Intracerebral haemorrhage accounts for 10% to 15% of all strokes, however it has a poor prognosis with higher rates of morbidity and mortality. Neurological deterioration is often observed during the first hours from onset, and determines the poor prognosis. Intracerebral haemorrhage, therefore, is a neurological emergency which must be diagnosed and treated properly as soon as possible. In this guide we review the diagnostic procedures and factors that influence the prognosis of patients with intracerebral haemorrhage and we establish recommendations for the therapeutic strategy, systematic diagnosis, acute treatment and secondary prevention for this condition.
La hemorragia intracerebral sólo representa entre el 10 y el 15% de todos los ictus, sin embargo condiciona un peor pronóstico, con unas tasas más elevadas de morbilidad y mortalidad. Es frecuente que durante las primeras horas tras el inicio de los síntomas se produzca un empeoramiento clínico, lo cual condiciona un peor pronóstico, por lo que la hemorragia intracerebral constituye una emergencia neurológica en la que debe realizarse un diagnóstico y tratamiento adecuado de manera precoz. En esta guía realizamos una revisión de los procedimientos diagnósticos y los factores que influyen en el pronóstico de los pacientes con hemorragia intracerebral y establecemos unas recomendaciones para la estrategia asistencial, sistemática diagnóstica, tratamiento en fase aguda y prevención secundaria en la hemorragia intracerebral.
Intracerebral haemorrhage (ICH) refers to the collection of blood within the cerebral parenchyma as the result of vascular rupture unrelated to trauma. Although the bleed may leak into the ventricular system or the subarachnoid space, it always begins in brain tissue. This trait distinguishes ICH from subarachnoid haemorrhage and primary intraventricular haemorrhage.
Haemorrhages are categorised as primary or secondary depending on the cause of the bleed. Primary ICHs are the most common and they are caused by the rupture of any blood vessel within the brain's normal vascular system after the vascular wall is weakened by degenerative processes secondary to arterial hypertension (AHT) or amyloid angiopathy. Secondary ICHs are caused by the rupture of blood vessels that are congenitally abnormal or newly formed, or of vessels that contain vascular wall abnormalities or weaknesses caused by coagulation disorders. They are associated with such entities as tumours, arteriovenous malformations (AVM), coagulation disorders, substance abuse, or haemorrhages inside areas of ischaemia.1
ICH incidence varies by country, race, age, and sex, and it is closely related to AHT prevalence. In Europe, its incidence rate is approximately 15 cases per 100000 inhabitants.2 While ICH is only present in 10% to 15% of all strokes, it is associated with a poorer prognosis and higher morbidity and mortality rates. The mortality rate during the first month after ICH is 40.4%.3 Most deaths occur in the first 2 days, and only 20% of the total patients are independent 6 months after having had an ICH.4 Mortality at 30 days is related to the size and location of the ICH. In patients with an initial haemorrhage volume greater than 60cm3, mortality for deep haemorrhages is 93%, and for lobar haemorrhages, 72%. If initial volume is less than 30cm3, mortality rates are 39% for deep haemorrhages, 7% for lobar haemorrhages, and 57% for cerebellar haemorrhages.3
The incidence of ICH is on the rise despite improved control over certain risk factors. This is related to the ageing of the population. However, the higher incidence rate among the elderly may also contribute to the decrease in mortality recorded in recent years, because of more pronounced cerebral atrophy.
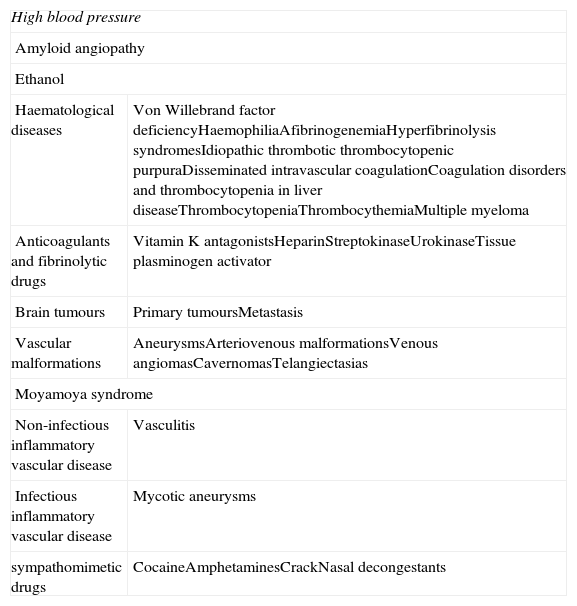
The most important risk factor for developing ICH in all age groups and both sexes is AHT, whether systolic or diastolic. AHT is present in 60% of all cases. Chronic AHT provokes degenerative changes in arteriole walls that favour vascular obstructions. This in turn causes the lacunar infarcts, leukoaraiosis, and vascular rupture that are responsible for the appearance of ICH.5 AHT may also be an acute cause of ICH by affecting small arterioles that are at risk due to hypertrophy of their walls. This leads to haemorrhages such as those caused by certain drugs or haemorrhages occurring after endarterectomy or angioplasty.6 Another important cause of ICH is cerebral amyloid angiopathy, which is the leading cause of lobar haemorrhage in elderly subjects. This degenerative process affects small arteries and arterioles located in the leptomeninges and cerebral cortex. Haemorrhages of this type are superficial, often recurring and multiple, and tend to be located in posterior areas of the brain. They appear in elderly subjects, and up to half of all patients present cognitive decline.7 Lastly, there are other less common causes of ICH which are listed in Table 1.
