| Wang 200569 | RCT | Yes | ND | ND | ND | ND | 1 |
| Pohl 200670 | RCT | Yes | Yes | No | No | No | 2 |
| Hiroaki 200971 | RCT | Yes | ND | ND | ND | ND | 1 |
| Lewallen 201072 | RCT | Yes | ND | ND | ND | ND | 1 |
ND: not described.
One study found that AFO use contributed no significant differences to results for postural sway67 measured with CDP and the postural sway index with patients standing still for 30 seconds and bending forward without moving their feet for 10 seconds. Another study found that AFO was associated with decreased swaying while eyes were open, but not while eyes were closed,70 this parameter was measured using CDP with the patients standing up straight with their arms at their sides. A third study found significantly improved balance for both open-eye and closed-eye trials, but only in acute patients treated with AFO69; this was measured using CDP with the patient standing and looking forward.
Regarding weight distribution in the affected leg, we observe that AFO caused significant improvement in lateral weight transfer in 2 studies,61,70 both of which were evaluated with CDP. In the first, the patient remained standing still for 30 seconds and then transferred weight from left to right during 10 seconds; in the second, patients remained standing with their arms to their sides and without moving their feet.
In one study, intervention resulted in significantly more symmetrical weight distribution during walking.70 Another study showed that patients treated with AFO displayed better weight shifting with a more symmetrical distribution, but only in acute patients.69 In another two studies, AFO did not change either weight distribution symmetry, transfer of weight to the affected leg, or dynamic postural stability as measured by CDP.56,61
Two studies concluded that AFO significantly improved scores on the TUG test,56,68 and two studies reported better scores on the Stair Test.57,68 One of those studies found no significant differences on the TUG test or the Functional Reach Test.57 Two studies observed that AFO use improved balance and stability as measured by the CDP and BBS.69,71 The first study, however, did not record improvements in patients at more than 12 months from the stroke event. Another study indicated that AFO improved balance as measured with BBS,56 whereas a different study found no improvements with BBS.70
Motor control and gaitIn 6 studies, researchers observed increased walking velocity among AFO users.17,56,57,61,69,71 This was measured with instrumental gait analysis systems17 or with walking speed tests.57,61,68,69,71 One study reported a decrease in velocity in patients treated with AFO.72 Three studies reported that AFO improved cadence.61,69,71 Two studies found a significant increase in step length,71,72 while another 2 studies found no differences in step length.17,61 According to 2 studies, AFO elicited an increase in stride length.61,71 Another study found a decrease in double stance time.70 A different study reported a greater range of joint motion in the ankle and a greater angle of dorsiflexion during the swing phase and at heel strike.17
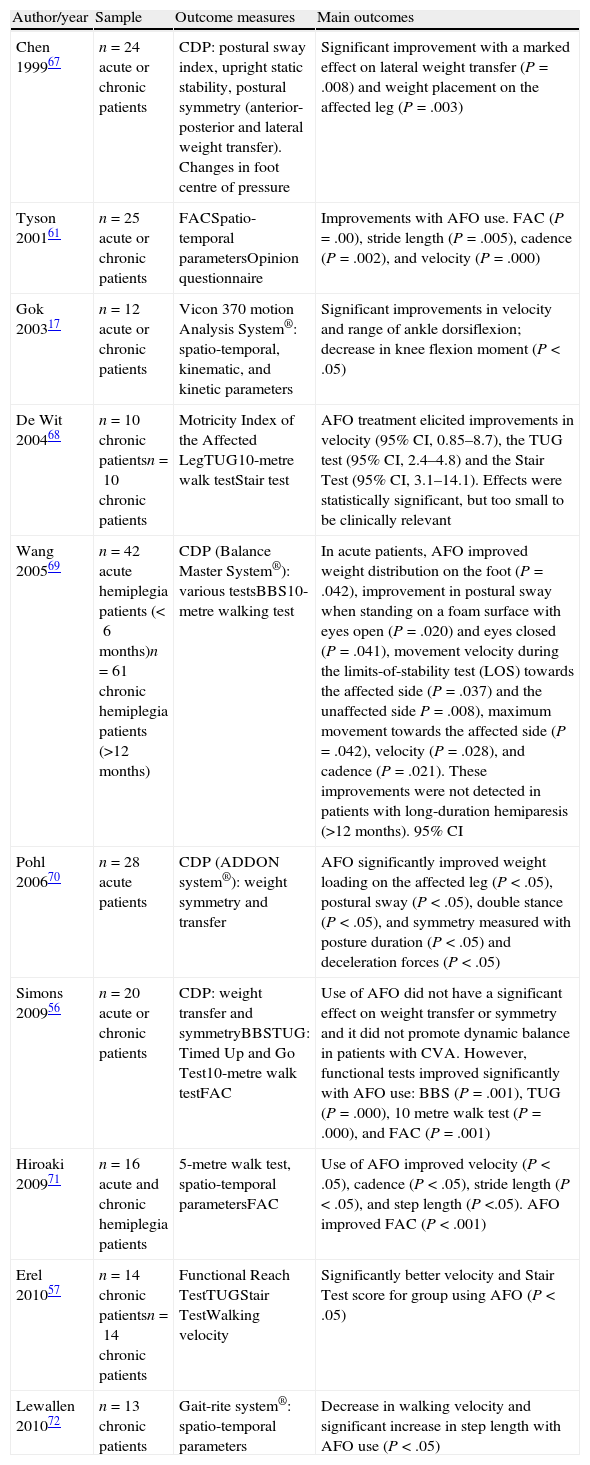
Both main outcomes and secondary outcome measures are listed in Table 2.
