Cerebellar infarction (CI) is uncommon, but may result in severe complications. The aim of our study was to determine the characteristics of patients with CI, as well as their outcomes as regards the territories affected.
Patients and methodsData were collected from 124 patients admitted to our department during a five-year period, with a radiological diagnosis of CI, and with or without involvement of other brain territories.
ResultsThe mean age in our series was 65.2 years, with most being male (68.5%). The posterior inferior cerebellar artery (PICA) was the most commonly affected territory at 49.2%, followed by superior cerebellar artery (SCA) at 17.7%, and anterior inferior cerebellar artery (AICA) at 10.5%. There was simultaneous supratentorial involvement in 13.7%, and two or three cerebellar arteries involved in 8.9%. The main aetiology in PICA was atherothrombosis (P=.02). On the other hand, cardio-embolism was the main origin in cases with more than one affected territory (P=.04). No particular aetiology could be found in SCA and AICA. There was haemorrhagic transformation in 29 patients (23.4%), particularly in the PICA and when other territories were involved. There was hydrocephalus in 15 patients (12.1%, 12 of them PICA; P=.02) in 2.9±1.5 days from stroke onset. At discharge, the degree of disability was worse if more than one arterial territory was involved (Rankin≥3, 64% versus 31–36%; P=.05). Four (3.2%) patients died.
ConclusionsCI is very heterogeneous. Nevertheless, it is noteworthy that PICA infarction is the most frequent type and its aetiology is usually atherothrombotic. Moreover, it is the territory most frequently associated with severe complications, which take place during the first week of the stroke.
Los infartos cerebelosos (IC) son infrecuentes pero pueden presentar complicaciones graves. Nuestro objetivo ha sido estudiar las características de los pacientes con IC, así como su evolución, en función del territorio afectado.
Pacientes y métodosSe han recogido datos de 124 pacientes ingresados en nuestro servicio durante un periodo de 5 años, con diagnóstico radiológico de IC, con y sin afectación de otras regiones cerebrales.
ResultadosLa edad media de nuestra serie es de 65,2 años, con predominio masculino (68,5%). El territorio más afectado fue la arteria cerebelosa posteroinferior (PICA) en el 49,2%, seguido de la arteria cerebelosa superior (ACS) en el 17,7% y cerebelosa anteroinferior (AICA) en el 10,5%. Se afectaron territorios supra-infratentoriales en el 13,7% y dos/tres territorios cerebelosos en el 8,9%. La etiología aterotrombótica fue más prevalente en PICA (p=0,02) y la cardioembólica en la afectación de múltiples territorios (p=0,04), siendo similares en ACS y AICA. Se produjo transformación hemorrágica en 29 pacientes (23,4%), sobretodo en la afectación de múltiples territorios y en PICA. Se asoció hidrocefalia en 15 pacientes (12,1%, 12 de ellos PICA; p=0,02), apareciendo de media a los 2,9±1,5 días del inicio del ictus. Al alta, la dependencia funcional (Rankin≥3) era mayor si la afectación territorial era múltiple (64% vs 31-36%; p=0,05). Se contabilizaron 4 defunciones (3,2%).
ConclusionesLos IC tienen gran heterogeneidad. Sin embargo, cabe destacar que los infartos de PICA son los más prevalentes, su etiología suele ser aterotrombótica y son los más asociados a complicaciones graves, que ocurren durante la primera semana del ictus.
Cerebellar infarcts account for between 1.5% and 3% of all ischaemic strokes.1–3 Their clinical manifestations are very diverse and often unspecific, meaning that this entity is often mistaken for other more benign conditions.2,3
At present, thanks to MRI brain scans, we can identify the affected territory more accurately, and detect other associated lesions. The mean age at which cerebellar infarcts appear is approximately 65 years. Two thirds of patients with this condition are male.3
The literature contains numerous studies of cerebellar infarcts in specific territories,5–12 and others written from a more global point of view.1,3,4,13–17
Our objective is to analyse the epidemiology, aetiological factors, complications, and functional condition in patients with cerebellar infarct at time of discharge. We will also examine the relationship between those factors and the affected vascular territory.