Causes of non-traumatic intracerebral haemorrhage.
| High blood pressure | |
| Amyloid angiopathy | |
| Ethanol | |
| Haematological diseases | Von Willebrand factor deficiencyHaemophiliaAfibrinogenemiaHyperfibrinolysis syndromesIdiopathic thrombotic thrombocytopenic purpuraDisseminated intravascular coagulationCoagulation disorders and thrombocytopenia in liver diseaseThrombocytopeniaThrombocythemiaMultiple myeloma |
| Anticoagulants and fibrinolytic drugs | Vitamin K antagonistsHeparinStreptokinaseUrokinaseTissue plasminogen activator |
| Brain tumours | Primary tumoursMetastasis |
| Vascular malformations | AneurysmsArteriovenous malformationsVenous angiomasCavernomasTelangiectasias |
| Moyamoya syndrome | |
| Non-infectious inflammatory vascular disease | Vasculitis |
| Infectious inflammatory vascular disease | Mycotic aneurysms |
| sympathomimetic drugs | CocaineAmphetaminesCrackNasal decongestants |
ICH is a neurological emergency, and therefore rapid diagnosis and management are fundamental; as mentioned before, clinical exacerbation is common in the first few hours following ICH. This factor is directly associated with a poorer functional prognosis. A number of observational studies show that 1 in 3 patients with supratentorial haemorrhage and most patients with a posterior fossa haemorrhage present an altered level of consciousness.8 Owing to the high risk of early neurological impairment, which is associated with poor long-term prognosis, care must be provided to ICH patients as quickly as possible.
Pre-hospital careThe main objective of pre-hospital care is maintaining correct cardiovascular and respiratory function and transporting the patient to the nearest hospital with facilities for acute-phase stroke patients. Additional objectives include taking the patient's medical history, especially events occurring at symptom onset and information about prior medical conditions. It is important to alert the receiving hospital prior to the arrival of a patient with a possible stroke so that staff can prepare the equipment needed to assess the stroke. This cuts down on delays in completing neuroimaging tests in the emergency department.9
Care in the emergency departmentOnce the haemodynamic and cardiorespiratory functions have been stabilised, further objectives include confirming the type of stroke to differentiate a haemorrhage from ischaemia or other brain lesions; gathering information about ICH aetiology; preventing potential complications; and starting appropriate treatment.
The clinical course of ICH may not offer data distinguishing that entity from other types of stroke unless there are pathognomonic clinical features pointing to a cerebral haemorrhage. However, certain signs and symptoms are more suggestive of ICH than of ischaemia. One symptom that appears frequently is headache, which is present in 40% of ICH cases and only 17% of ischaemic stroke cases. Other common symptoms, present in 50% of ICH cases, include nausea, vomiting, and decreased level of consciousness; these signs are exceptional in ischaemic stroke. We also find increased arterial blood pressure in almost 90% of ICH cases.10
When taking medical histories, doctors must emphasise data such as time of symptom onset, vascular risk factors (AHT, diabetes, hypercholesterolaemia), substance abuse (tobacco, alcohol, cocaine, amphetamines), drugs (anticoagulants, antiplatelet drugs, nasal decongestants, diet pills, stimulants, sympathomimetic drugs), prior traumatic event or recent surgery (especially endarterectomy or carotid angioplasty, which may be associated with reperfusion syndrome), pre-existing cognitive decline (related to amyloid angiopathy), seizures, systemic illnesses related to coagulation disorders (liver disease, vasculitis, cancer, blood dyscrasia), and any family history of neurological diseases associated with increased risk of cerebral bleeding (including arteriovenous malformations and intracranial aneurysms).
In addition to assessing neurological deficit in the initial examination, doctors must evaluate respiration and the haemodynamic state. To this end, an electrocardiogram and chest radiography are needed. A detailed physical examination that includes a cardiovascular study and ophthalmoscopy is often helpful for establishing an aetiological diagnosis. In cases in which the patient has remained bedridden during long periods of time, doctors should check for potential associated complications, including pressure ulcers, compartment syndromes, rhabdomyolysis, and traumatic lesions.
Laboratory testsIt is important to perform blood tests to gather results for complete blood count, electrolytes, urea, creatinine, liver function parameters, and glucose. High creatinine and glucose levels are associated with haemorrhage growth and a poor functional prognosis.11,12 Doctors should also complete a coagulation study including activated partial thromboplastin time (APTT) and INR. This is done because haemorrhages associated with anticoagulant treatment are accompanied by increased risk of morbidity and mortality13,14 and require urgent treatment to reverse the coagulation disorder.
Younger patients should undergo urine screening to detect toxic substances such as cocaine and other sympathomimetic drugs and women of childbearing age will require a pregnancy test.
NeuroimagingThe presence of sudden-onset focal neurological deficit suggests a vascular origin unless there is another proven cause. Although some of the symptoms described above, such as headache, vomiting, and decreased level of consciousness, are suggestive of ICH, these findings are not specific and they do not enable us to differentiate between neurological deficit caused by cerebral ischaemia and that caused by a haemorrhage. For this reason, neuroimaging studies are fundamental. Both computed tomography (CT) and magnetic resonance imaging (MRI) may be used for the initial diagnosis. CT is highly sensitive for identifying haemorrhage during the acute phase, and it is regarded as the technique of choice. Gradient echo MRI sequences are as sensitive as CT for detecting blood during the acute phase of stroke, and they are even more sensitive than CT for detecting old haemorrhages.15 However, the availability, lower costs, and shorter times associated with CT mean that this technique is more commonly used than MRI.
