Daratumumab (DARA) is a medication that in 2016 proved its efficacy and safety in the treatment of multiple myeloma. This product, based on a monoclonal antibody, technically generates an interference in most blood compatibility tests, artificially producing high blood pan-reactivity and incompatibility. The difficulty in finding blood compatible with the patient's serum can also mask the presence of other clinically significant alloantibodies and, above all, delays the timely delivery of erythrocyte concentrate, which, obviously, endangers the patient's life.
In transfusion medicine, very different strategies have recently been described for the resolution of this technical interference. We adopted the use of dithiothreitol (or DTT) to treat the red blood cells of the possible blood donors for the patient, thus achieving the elimination of this technical discrepancy and the adequate, safe and timely selection of this blood component. In the present paper, we describe the first case of a patient with a diagnosis of multiple myeloma treated with DARA, in which the interference caused by this drug in pretransfusion tests was successfully resolved.
Communication is a key element in the management of these patients. The doctor should inform the patient and the blood bank about the risk of presenting incompatible cross-tests when they are being treated with this medication. It is additionally recommended that all patients with multiple myeloma should undergo an erythrocyte phenotyping before receiving their treatment with DARA, and carry a card indicating that they are receiving this medication. Fortunately, hemolytic transfusion reactions have not yet been reported in these types of patients at the time of this publication.
For the staff at blood banks, it is evident that an additional operating procedure must be in place to describe the technical and administrative operation that must be followed from the admission of these patients to the hospital.
Technically speaking, we recommend, based on our experience reported in this case, the use of the DTT technique to resolve this discrepancy and technical difficulty in pretransfusion testing in patients treated with DARA.
Daratumumab (DARA) is a medication which was recently approved by the FDA and patented by Janssen for the treatment of multiple myeloma. This drug is an lgG1k CD38-targeted monoclonal antibody (MoAb) which is expressed in myeloma cells; it is highly cytotoxic for the tumor cells in these patients.1–4 The presence of CD38 antigen on the surfaces of the erythrocytes of healthy individuals has also been proven by flow cytometry.5 Additionally, panreactivity has been detected in the plasma of patients treated with DARA in routine compatibility tests.6 This is a new problem in transfusion medicine which delays timely delivery of blood components, thus representing a potential danger to the patient's life. Being a topic of recent emergence, technical procedure manuals (i.e., the AABB), do not contemplate a specific methodology for the resolution of these types of problems.
For over 20 years, red blood cells treatment with the redox agent dithiothreitol (DDT) have been commonly used to denature the Kell antigen, as well as other less clinically-significant antigen groups as a result of a hemolytic transfusion reaction. We can see this as in the cases of Landsteiner-Wiener, Cartwright, Dombrock, Indian, Jhon Milton Hagen, Lutheran and Raph. This feature is useful in the research of allo-antibodies (technical manual of the AABB).7,8
More recently, the CD38 antigen has been proven to also be sensitive to denaturing by the redox agent dithiothreitol (DDT),9 without affecting the rest of the clinically significant erythrocyte antigens. Among the possibilities to eradicate the interference, we apply the DTT technique, which allows us to detect the presence of clinically significant antibodies, thus providing blood components with a lower transfusion risk for the patient.
Materials and methodsThe blood bank at the Zambrano Hellion hospital received a transfusion request of 2 units of globular packages and 4 platelet concentrates. The patient, a 64-year-old male without transfusions in his background, had a diagnosis of multiple myeloma with a 4-year evolution and was resistant to conventional medical treatment. Venous blood was collected with EDTA. The samples were studied for routine pre-transfusion tests through the conventional methods and techniques used in the blood bank (i.e., micro-agglutination techniques in gel). ABO blood group studies and Rh were performed with Grifols cards.