Patients and methodsWe performed a retrospective analysis of the 2480 patients admitted to our neurology department with a diagnosis of ischaemic stroke during a 5-year period (March 2005 to March 2010). Within this patient group, we selected and analysed the 124 patients (5%) with a radiological diagnosis of acute cerebellar infarct, with or without involvement of other vascular territories of the central nervous system. Radiological diagnosis was performed using computed tomography (CT) and/or brain MRI during hospitalisation.
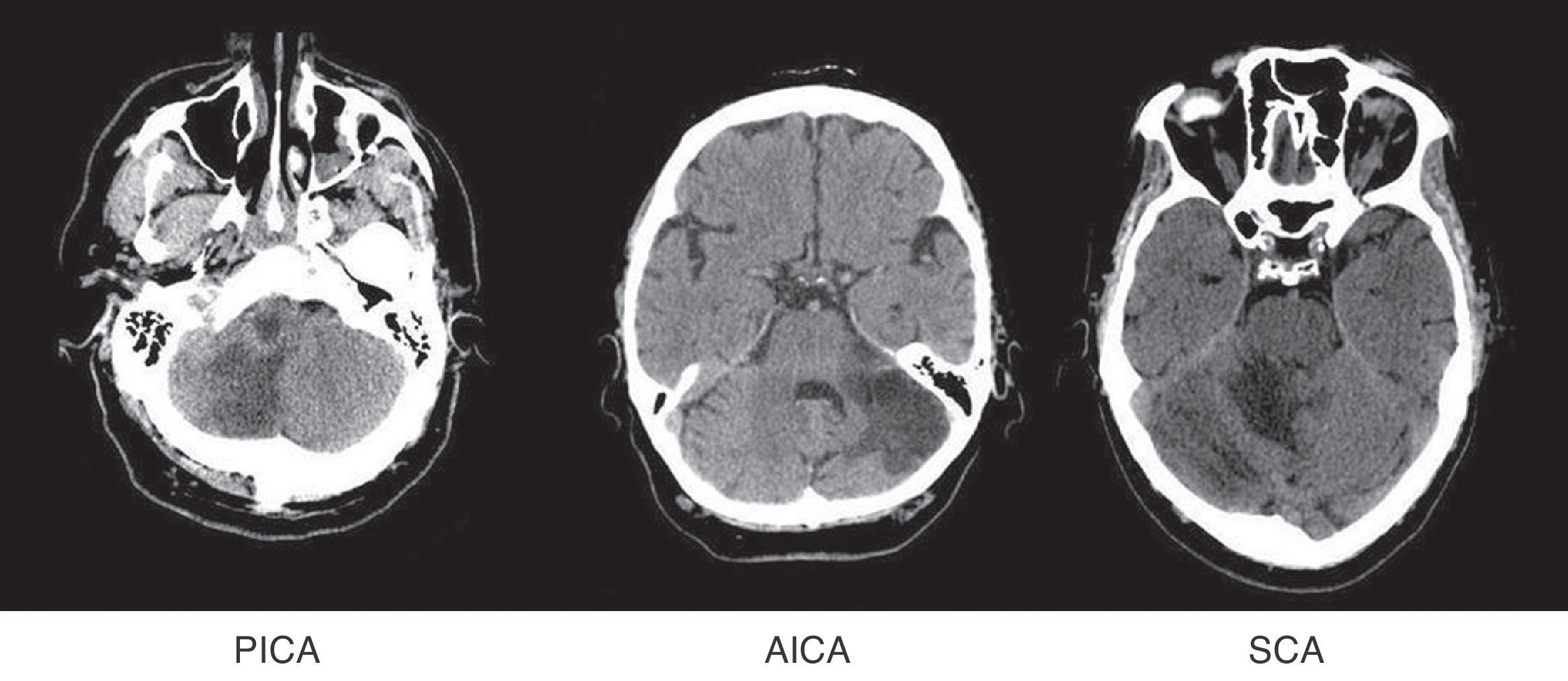
Patients were grouped by affected territory using Amarenco's Diagrams14: superior cerebellar artery (SCA), anterior inferior cerebellar artery (AICA), posterior inferior cerebellar artery (PICA), more than one affected territory (CA+) and simultaneous affectation of supratentorial territories (ST) (Fig. 1).
Small vessel infarcts and boundary zone infarcts were not considered.