CT allows us to pinpoint the location of the haemorrhage and identify its effects (mass effect, oedema, ventricular extension, and subarachnoid extension). Furthermore, administering contrast intravenously lets us diagnose certain secondary causes of ICH, such as AVMs or tumours. In the first hours of the process, ICH presents as increased density in the cerebral parenchyma, which is explained by the haemoglobin in the escaped blood. As the days pass, the haemorrhage can be found in the centre of a hypodense ring. At first, this appearance is caused by retraction of the clot; at a later point, it is caused by vasogenic oedema. At the end of several weeks, the high initial density of the haemorrhage begins to decrease from the perimeter toward the centre. The final stage of an ICH as viewed by CT is total reabsorption of haemorrhagic tissue. This produces a residual cavity that is indistinguishable from that left by an old cerebral infarct.16
Some data on the location and morphology of ICHs detected using CT may be important to establish an aetiological diagnosis. The most common location of hypertensive ICH is the putamen (30%–50%), followed by subcortical white matter (30%) and the cerebellum (16%). If the location is lobar, the role played by AHT is less significant and amyloid angiopathy is more likely to be the cause. This is especially true in patients older than 60 years with a certain level of cognitive decline.17 Other common causes of lobar haemorrhages are arteriovenous malformations (7%–14%), tumours (7%–9%), and blood dyscrasias, including anticoagulant treatment (5%–20%). In 3% of all patients, the haemorrhage remains limited to the intraventricular system.18
Since the haemorrhage often grows during the acute phase, and this phenomenon is associated with neurological deterioration and increased morbidity and mortality,19 research is being done on techniques that may help us predict haemorrhage growth. The use of CT angiography with contrast may help identify patients at risk for haemorrhage expansion based on the presence of isolated contrast in the haemorrhage (spot sign).20,21 This technique is also useful for detecting secondary causes of ICH, such as arteriovenous malformations, tumours, or venous thrombosis.
MRI scans contribute further information about the stage of development of the ICH. Differences in these scans have to do with the way that images of haemoglobin change throughout the catabolism process. In the early stages of acute-phase ICH (initial hours), oxyhaemoglobin levels in the haemorrhage are high and the MRI shows hypointensities in T1 and hyperintensities in T2. In later stages of acute-phase ICH (first few days), oxyhaemoglobin converts to deoxyhaemoglobin from the centre of the bleed to its perimeter. In the MR image, this appears as a hypointense area in T2, surrounded by a hyperintense ring corresponding to the oedema. In late stages of ICH (after several weeks), deoxyhaemoglobin is transformed into methaemoglobin from the perimeter to the centre. The change appears as a peripheral hyperintense signal in T1 which progressively extends to the entire area of the haemorrhage. In the recovery phase (months after onset), all of the haemoglobin has been transformed into haemosiderin, which creates a pronounced hypointense signal in T2-weighted sequences. MRI gradient-echo sequences are highly sensitive for detecting small chronic haemorrhages, called microbleeds, which measure less than 5mm. Microbleeds appear as hypointense pinpoint lesions and indicate the presence of chronic haemosiderin deposition.22 Magnetic resonance angiography (MRA) is a useful technique for detecting vascular lesions associated with ICH. It has a high sensitivity for detecting aneurysms and AVMs.23 MRA is also useful during the venous phase when there is a suspicion of sinus thrombosis as the cause of the haemorrhage. The technique is as reliable as CT angiography with contrast during the venous phase.
Conventional arteriography may be useful when there is a strong suspicion of a secondary cause and results from non-invasive studies are negative. Radiological signs that suggest a secondary cause are presence of subarachnoid haemorrhage, unusual haemorrhage shape (non-circular), oedema size not proportional to haemorrhage evolution time, uncommon location, or presence of abnormal structures. The probability of detecting a secondary cause by using angiography is higher in these cases.24 For suspected vasculitis, conventional angiography is the technique of choice. In some cases, as with cavernous angiomas, conventional angiography may yield negative results. Arteriography is not useful, however, in hypertensive patients older than 45 with haemorrhages in the putamen, thalamus, or posterior fossa.25
- 1.
An emergency brain CT or MRI scan is recommended on an emergency basis in order to distinguish between ICH and other ischaemic or structural lesions (level of evidence 1, grade A recommendation).
- 2.
CT angiography with contrast may be useful for identifying patients who are at risk for haemorrhage growth (level of evidence 2b, grade B recommendation).
- 3.
CT angiography and/or MRI angiography may be useful for identifying structural lesions that are aetiologically related to ICH when there is a suspicion based on radiological findings (level of evidence 2a, grade B recommendation).
- 4.
Conventional angiography must be considered in patients when the ICH aetiology has not been determined by non-invasive methods and radiological signs are suggestive of a structural lesion (level of evidence 4, grade C recommendation).
Treatment for patients with ICH is fundamentally medical. It is based on maintaining vital functions, neurological monitoring, maintaining homeostasis, and preventing complications.26 The key objective of all of these activities is to prevent increases in haemorrhage size, which would provoke a mass effect, increase intracranial pressure, and cause secondary neurological impairment. All ICH patients must be cared for in hospitals that include a neurologist, neurosurgeon, CT, stroke unit, and intensive care units that are available 24hours a day. If the patient does not require mechanical ventilation, care measures should be carried out in the stroke unit,27–30 provided that the patient can be examined by a neurosurgeon and has the option of being transferred to the intensive care unit at any time of day should it become necessary.
General careResuscitationAll patients with ICH must be cared for in hospitals with stroke units and ICUs available 24hours a day. If the patient does not require mechanical ventilation, life support measures should be applied in the stroke unit, provided that the patient can be examined by a neurosurgeon and has the option of being transferred to the intensive care unit at any time of day if necessary. Admission to a general ICU rather than a specialised neurological ICU increases risk of death by a factor of 3.4.31 Likewise, admission to a stroke unit increases probability of survival and a good functional prognosis by 64%.32 Recent population-based studies suggest that good medical care has a significant impact on mortality and morbidity in ICH.33
An initial assessment of the patient will allow us to evaluate the patient's level of consciousness and ability to maintain spontaneous breathing. However, even in patients with a high level of consciousness, it is recommended that doctors know the oxygen saturation level. The simplest means of measuring oxygen saturation is by using a pulse oximeter. If arterial oxygen saturation is less than 92%, the patient will require an oxygen mask with a flow that will raise oxygen saturation to above that threshold. Performing an arterial blood gasometry study is optional and depends on the patient's condition. Up to a third of the patients with supratentorial haemorrhage and almost all patients with a posterior fossa haemorrhage present decreased level of consciousness or bulbar muscle dysfunction that results in the need for intubation.34 Early intubation in patients who have suffered from very large haemorrhages accompanied by decreased level of consciousness may help prevent aspiration pneumonia. In general, endotracheal intubation and gastric aspiration are indicated in patients with a score of less than 8 on the Glasgow coma scale (GCS). Intubation should be performed after administration of drugs that suppress the tracheal reflex, since that reflex may cause increased intracranial pressure and exacerbate the neurological lesion. In any case, the indication for orotracheal intubation is debatable. There may be reason to consider it only if doctors plan to apply other treatment measures in order to improve the patient's neurological situation.