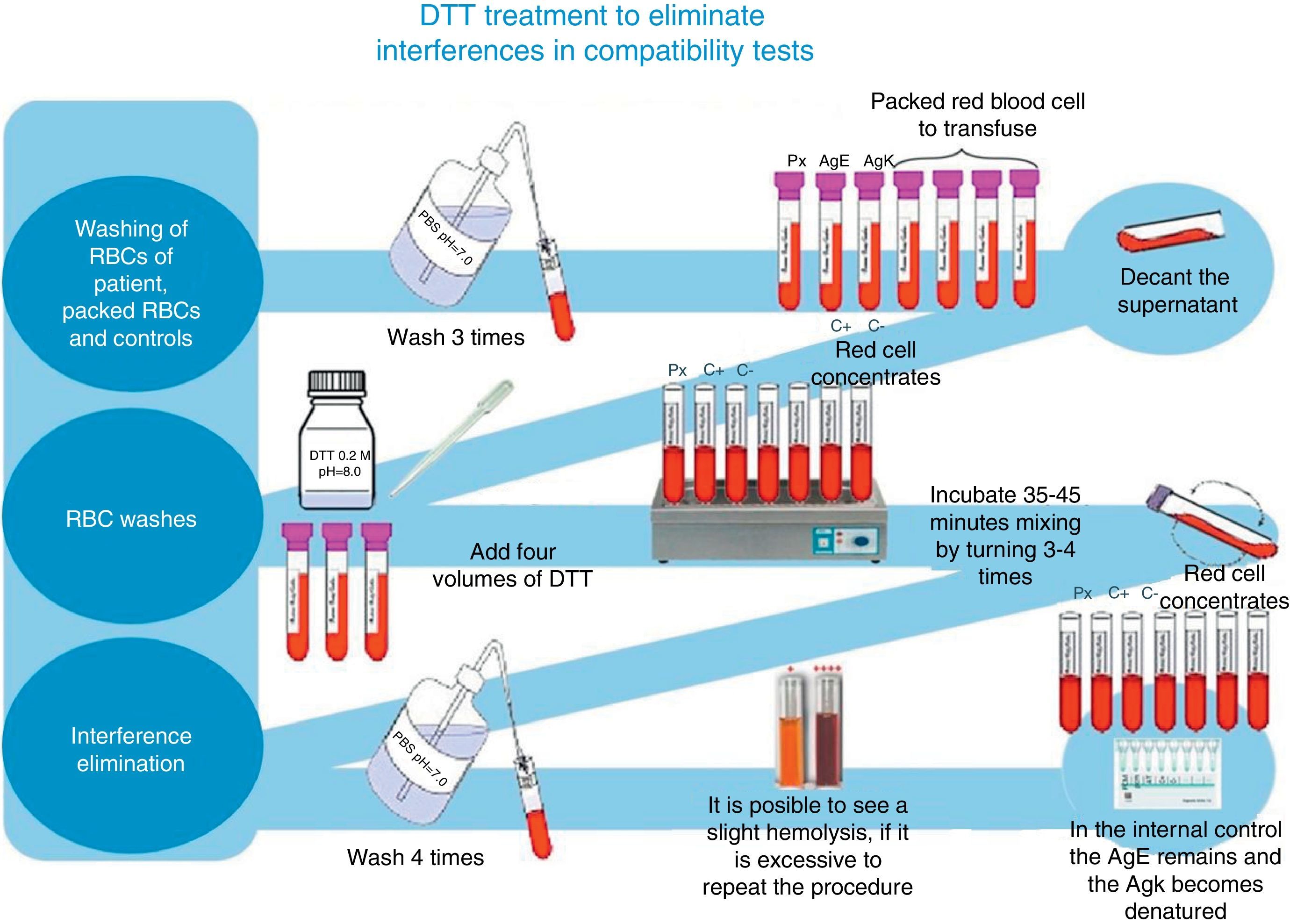
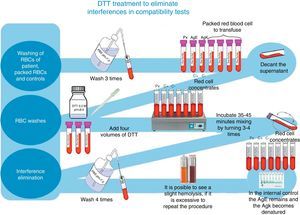
For irregular antibody tracking and identification, we used a complete Grifols erythrocytes panel, as well as Grifols DG Gel Coombs columns. Additionally, pre-transfusion cross-matching tests were processed according to the technique described in the DG Coombs insert (antihuman globulin poly-specific), for the treatment of erythrocytes with dithiothreitol (DTT) at 0.2M, pH 8, utilizing the 4.6 method of the ABB's technical manual (12th edition of the general laboratory methods sections)8 (see Fig. 1).