We recorded the following epidemiological variables from each patient: sex, age, presence of cardiovascular risk factors (smoking, high blood pressure, diabetes mellitus, dyslipidaemia, presence of cardioembolism) and aetiology (TOAST criteria18). We recorded the incidence rate of complications such as hydrocephalus, including the date of appearance or onset of haemorrhagic transformation. Prognosis was also assessed by analysing the baseline status and discharge status using the modified Rankin scale (mRS). Neurological sequelae were measured on the NIHSS scale.
Patients with a baseline mRS of 4 or 5 were excluded because their functional state limited their ability to be evaluated and treated.
Aetiology was determined by means of a vascular study (echo Doppler study of the supra-aortic trunks and CT angiography or MR angiography of the circle of Willis) and transthoracic echocardiogram. Patients were telemetrically monitored during their stay in a stroke unit. Where necessary, the study was expanded to include transoesophageal echocardiogram and transcranial Doppler ultrasound (TCD) with saline contrast to assess right-to-left shunt.
We checked for hydrocephalus and/or haemorrhagic transformation by performing sequential neuroimaging studies on patients whose clinical state worsened during hospitalisation.
Neurological examinations to calculate mRS and NIHSS scores upon admission and discharge were performed by neurologists in our stroke unit.
These variables were studied using StatView statistical software, which performed univariate and multivariate analysis with a significance level of α=0.05.
ResultsWe collected data from 124 consecutive patients with a male/female ratio of 2.2:1. Mean age at time of diagnosis was 65.25±14.9 years, with no significant differences between the sexes.
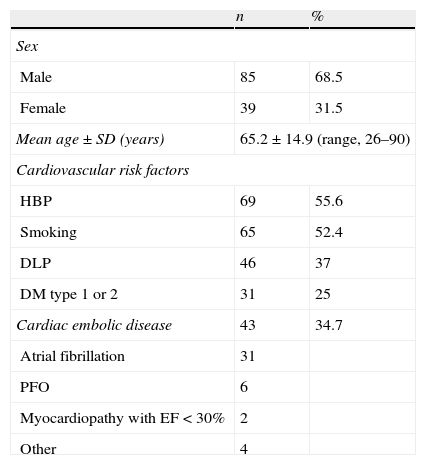
Regarding cardiovascular risk factors, slightly more than half of the patients had a history of high blood pressure and tobacco use. Approximately one third of the cases had dyslipidaemia and one fourth had type 1 or 2 diabetes. Cardioembolism was observed in 43 patients (34.7%); most had atrial fibrillation, and patent foramen ovale (PFO) was discovered in 6 of them (Table 1).
Demographic data and presence of cardiovascular risk factors/cardioembolism in 124 patients with cerebellar infarct.
| n | % | |
| Sex | ||
| Male | 85 | 68.5 |
| Female | 39 | 31.5 |
| Mean age±SD (years) | 65.2±14.9 (range, 26–90) | |
| Cardiovascular risk factors | ||
| HBP | 69 | 55.6 |
| Smoking | 65 | 52.4 |
| DLP | 46 | 37 |
| DM type 1 or 2 | 31 | 25 |
| Cardiac embolic disease | 43 | 34.7 |
| Atrial fibrillation | 31 | |
| PFO | 6 | |
| Myocardiopathy with EF<30% | 2 | |
| Other | 4 | |
n: number of patients; PFO: patent foramen ovale; EF: ejection fraction.
The most frequent initial clinical manifestations were feeling of instability and/or changes in gait (71%), vomiting (36.3%), dysarthria (33.9%), and headache (25%). To a lesser extent, in order of frequency, patients also presented cranial nerve impairment, motor or sensory deficits, altered level of consciousness, and cervical pain.
The mean score on the NIHSS at onset was 3.3. In particular, 96 patients (77.4%) had an NIHSS score ≤3 and only 3 patients had NIHSS>25.
Diagnosis was performed by MR imaging in 93 patients (75%) and by CT in the remaining 31 (25%). Mean time to establish a correct diagnosis was 41hours (range, 1hour to 3 weeks).