Neurological monitoringSince a high number of patients experience a decline in the hours following the stroke, periodic monitoring of the level of consciousness and the neurological deficit should be performed during at least the first 72hours. The most widely recommended scales are the NIH stroke scale (NIHSS) for measuring neurological deficit35 and the GCS for measuring level of consciousness due to its simplicity and reliability.36
Control over arterial pressureIn most patients with an intracerebral haemorrhage, arterial pressure readings are elevated during the acute phase. In fact, values are often higher than those observed in cases of ischaemic stroke.37 Although blood pressure generally decreases spontaneously in the days following the haemorrhage, high readings persist in many patients. Potential pathophysiological mechanisms that promote increases in arterial pressure include activation of the neuroendocrine system (sympathetic, renin-angiotensin, or glucocorticoid systems) due to stress and the elevation of intracranial pressure (Cushing effect).
High arterial pressure values in patients with ICH may be associated with increased haemorrhage growth,38 which is another sign of poor patient prognosis. Arterial pressure readings in cases of ischaemic stroke follow a U-shaped curve, and both high and low values increase the risk of neurological damage, mortality, and poor functional prognosis.39 Animal models of ICH have described secondary damage, possibly caused by mechanical compression of microvessels, which induces an ischaemic area around the haemorrhage.40 This leads us to think that decreased arterial pressure could contribute to reduced blood flow in the peri-haemorrhagic region, thereby producing more pronounced neurological impairment. Based on these data, we recommend maintaining systolic blood pressure below 180mmHg during the acute phase of ICH. However, neuroimaging studies have been unable to identify ischaemia around the haemorrhage site in human clinical data,41,42 and this aspect remains controversial.
The INTERACT study43 provides new data regarding blood pressure management during the acute phase of ICH. This study was designed in order to evaluate how stricter control over arterial pressure would affect haemorrhage growth and the development of peri-lesional oedema. To that end, the study included 404 patients with spontaneous ICHs that had appeared in the preceding 6hours. Their systolic blood pressure values were ≥150mmHg and ≤220mmHg. Patients were randomly assigned to receive blood pressure treatment either according to recommendations in international guidelines or according to stricter standards. In patients whose blood pressure was controlled according to international guidelines, systolic pressure was kept below 180mmHg. The objective in the group of patients under stricter standards was to reach a systolic blood pressure of 140mmHg during the first hour and maintain levels below that threshold during the following 7 days. Results from the study show that patients assigned to the group with stricter blood pressure control presented less haemorrhage growth and a tendency for the peri-haemorrhagic oedema to decrease. There were no signs of increased neurological decline or poorer functional prognosis, but the study was not designed to evaluate these parameters. Data from the study seem to indicate that strict control over blood pressure is safe. However, we have yet to determine the most appropriate level of arterial pressure, the correct duration for antihypertensive treatment, and the effect of these parameters on the functional prognosis of ICH patients. The study INTERACT 2 is currently underway44 and its main objective is to evaluate how maintaining strict control over blood pressure during the acute phase affects functional prognosis in ICH patients.
The drugs recommended for blood pressure control are those that do not induce cerebral vasodilation or sudden hypotension, such as intravenous labetalol (loading doses of 10 to 20mg in 1 to 2minutes, repeated every 1 to 20minutes until the blood pressure reaches the desired level or the maximum dose of 200mg has been given); intravenous enalapril (1mg bolus); or intravenous urapidil (25mg bolus in 20s, repeating procedure after 5minutes in absence of a response).
Glycaemic controlHigh glycaemic levels upon admission are associated with increased risk of mortality and poor prognosis in patients with intracerebral haemorrhage.45,46 One clinical trial in critical care patients with or without acute stroke shows that maintaining glucose levels between 80 and 110mg/dL using intravenous insulin has been associated with higher incidence rates of hypoglycaemia episodes, whether systemic or cerebral. It may also be linked to an even higher risk of mortality among the patients receiving this treatment.47,48
There are no intervention studies designed specifically for ICH, and as a result, the target level for glycaemic control in ICH patients is not completely clear. However, glucose levels above 155mg/dL in ischaemic stroke have been associated with poor prognosis,49 and it is therefore appropriate to correct levels above this threshold. Hypoglycaemia must be prevented by administering a 10% to 20% dextrose solution.
Temperature controlFever owing to any cause is associated with neurological impairment and poor prognosis.50 Although there is no evidence that treatment decreases this risk, symptomatic treatment with antipyretic drugs such as paracetamol is recommended. For patients with fever, we recommend ordering a chest radiography, cultures of blood, sputum, and urine, and urine sediment analysis in order to identify and treat associated infectious processes. Peripheral blood vessels should be systematically checked to rule out phlebitis.
Some recent studies have demonstrated benefits of moderate hypothermia in certain conditions including head trauma. However, its effects have not been explored in cases of patients with ICH.
Managing haemostasisHaemostatic alterations, such as treatment with oral anticoagulants, coagulation factor deficiencies, or platelet abnormalities, can contribute to haemorrhage growth, which in turn leads to neurological impairment. It is therefore important to correct these factors as quickly as possible.