Summary of results.
| Author/year | Sample | Outcome measures | Main outcomes |
| Chen 199967 | n=24 acute or chronic patients | CDP: postural sway index, upright static stability, postural symmetry (anterior-posterior and lateral weight transfer). Changes in foot centre of pressure | Significant improvement with a marked effect on lateral weight transfer (P=.008) and weight placement on the affected leg (P=.003) |
| Tyson 200161 | n=25 acute or chronic patients | FACSpatio-temporal parametersOpinion questionnaire | Improvements with AFO use. FAC (P=.00), stride length (P=.005), cadence (P=.002), and velocity (P=.000) |
| Gok 200317 | n=12 acute or chronic patients | Vicon 370 motion Analysis System®: spatio-temporal, kinematic, and kinetic parameters | Significant improvements in velocity and range of ankle dorsiflexion; decrease in knee flexion moment (P<.05) |
| De Wit 200468 | n=10 chronic patientsn=10 chronic patients | Motricity Index of the Affected LegTUG10-metre walk testStair test | AFO treatment elicited improvements in velocity (95% CI, 0.85–8.7), the TUG test (95% CI, 2.4–4.8) and the Stair Test (95% CI, 3.1–14.1). Effects were statistically significant, but too small to be clinically relevant |
| Wang 200569 | n=42 acute hemiplegia patients (<6months)n=61 chronic hemiplegia patients (>12months) | CDP (Balance Master System®): various testsBBS10-metre walking test | In acute patients, AFO improved weight distribution on the foot (P=.042), improvement in postural sway when standing on a foam surface with eyes open (P=.020) and eyes closed (P=.041), movement velocity during the limits-of-stability test (LOS) towards the affected side (P=.037) and the unaffected side P=.008), maximum movement towards the affected side (P=.042), velocity (P=.028), and cadence (P=.021). These improvements were not detected in patients with long-duration hemiparesis (>12 months). 95% CI |
| Pohl 200670 | n=28 acute patients | CDP (ADDON system®): weight symmetry and transfer | AFO significantly improved weight loading on the affected leg (P<.05), postural sway (P<.05), double stance (P<.05), and symmetry measured with posture duration (P<.05) and deceleration forces (P<.05) |
| Simons 200956 | n=20 acute or chronic patients | CDP: weight transfer and symmetryBBSTUG: Timed Up and Go Test10-metre walk testFAC | Use of AFO did not have a significant effect on weight transfer or symmetry and it did not promote dynamic balance in patients with CVA. However, functional tests improved significantly with AFO use: BBS (P=.001), TUG (P=.000), 10 metre walk test (P=.000), and FAC (P=.001) |
| Hiroaki 200971 | n=16 acute and chronic hemiplegia patients | 5-metre walk test, spatio-temporal parametersFAC | Use of AFO improved velocity (P<.05), cadence (P<.05), stride length (P<.05), and step length (P<.05). AFO improved FAC (P<.001) |
| Erel 201057 | n=14 chronic patientsn=14 chronic patients | Functional Reach TestTUGStair TestWalking velocity | Significantly better velocity and Stair Test score for group using AFO (P<.05) |
| Lewallen 201072 | n=13 chronic patients | Gait-rite system®: spatio-temporal parameters | Decrease in walking velocity and significant increase in step length with AFO use (P<.05) |
AFO: ankle foot orthosis; BBS: Berg Balance Scale. TUG: Timed Up and Go; FAC: Functional Ambulatory Classification; CDP: computerised dynamic posturography.
The purpose of this study was to complete a systematic review that would combine existing published studies to permit an assessment of the effects of AFO on postural control and gait in stroke survivors.
We believe that the specific search protocol identified all relevant trials. While not all of the grey literature may have been identified, this was not likely to have a significant impact on results. Since our study only included randomised controlled trials from one population and treated with a specific intervention, it was designed to provide answers to a specific clinical question.
The visual, proprioceptive, and vestibular systems are critical sources of afferent information that affect postural control and spatial orientation.3 The 2 studies that included closed-eye evaluations69,70 yielded disparate results. One concluded that use of AFO led to better results on CDP tests in acute patients only,69 whereas the other study did not record any significant improvements in postural control.70 Better sensory control, and improvement of levels related to automaticity and confidence in balancing and walking, may contribute to functional recovery.56
Most patients have problems with motor control and balance after a stroke, and consequences may include difficulty in walking and increased risk of falls.74 At the skeletal muscle level, insufficient dorsiflexion during the swing phase, ankle instability, and poor lift during the last phase of walking all disturb the normal walking pattern. AFOs may be the best orthoses for improving postural control since they compensate for the weakness of muscles around the affected foot and improve peripheral stability.17 However, none of the included studies use electromyography to evaluate muscular condition, and it therefore remains unclear whether the mechanical properties of the AFO affect the level of muscular activity in the lower limbs. It is therefore crucial that future studies examine the baseline condition of the muscles and the effects of using that device on the musculoskeletal system.