The diagnosis was established early enough to permit use of thrombolysis in 27 patients (21.8%). However, intravenous thrombolysis was only performed in 6 patients (5% of the total). Exclusion criteria were as follows: low NIHSS score (≤3), 10 patients; coma, 2; signs of improvement, 2; extensive infarct shown by brain CT, 2; and in 1 case each, severe thrombocytopenia, anticoagulant treatments, acute coronary syndrome, recent major surgery and uncontrolled high blood pressure.
Initial diagnosis was erroneous in 35 patients (28.2%). The condition was first thought to be benign positional vertigo in 20 patients. Other proposed diagnoses included hypertensive crisis, infections (respiratory, urinary, gastrointestinal, unknown origin); cerebral metastasis, encephalopathy, syncope, and vasovagal episode. The remaining 62 patients (50%) were diagnosed correctly, but diagnosis was not performed within the thrombolysis time window.
Once diagnosed, all patients were admitted to the stroke unit.
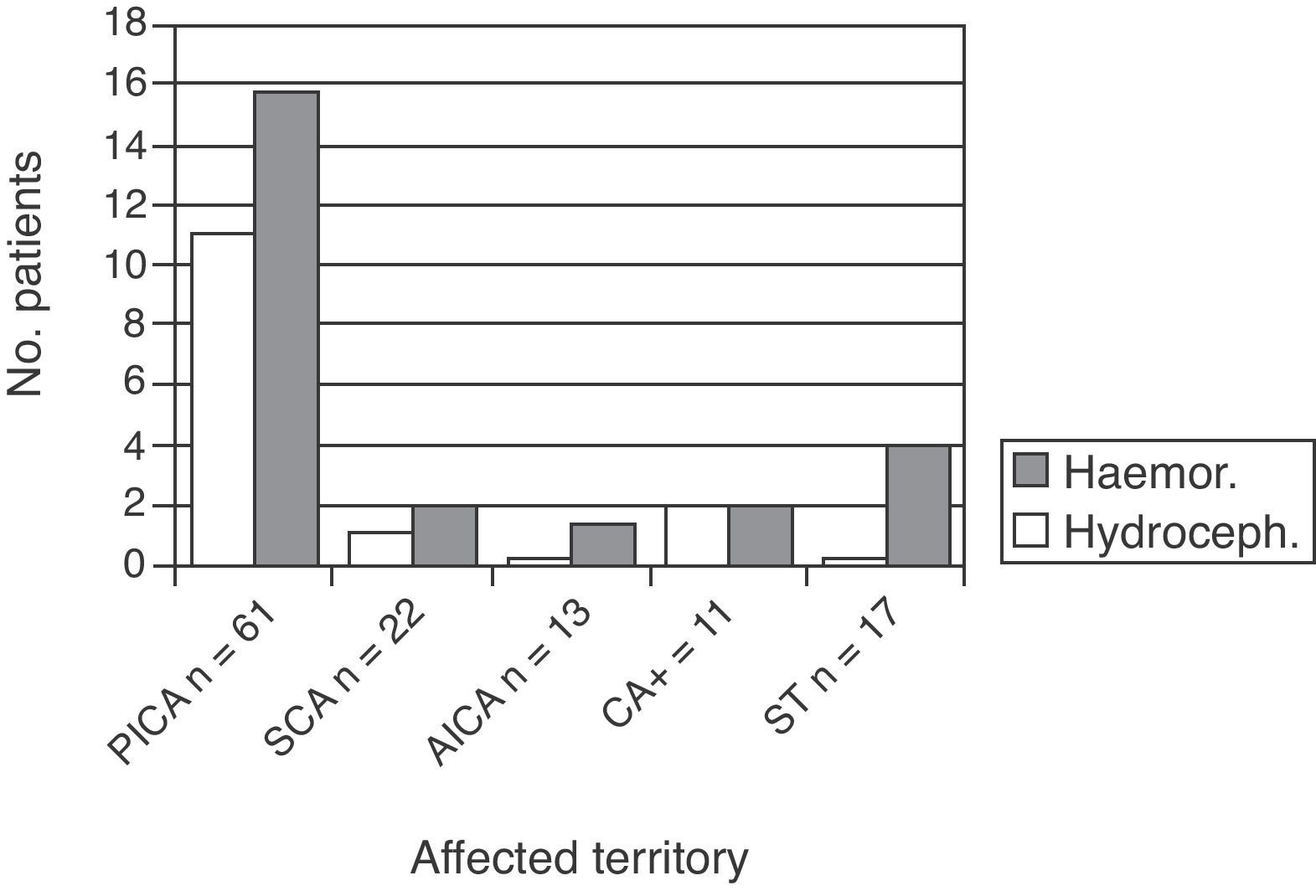
Affected vascular territories in order of prevalence were PICA (61, 49.2%), SCA (22, 17.7%), and AICA (13, 10.5%). Eleven patients were placed in the CA+ group (8.9%) and 17 (13.7%) experienced simultaneous affectation of supratentorial territories.