In cases in which the patient is being treated with oral anticoagulants, the INR must be corrected to reach normal values as soon as possible.51 This should be done using intravenous vitamin K and/or fresh frozen plasma and/or prothrombin complex concentrate.52,53 The effectiveness of fresh frozen plasma is limited by the risk of allergic reactions and infections, as well as by the considerations of processing time and hypervolaemia. Prothrombin complex concentrates also contain factors II, VII, IX, and X, and they are able to normalise INR values quickly. On this basis, they constitute the treatment of choice for cases of ICH related to oral anticoagulants.54 However, they must be combined with vitamin K, since the half-life of oral anticoagulants is much longer than those of vitamin K-dependent clotting factors. In cases in which patients have received intravenous heparin and display prolonged APTT, doctors should administer protamine sulphate. If ICH has occurred due to fibrinolytic treatment, it may be necessary to administer fresh frozen plasma, platelets, or antifibrinolytics such as aminocaproic acid or tranexamic acid. Recombinant activated factor VII should be administered to patients with both ICH and haemophilia.
Recombinant activated factor VII has also been investigated in ICH patients without disorders of haemostasis. A phase 2 study has shown that recombinant activated factor VII limits haemorrhage growth and improves patients’ functional prognosis compared to a placebo, despite increasing the frequency of thromboembolic complications.55 A phase 3 study confirmed that delivering recombinant activated factor VII limits haemorrhage growth, but no significant differences in prognosis could be found with respect to a placebo.56 It has not yet been shown whether recombinant activated factor VII can deliver benefits in selected patients, but in any case, administering this treatment to all ICH patients indiscriminately does not improve their prognosis and furthermore, it increases the risk of thromboembolic complications.
Patients with ICH and thrombocytopenia must receive transfusions of platelet concentrate. Data from patients without thrombocytopenia who are being treated with antiplatelet drugs are contradictory. Platelet dysfunction has been linked to increased haemorrhage volume and a poor functional result57; however, one clinical trial investigating neuroprotection in cerebral haemorrhages58 found no differences between patients receiving a placebo and those previously treated with antiplatelet drugs. Therefore, platelet replacement therapy is not indicated for patients taking antiplatelet drugs and those whose platelet count is normal.
Preventing complicationsDeep vein thrombosis and pulmonary embolismPatients with ICH are at an increased risk of suffering thromboembolic complications.59 Sporadic use of compression stockings has not been shown to be effective for preventing deep vein thrombosis.60 However, the combination of intermittent mechanical compression and compression stockings is much more effective.61 The use of low molecular weight heparin beginning on day 1 after a cerebral haemorrhage decreases the risk of thromboembolic complications in ICH patients and does not increase the risk of bleeding.62
SeizuresThe presence of seizures increases the brain's metabolic demand and exacerbates neurological damage in patients with ICH. If seizures appear, they should initially be treated with benzodiazepine, followed by antiepileptic drugs. However, administering antiepileptic drugs to ICH patients who have not experienced a seizure is associated with increased morbidity and mortality, especially in the case of phenytoin.63,64 Prophylactic treatment for seizures is not recommended.
Managing intracranial pressureControl over intracranial pressure (ICP) is one of the specific treatment objectives in ICH, and the approach should be directed at the underlying cause. The most common causes of high ICP are hydrocephalus due to intraventricular haemorrhage and the mass effect of the haemorrhage.
Placing devices that measure ICP increases the risk of haemorrhage and infection, and such devices should not be used as routine treatment. However, there are also non-invasive techniques that allow us to estimate intracranial pressure in patients with ICH, such as the transcranial Doppler test. An increase in the pulsatility index in the middle cerebral artery of the unaffected hemisphere indicates intracranial hypertension, and this has been shown to predict mortality.65
Data on managing ICP in ICH are limited; recommendations have been extrapolated from those used in the management of patients with head trauma.66 Doctors recommend considering ICP treatment and management in patients with ICH with a Glasgow scale score ≤8, clinical evidence of transtentorial herniation, significant intraventricular haemorrhage, or hydrocephalus. Nevertheless, we must be aware that very few studies attempt to show the utility of ICP monitoring in ICH patients. Most such studies were unable to discriminate between patients who might be candidates for surgical evacuation of the haemorrhage and candidates for medical treatment only.
Centring the head and raising the headboard to an angle of 20° to 30° improves venous return and may decrease PIC slightly. Hyperventilation decreases partial pressure of oxygen in arterial blood, which leads to cerebral vasoconstriction and a lowered ICP. The target is to reach partial pressure of CO2 between 28 and 35mmHg and subsequently maintain pressure between 25 and 30mmHg if the ICP remains high. This results in rapid decrease of ICP, although the effect is temporary and other measures will have to be taken in order for ICP to remain under control. Conditions that can cause increased ICP must be avoided, including fever, Valsalva-like manoeuvres (coughing or vomiting), seizures, stress, pain, AHT, and hyponatraemia. Osmotherapy reduces ICP by increasing osmolarity in plasma, which in turn displaces water from healthy brain tissue into the vascular compartment. The most commonly employed drugs of this type are mannitol and loop diuretics such as furosemide. Recommendations for dosing 20% mannitol range from 0.7 to 1g/kg (250mL) followed by 0.3 to 0.5g/kg (125mL) every 3 to 8hours. Treatment should not be extended beyond 5 days so as to avoid the rebound effect. Furosemide (10mg every 2–8hours) may be used simultaneously to maintain the osmotic gradient. Using corticosteroids for this purpose is not effective and may even increase the number of complications.67 Sedation with intravenous drugs, such as benzodiazepines, barbiturates, narcotics, and butyrophenones, reduces brain metabolism and decreases cerebral blood flow and ICP. In contrast, sedation also gives rise to numerous complications which include arterial hypotension and respiratory infections.
Hydrocephalus caused by the presence of an intraventricular bleed is one of the factors associated with a poor prognosis and increased mortality.68,69 Ventriculostomy must be considered in cases in which hydrocephalus and a decreased level of consciousness are both present. A randomised study named CLEAR III, currently underway, is evaluating the efficacy and safety of intraventricular infusion of thrombolytic drugs in patients with intraparenchymal haemorrhage and ventricular invasion.