AFOs appear to exert positive effects on hip, knee, and foot alignment,17 and they improve gait parameters such as velocity, cadence, and step length. Although these parameters by themselves cannot indicate improved stability during walking,71 they can contribute to increasing the patient's walking ability both as part of and outside of a physiotherapy programme. This is critical for improving motor ability,75 and a secondary benefit is reduced risk of falling.74 Furthermore, the positive effect of AFO use on the patient's self-confidence and the decreased fear of engaging in different activities60 suggest that safety plays a major role in the patient's motivation to use the device.68
Although slight variability could be observed in some outcome measures such as velocity or cadence, results are more debatable for other parameters such as weight symmetry or postural sway. Studies evaluating weight symmetry during walking have yielded contradictory results. Two such studies pointed to improvements in weight symmetry.69,70 The first of these studies evaluated patients with both acute and chronic hemiplegia but only observed improvements in the first group. The second study only assessed patients with acute hemiplegia. Two other studies reported no improvements in either acute or chronic hemiparetic patients.56,61 This may be due to different factors that include variability in patient characteristics (age, time since onset, aetiology) and interindividual variations in step length that may affect symmetry. In any case, it is clear that weight asymmetry during walking requires additional muscular effort and therefore more metabolic expenditure.57 This is to be avoided in stroke patients.
Since all included studies were performed on subjects able to walk without an AFO, evidence is not sufficient to suggest that AFO treatment would be effective in patients with more severe hemiplegia. Further research is needed on the effect of AFO use in this segment of the population. These studies did not measure the effectiveness of interventions after stroke according to whether patients had acute or chronic hemiplegia. Furthermore, studies did not take patients’ previous condition into account, and this key factor may have an impact on outcomes with regard to the supervision required, score on the FAC, percentage of time post-stroke using the AFO, or adaptation time permitted for AFO use. Furthermore, studies did not indicate whether patients were receiving pharmacological treatment concomitantly with rehabilitation. It is extremely important that the above factors be taken into account in order to direct future research on the impact of AFO use on postural and gait control in patients who have had a stroke.
The limitations of this systematic review are mainly the considerable clinical diversity of the studies it includes, and the methodological limitations of each individual study. These studies present certain deficiencies that should be noted and which require cautious interpretation of their results. As stated in the review, all included studies are randomised controlled trials; however, procedures for random selection are not described for most articles, and allocation was either not concealed or this information was not provided. As for blinding techniques, they are difficult to apply to the patients and therapists involved in the intervention. Blinding evaluators is also extremely complicated. This being the case, studies assume the risk of a classification bias that may affect measurements of variables. Although subjects’ eligibility criteria were listed by most studies, none of the included studies provided details about the process used to select its subjects. We therefore cannot rule out the possibility of a selection bias. Only one article gives details on drop-outs and patients lost to follow-up.17 None of the articles describes potential adverse effects. It seems reasonable to believe that using AFO is a safe procedure. Studies with larger sample sizes should be carried out to provide a more precise evaluation of this intervention's safety profile.
In this review, the authors were exclusively responsible for developing selection criteria and screening the studies for inclusion. On the other hand, cross-checking may improve the quality of this review.
Future studies will require a longitudinal design in order to evaluate effects of AFO over time and in a larger population than that found in most of the reviewed articles. They should also take the following into account: aetiology, lesion severity, cognitive abilities, age, time since stroke, and other factors. Functional analysis of each patient's abilities and motor functions should be performed in order to select the appropriate aids. In addition to spatio-temporal parameters, future studies should also consider kinematic analysis or energy expenditure.72 Lastly, we should be aware of the different repercussions that different types of AFO may have on different patients. This is an important objective for future reviews and clinical guidelines.
ConclusionsScientific evidence shows that balance training after stroke improves postural control. However, scientists do not agree on what type of training offers the most effective result.
An AFO may be prescribed soon after a stroke event to improve certain gait parameters, such as velocity and cadence. It may also offer the patient greater stability, which will increase his/her self-confidence and postural control by enabling participation in more activities. Most studies agree on the above points. However, we must exercise caution when interpreting these results because the deficiencies in the studies reviewed here have left gaps in the research.
There is an urgent need for well-designed longitudinal studies that evaluate the effects of AFO on stroke patients. Such studies must use precisely defined eligibility criteria and ensure that study groups are similar through randomised concealed allocation, and this procedure should be described. Furthermore, new studies must at the very least be able to ensure that evaluators are blinded.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Guerra Padilla M, Molina Rueda F, Alguacil Diego IM. Efecto de la ortesis de tobillo pie en el control postural tras el accidente cerebrovascular: revisión sistemática. Neurología. 2014;29:423–432.