The brainstem was affected in 25 patients (20.2% of the total). This was isolated in 5 cases (lateral medullary infarct). Most of these events (16) occurred with PICA infarcts. The rest involved the AICA (4), the CA+ group (2), ST (2) and the SCA (1).
Bilateral impairment of the same territory was present in 7 patients (PICA in 4, SCA in 3). Unlike in other studies, the above were not considered a separate group for the purpose of our calculations.17
With regard to aetiology, we found 41 cardioembolic strokes (33%), including patients with PFO and no other causes of stroke; 40 atherothrombotic strokes (32.2%); and 37 of undetermined origin (29.8%). Of the latter, 21 were of unknown aetiology, 12 corresponded to incomplete studies, and 4 had double causes. Lastly, strokes in 6 patients (4.8%) were caused by unusual events –specifically, arterial dissection– in a vertebral artery and/or the PICA.
A vascular study with CT angiography and/or MR angiography was performed in 106 patients (85.5%). Atherothrombotic aetiology was determined in the 40 cases mentioned above based on the following findings: occlusion or stenosis >50% in vertebrobasilar system arteries in 26 individuals and stenosis ≤50% secondary to platelets. No other mechanisms were found in the other 14 cases.
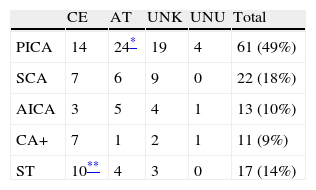
When examining affected territories separately, we find that atherothrombotic aetiology is predominant with PICA infarct (P=.02), and cardioembolic aetiology is predominant with simultaneous impairment of supratentorial territories (P=.04). There are no significant differences in territory for the other aetiologies (Table 2).
Aetiology according to TOAST criteriaa for cerebellar infarcts and distribution by affected territory.
| CE | AT | UNK | UNU | Total | |
| PICA | 14 | 24* | 19 | 4 | 61 (49%) |
| SCA | 7 | 6 | 9 | 0 | 22 (18%) |
| AICA | 3 | 5 | 4 | 1 | 13 (10%) |
| CA+ | 7 | 1 | 2 | 1 | 11 (9%) |
| ST | 10** | 4 | 3 | 0 | 17 (14%) |
CA+: 2 to 3 cerebellar territories affected; AT: atherothrombosis; CE: cardioembolic; UNK: unknown (including multiple causes and incomplete studies); UNU: unusual; ST: simultaneous impairment of supratentorial territories.
Fifteen patients experienced hydrocephalus (12.1% of the total). This complication was identified by brain CT performed due to worsened clinical state. These patients were examined by the neurosurgery department, and all were fitted with a temporary external ventricular drain. This group comprised 12 patients with PICA infarct, 1 with an SCA infarct, and 2 with CA+ (P=.02).
The mean number of days between the vascular event and appearance of hydrocephalus, understood as symptom onset, was 2.9 (range, 1–6). Of the 15 patients with hydrocephalus, 2 died.
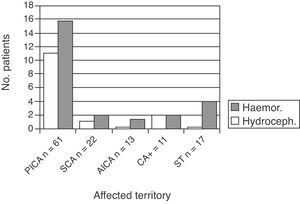
Haemorrhagic transformation also occurred in 29 patients (23.4%). Of the 29, 19 were in the PICA group, 4 in the CA+ group, 1 in the SCA group, and 1 in the AICA group (the latter was the only patient taking oral anticoagulants as baseline treatment) (Fig. 2).