ICH prognosisMany different studies have turned up factors that may be related to patient prognosis. These variables include age, scores on the GCS and NIHSS scales, haemorrhage volume and location, and the presence of intraventricular haemorrhage.70 Data which may reflect a poor prognosis may be interpreted as a reason for limiting care. This decision affects mortality, and early mortality in particular.71–73 Current evidence suggests that establishing a sure prognosis is impossible. We therefore do not recommend deciding to limit care in early stages.
- 1.
If arterial oxygen saturation is less than 92%, the patient will require an oxygen mask with a flow sufficient to maintain oxygen saturation above that threshold.
- 2.
Early intubation is recommended in patients with a massive ICH and low level of consciousness (GCS<8) if the patient's prior functional state is good, but not if all brainstem signs have disappeared (level of evidence 5, grade C recommendation).
- 1.
Level of consciousness and neurological deficit must be evaluated periodically during at least the first 72hours after the stroke. Neurological impairment should be measured using the NIHSS scale; level of consciousness is monitored using the Glasgow coma scale (level of evidence 5, grade C recommendation).
- 1.
The current recommendation, as we await results from new clinical trials, is to treat patients whose systolic blood pressure exceeds 180mmHg (level of evidence 2b, grade C recommendation).
- 2.
Rapid reduction of systolic blood pressure to the limit of 140mmHg is safe in patients whose systolic blood pressure readings fall between 150 and 220mmHg (level of evidence 2a, grade B recommendation).
- 1.
Blood glucose levels must be checked regularly and hyperglycaemia above 155mg/dL is to be avoided (level of evidence 2c, grade C recommendation). If the glucose level exceeds that threshold, it should be corrected with insulin. Glucose levels below 70mg/dL must be corrected with 10% to 20% dextrose (level of evidence 5, grade C recommendation).
- 1.
Doctors recommend treating hyperthermia above 37.5°C with intravenous paracetamol (level of evidence 5, grade C recommendation).
- 1.
Patients with coagulation factor deficiency or severe thrombocytopenia should be treated with the lacking coagulation factors or platelets, respectively (level of evidence 1, grade B recommendation).
- 2.
Patients on anticoagulant treatment with ICH and elevated INR should receive intravenous prothrombin complex concentrate and vitamin K, plus fresh plasma if necessary, to replace vitamin K-dependent factors until INR level is normalised (level of evidence 1, grade B recommendation).
- 3.
Patients who have undergone intravenous heparin treatment and have a prolonged APTT should receive treatment with protamine sulphate (level of evidence 5, grade C recommendation).
- 4.
Patients with ICH who have undergone thrombolytic treatment should receive a transfusion of fresh plasma and platelets or antifibrinolytic drugs such as aminocaproic acid or tranexamic acid (level of evidence 5, grade C recommendation).
- 1.
A combination of intermittent mechanical compression and compression stockings should be used to prevent deep vein thrombosis (level of evidence 1, recommendation level B). Beginning on day 1, it is possible to administer prophylactic treatment with low molecular weight heparin (level of evidence 2b, grade B recommendation).
- 1.
If seizures appear, the patient will require antiepileptic drugs (level of evidence 1, grade A recommendation).
- 2.
Prophylactic treatment with antiepileptic drugs is not indicated (level of evidence 3, grade B recommendation).
- 1.
ICP must be monitored in patients with a GCS≤8 and signs of transtentorial herniation or hydrocephalus (level of evidence 2b, grade C recommendation).
- 2.
Placing a ventricular shunt should be considered for patients with hydrocephalus (level of evidence 2a, grade B recommendation).
- 3.
Although osmotic diuretics are recommended as the first treatment option, they are not indicated for prophylactic use (level of evidence 5, grade C recommendation).
- 4.
Doctors recommend hyperventilation in cases that do not respond to treatment with osmotic diuretics, provided that the patient has a good functional prognosis (level of evidence 5, grade C recommendation).
- 5.
Corticosteroids are not recommended as treatment for primary ICH (level of evidence 2, grade B recommendation).
The question of whether or not an ICH patient should be treated surgically is controversial. While surgery may reduce the effects stemming from the mechanical compression exerted by the haemorrhage and also decrease the toxic effect of blood on nearby brain tissue, surgical risks may be high. In most patients, the benefits of surgery do not outweigh the procedure's potential for harm.
One important factor in the decision of whether to treat ICH surgically is the haemorrhage location. Cerebellar haemorrhages larger than 3cm in diameter, those compressing the brainstem, or those with hydrocephalus respond better to surgical treatment than to medical treatment.74,75 In these cases, placing a ventricular shunt without evacuating the haemorrhage is insufficient, and shunt placement with no additional actions is not recommended. In contrast, surgery is not indicated for cerebellar haemorrhages that measure less than 3cm and do not compress the brainstem or involve hydrocephalus.
The clinical trial STICH observed that patients with a lobar haemorrhage located less than 1cm from the cerebral cortex tended to benefit from surgical treatment, but the tendency was not statistically significant.76 They also discovered a non-statistically significant tendency toward benefiting from surgery in patients with lobar haemorrhage and GCS scores between 9 and 12. However, further clinical trials will be needed to demonstrate this benefit. In cases of haemorrhages located more than 1cm from the cerebral cortex and a GCS score ≤8, prognosis is poorer in patients who undergo surgical treatment.77
Related studies on haemorrhages located in basal ganglia do not show better results with surgical treatment. We must also be mindful of the fact that gaining access to the haemorrhage will involve passing through healthy brain tissue, meaning that the procedure will produce more severe sequelae.77,78
Recommended surgical treatment techniques include performing a craniectomy with decompression and evacuation of the haemorrhage, but attempts have been made at developing less invasive techniques. Certain projects have studied the benefits of stereotactic surgery combined with local thrombolysis78,80 or endoscopic aspiration.81,82 These techniques eliminate the haemorrhage more fully and decrease mortality when they are performed in the first 72hours. Nevertheless, they have not been shown to improve patients’ functional prognosis. One clinical trial compared surgery using minimally invasive craniopuncture with medical treatment in cases of small-volume haemorrhages in the basal ganglia. The report observes that the technique is safe and may improve functional prognosis in patients with this type of haemorrhage.79
The optimal moment in which to surgically evacuate the haemorrhage is also a matter of debate. Studies of surgical procedures performed within 24, 48, 72, or 96hours of the haemorrhage have found no differences in outcome except with regard to patients treated with minimally invasive techniques, as indicated above.