Only 2 individuals showed clinical manifestations, both of which were decreased level of consciousness; 4 cases were detected by the emergency department in its initial CT. The rest (72.4%) were asymptomatic, and illness was detected by routine CT or MR scans. We did not classify patients by bleed volume or whether or not ventricular invasion was present. Regarding the underlying aetiology, there were 12 cases of cardioembolic stroke (41.4%), 10 cases due to undetermined causes (34.5%), and 7 due to atherothrombosis (24.1%).
Lastly, we assessed degree of dependence upon discharge. Patients were classified according to their scores on the mRS as able to carry out daily living activities (mRS=0–2) or functionally dependent (mRS=3–5).
At discharge, 41.9% of the patients were functionally dependent (only 4.5% retained their baseline Rankin scores); 4 patients died, all while hospitalised. NIHSS was ≤5 in 115 patients (92.7%) upon discharge.
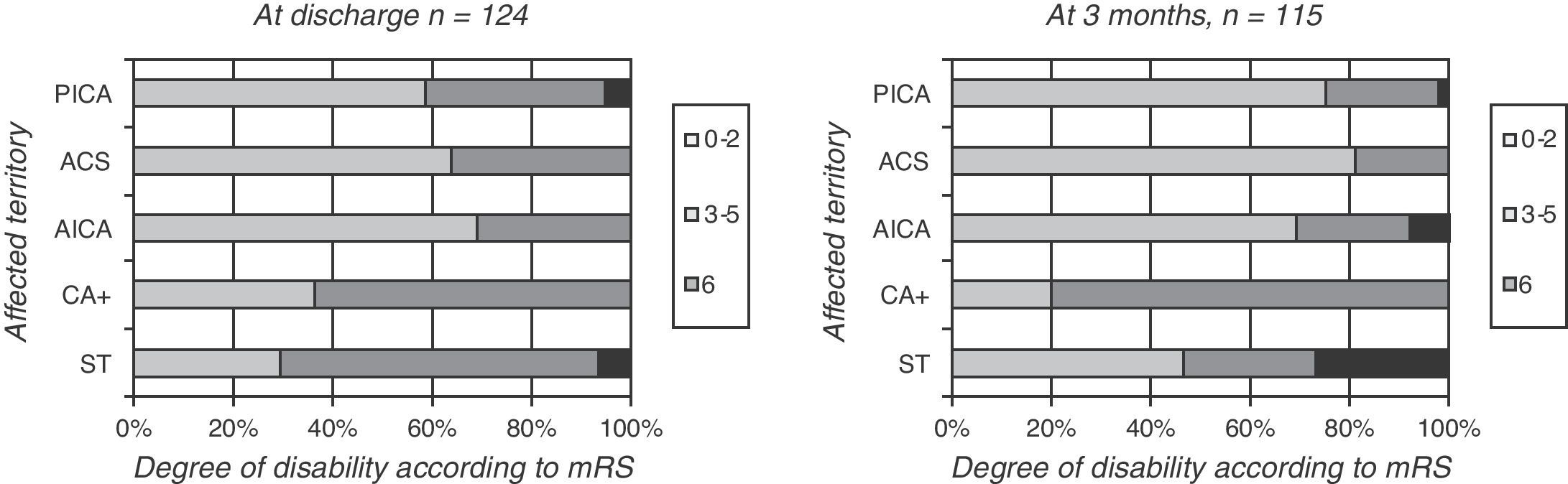
Patients with more than one affected cerebellar territory, or simultaneous impairment of supratentorial territory, had a poorer functional prognosis (mRS of 3–5 in 64%) than patients with only one affected cerebellar territory. This was true for PICA, SCA, and AICA territories (mRS of 3–5 in 31%–36%, P=.05) (Fig. 3, left).
If patients with brainstem infarcts are placed in a separate category, they account for 3 of the 4 total deaths. Likewise, 48% of these patients had an mRS of 3–5 at discharge; 40% had an mRS ≤2.
Three months after suffering stroke, 115 patients were still being monitored (4 died during the acute phase and 5 were lost to follow-up). During this time, 6 patients died (3 stroke-related, 1 of undetermined causes, 1 due to respiratory infection, and 1 due to lung cancer). Of the remaining patients, 32 (25.8% of the initial series) remained functionally dependent.
Differences related to the affected territory were even more marked at 3 months of follow-up; impairment of multiple territories was associated with increased functional dependence (P=.03 (Fig. 3, right)).
Mean hospital stay (Sm) was 14.2 days (range, 3–74). Sm for patients with complications was longer than for patients who did not experience hydrocephalus or haemorrhagic transformation (20.7 vs 11.7 days).
DiscussionCerebellar infarcts affect more males than females, which is true of ischaemic strokes in general. Our data for age of onset and male/female ratio are similar to those published in other studies.1,3,4,13
Thrombolytic treatment was provided to less than a fourth of the patient total. On the one hand, the lack of specificity of clinical symptoms means that wrong diagnoses are sometimes considered,2 and also causes patients to come to the hospital too late (on average, more than a day and a half late in our group). On the other hand, even when patients visit within the time window, treatment is often discarded due to the NIHSS being too low.
Our results, unlike those from other studies,1,13,14 showed a predominance of PICA territory infarcts (present in nearly half of all patients). It is possible that the advent of brain MRI has made it easier to identify affected territories precisely, which would explain the difference in proportion.
We also identified different prevalent aetiologies for events in each of the territories. PICA infarcts were generally caused by atherothrombosis; there was no single predominant aetiology for SCA and AICA infarcts.
As might be expected, cardioembolic disease was the most frequent aetiology in patients with simultaneous affectation of multiple territories.
The complication rate and time of appearance are similar to those reported by the literature.1,3,4,16 Most of the hydrocephalus cases appeared between 48 and 72hours after stroke onset; none presented later than day 6 following the stroke. Monitoring in a stroke unit is therefore especially important in the first week following the event; after that, it is optional.
The appearance of hydrocephalus was mainly associated with PICA infarcts. Four of the 15 patients had associated brainstem impairment. All patients were evaluated by the neurosurgery department. The general consensus was to use a temporary external ventricular drain rather than decompressive craniectomy. There were 2 deaths and 8 patients were functionally dependent at discharge.
In cases of haemorrhagic transformation without hydrocephalus, we used only the routine measures employed in the stroke unit. This complication arose primarily in patients with PICA infarcts and those with simultaneous supratentorial impairment. We found no reasons explaining haemorrhagic transformation in the first group; however, the risk of this complication is higher with cardioembolic infarcts, like the ones affecting the second group of patients.19 In either case, the complication was not associated with use of antiplatelet or anticoagulant drugs.
A functional assessment scale such as the Rankin scale is more suitable for determining prognosis than a neurological scale like the NIHSS. Considering the symptoms which a cerebellar infarct causes, the NIHSS is not as useful for evaluating patient prognosis. Nearly all of our patients had low NIHSS scores, including those with major sequelae.
Some authors have written that the appearance of complications implies an increased risk of death.4,13 In our case, there were no significant differences between groups with complications and groups without. It could well be that use of aggressive methods to resolve hydrocephalus and continuous monitoring in stroke units had an impact on our results. This possibility would have to be confirmed in a larger patient sample.
Meanwhile, other authors have observed that prognosis has more to do with whether or not lesions are bilateral, and less to do with which territory is affected.17 Our study has a very low prevalence of bilateral lesions. However, it clearly shows that patient condition upon discharge, and most of all, after 3 months of follow-up, was worse when various territories were affected than when the infarct was limited to a single territory. Prognosis is also poorer when the brainstem is affected than when it is not.
This study has the typical limitations of a retrospective analysis examining multiple aetiologies. However, it was carried out in a single centre, which decreases the number of deviations from protocol and assures a more homogeneous use of treatments and evaluations.
In conclusion, the most commonly affected territory was PICA, and the predominant cause in this territory was atherothrombosis. In contrast, we did not find a predominant aetiology for either SCA or AICA infarcts. PICA was also the territory with the greatest risk of complications, most of which took place during the first week. Strangely enough, the development of complications did not serve to worsen patient prognosis; prognosis mainly depended on the number of affected territories and the presence of brainstem impairment.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Cano LM, et al. Infarto cerebeloso: pronóstico y complicaciones de sus territorios vasculares. Neurología. 2012;27:330–5.
Part of this study was presented in poster format at the 62nd annual meeting of the Spanish Society of Neurology.