- 1.
Surgical treatment is recommended as soon as possible for patients with cerebellar haemorrhages who present with neurological impairment, brainstem compression, or hydrocephalus (level of evidence 1, grade B recommendation).
- 2.
In patients with neurological impairment and a lobar haemorrhage exceeding 30mL in volume and located less than 1cm from the cerebral cortex, surgical treatment should also be considered (level of evidence 2b, grade B recommendation).
- 3.
Evacuation procedures are not recommended for deep haemorrhages (level of evidence 2, grade B recommendation). Although minimally invasive surgery may be an alternative in the future, data are not sufficient to recommend stereotactic surgery to evacuate haemorrhages at the present time (level of evidence 2, grade B recommendation).
The risk of recurrence after a first ICH is between 2.1% and 3.7% yearly.83,84 In addition, lobar haemorrhages related to amyloid angiopathy,85 haemorrhages secondary to anticoagulant treatment,84 history of prior cerebral haemorrhage,86 advanced age,84 and microbleeds detected by gradient echo MRI87 increase the risk of recurrence.
AHT is the modifiable factor with the most influence on risk of ICH recurrence, which is why proper blood pressure control is so important. Good control over blood pressure lowers risk of ICH recurrence, whether for hypertensive haemorrhages or for bleeds secondary to amyloid angiopathy.88 Although the optimal blood pressure value for reducing risk of ICH recurrence is unknown, maintaining normal blood pressure values (below 120/80mmHg) seems to be a reasonable choice.89
Oral anticoagulants increase risk of ICH recurrence,84 and the benefits of anticoagulation to prevent thromboembolic events must therefore be weighed against the risk of future ICHs. Risk of recurrence is higher in lobar haemorrhages, which is why anticoagulant treatment should be suspended definitively in patients with atrial fibrillation.90 In cases of deep haemorrhages, risk of recurrence is lower. Generally speaking, doctors should consider suspending anticoagulants during the acute phase except in patients at high risk for thromboembolic events (for example, those fitted with mechanical valves) and at low risk for a haemorrhage.90 When thromboembolic risk is high (CHA2DS2-VASc score ≥2), doctors recommend recommencing oral anticoagulants 7 to 10 days after the stroke.91 Antiplatelet drugs have a less pronounced effect on haemorrhage risk and severity than oral anticoagulants do.92 They may therefore constitute a treatment alternative in patients who have a moderate level of risk (CHA2DS2-VASc ≤1) or who are functionally dependent (modified Rankin scale 4–5).91
In haemorrhages secondary to an underlying lesion, specific treatment decreases risk of recurrence. For example, surgery may be recommended for cavernous angiomas that are surgically accessible and have a bleed rate of 0.7% per year per lesion,93 depending on the risk of a new haemorrhage. A better approach for deep lesions is close monitoring; surgery should be reserved for cases in which impairment is progressive or bleeding is recurrent. Risk of rebleeding in AVMs is high at 18% the first year94 and 2% per year in later years.95 Treatment that excludes the AVM from the circulatory system is recommended where possible. In this case, alternatives include surgical treatment, endovascular therapy, and radiosurgery. Surgical treatment of ICH depends on the location. Haemorrhages located in the basal ganglia, diencephalon, or brainstem are typically inoperable. Endovascular treatment was initially developed to facilitate resection of very large AVMs, or as an alternative to high-risk surgery.96 However, when lesions are small, complete occlusion may be achieved with endovascular therapy. Radiosurgery is more effective in small AVMs (<3cm)97 and may also be used for AVMs that cannot be reached with any other technique. In ICH secondary to neoplasia, surgical treatment is generally used to excise the underlying tumour. Nevertheless, treatment depends on the patient's functional condition and the tumour type and location.
- 1.
Maintaining blood pressure values below 120/80mmHg is recommended for all patients with ICH (level of evidence 2a, grade B recommendation).
- 2.
Anticoagulants should not be administered following a lobar ICH in cases with non-valvular atrial fibrillation (level of evidence 2a, grade B recommendation). Antiplatelet drugs may be administered to these patients as an alternative to anticoagulants (level of evidence 2, grade B recommendation).
- 3.
In cases of accessible cavernous angiomas, doctors should evaluate surgical treatment according to the risk of bleeding (level of evidence 5, grade D recommendation). Monitoring is recommended for haemorrhages at deep locations; surgery should be considered in cases of rebleeding or increasing neurological deficit (level of evidence 5, grade D recommendation).
- 4.
Recommended treatments for AVMs may be surgical, endovascular, and/or radiosurgical depending on the surgical risk and the size and location of the lesion (level of evidence 5, grade D recommendation).
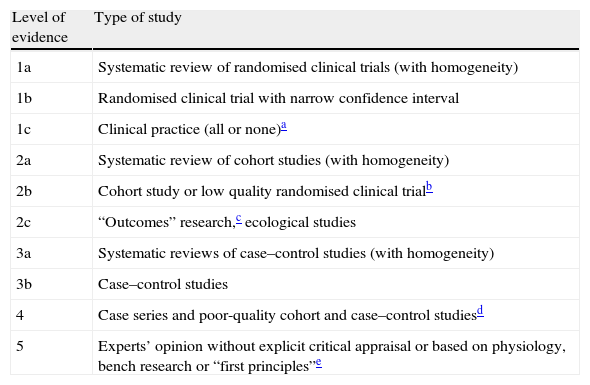
These clinical guidelines are rewritten periodically because they must reflect a continuous series of advances in clinical trials. They therefore draw from previous SEN recommendations, as well as from current recommendations by the European Stroke Initiative98 and the American Heart Association Stroke Council.99 These recommendations were taken into account in the process of elaborating the guidelines we present here. Likewise, in order to prepare guidelines according to the standard set by international publications, we used the levels of evidence and grades of recommendation published by the Centre for Evidence-Based Medicine at the University of Oxford (Addenda 2 and 3).100
Conflict of interestThe authors have no conflicts of interest to declare.
Coordinator: Exuperio Díez-Tejedor, Hospital Universitario La Paz, Madrid.
Exuperio Díez-Tejedor (Coord), Hospital Universitario La Paz, Madrid; Blanca Fuentes (Secretary), Hospital Universitario La Paz, Madrid; María Alonso de Leciñana, Hospital Universitario Ramón y Cajal, Madrid; José Álvarez-Sabin, Hospital Universitari Vall d’Hebron, Barcelona; Juan Arenillas, Hospital Universitario Clínico de Valladolid; Sergio Calleja, Hospital Universitario Central de Asturias, Oviedo; Ignacio Casado, Hospital San Pedro, Cáceres; Mar Castellanos, Hospital Josep Trueta, Girona; José Castillo, Hospital Clínico Universitario, Santiago de Compostela; Antonio Dávalos, Hospital Universitari Germans Trias i Pujol, Badalona; Fernando Díaz-Otero, Hospital Universitario Gregorio Marañón, Madrid; Exuperio Díez-Tejedor, Hospital Universitario La Paz, Madrid; José Antonio Egido, Hospital Clínico Universitario San Carlos, Madrid; Juan Carlos Fernández, Hospital Universitario Dr. Negrín, Las Palmas; Mar Freijo, Hospital Universitario de Basurto, Bilbao; Blanca Fuentes, Hospital Universitario La Paz, Madrid; Jaime Gállego, Hospital General de Navarra, Pamplona; Andrés García Pastor, Hospital Universitario Gregorio Marañón, Madrid; Antonio Gil-Núñez, Hospital Universitario Gregorio Marañón, Madrid; Francisco Gilo, Hospital Universitario La Princesa, Madrid; Pablo Irimia, Clínica Universitaria de Navarra, Pamplona; Aida Lago, Hospital Universitario La Fe, Valencia; José Maestre, Hospital Universitario Virgen de las Nieves, Granada; Jaime Masjuan, Hospital Universitario Ramón y Cajal, Madrid; Joan Martí-Fábregas, Hospital de la Santa Creu i Sant Pau, Barcelona; Patricia Martínez-Sánchez, Hospital Universitario La Paz, Madrid; Eduardo Martínez-Vila, Clínica Universitaria de Navarra, Pamplona; Carlos Molina, Hospital Universitario Vall d’Hebron, Barcelona; Ana Morales, Hospital Universitario Virgen de la Arrixaca, Murcia; Florentino Nombela, Hospital Universitario La Princesa, Madrid; Francisco Purroy, Hospital Universitario Arnau de Vilanova, Lérida; Marc Ribó, Hospital Universitari Vall d’Hebron, Barcelona; Manuel Rodríguez-Yáñez, Hospital Clínico Universitario, Santiago de Compostela; Jaime Roquer, Hospital del Mar, Barcelona; Francisco Rubio, Hospital Universitario de Bellvitge, Barcelona; Tomás Segura, Hospital Universitario de Albacete, Albacete; Joaquín Serena, Hospital Josep Trueta, Gerona; Patricia Simal, Hospital Clínico Universitario San Carlos, Madrid; Javier Tejada, Hospital Universitario de León, León; José Vivancos, Hospital Universitario La Princesa, Madrid.
José Álvarez-Sabín, Hospital Universitari Vall d’Hebron, Barcelona; José Castillo, Hospital Clínico Universitario, Santiago de Compostela; Exuperio Díez-Tejedor, Hospital Universitario La Paz, Madrid; Antonio Gil-Núñez, Hospital Universitario Gregorio Marañón, Madrid; José Larracoechea, Hospital de Cruces, Bilbao; Eduardo Martínez-Vila, Clínica Universitaria de Navarra, Pamplona; Jaime Masjuan, Hospital Universitario Ramón y Cajal, Madrid; Jorge Matías-Guiu, Hospital Clínico Universitario San Carlos, Madrid; Francisco Rubio, Hospital de Bellvitge, Barcelona.
| Level of evidence | Type of study |
| 1a | Systematic review of randomised clinical trials (with homogeneity) |
| 1b | Randomised clinical trial with narrow confidence interval |
| 1c | Clinical practice (all or none)a |
| 2a | Systematic review of cohort studies (with homogeneity) |
| 2b | Cohort study or low quality randomised clinical trialb |
| 2c | “Outcomes” research,c ecological studies |
| 3a | Systematic reviews of case–control studies (with homogeneity) |
| 3b | Case–control studies |
| 4 | Case series and poor-quality cohort and case–control studiesd |
| 5 | Experts’ opinion without explicit critical appraisal or based on physiology, bench research or “first principles”e |
When all patients died before a certain treatment became available, and some who received the treatment survived; or if some patients died before the treatment existed, and none of those receiving the treatment died.
The term ‘outcomes research’ refers to cohort studies in patients with the same diagnosis in which the events that occur are related to treatments delivered to the patients.
Cohort study: no clear definition of the groups being compared and/or no objective measurement of treatments and events (preferably blinded) and/or without properly identifying or controlling for known confounders and/or complete and sufficient follow-up period. Case–control study: no clear definition of the groups being compared and/or no objective measurement of treatments and events (preferably blinded) and/or without properly identifying or controlling for known confounders.