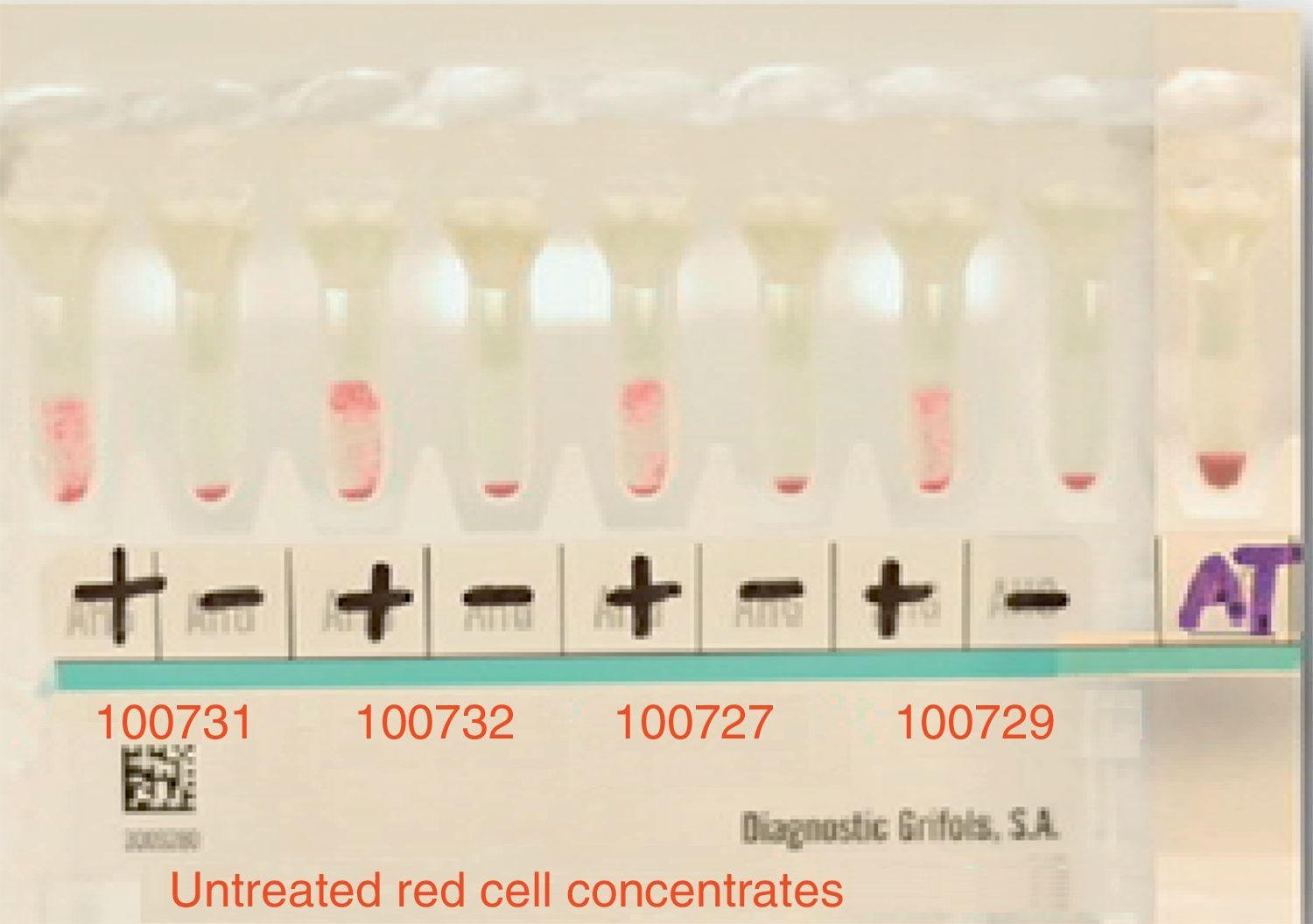
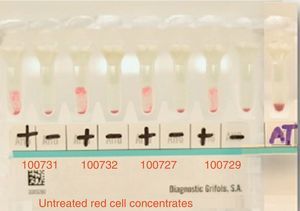
ResultsThe patient's plasma presented pan-reactivity with the panel erythrocytes and known antigens, which showed a reaction pattern with a 3+ intensity (grade 0–4) homogenously (see Fig. 2).
The main cross-matching test resulted in incompatibility with all studied donors (15 in total).
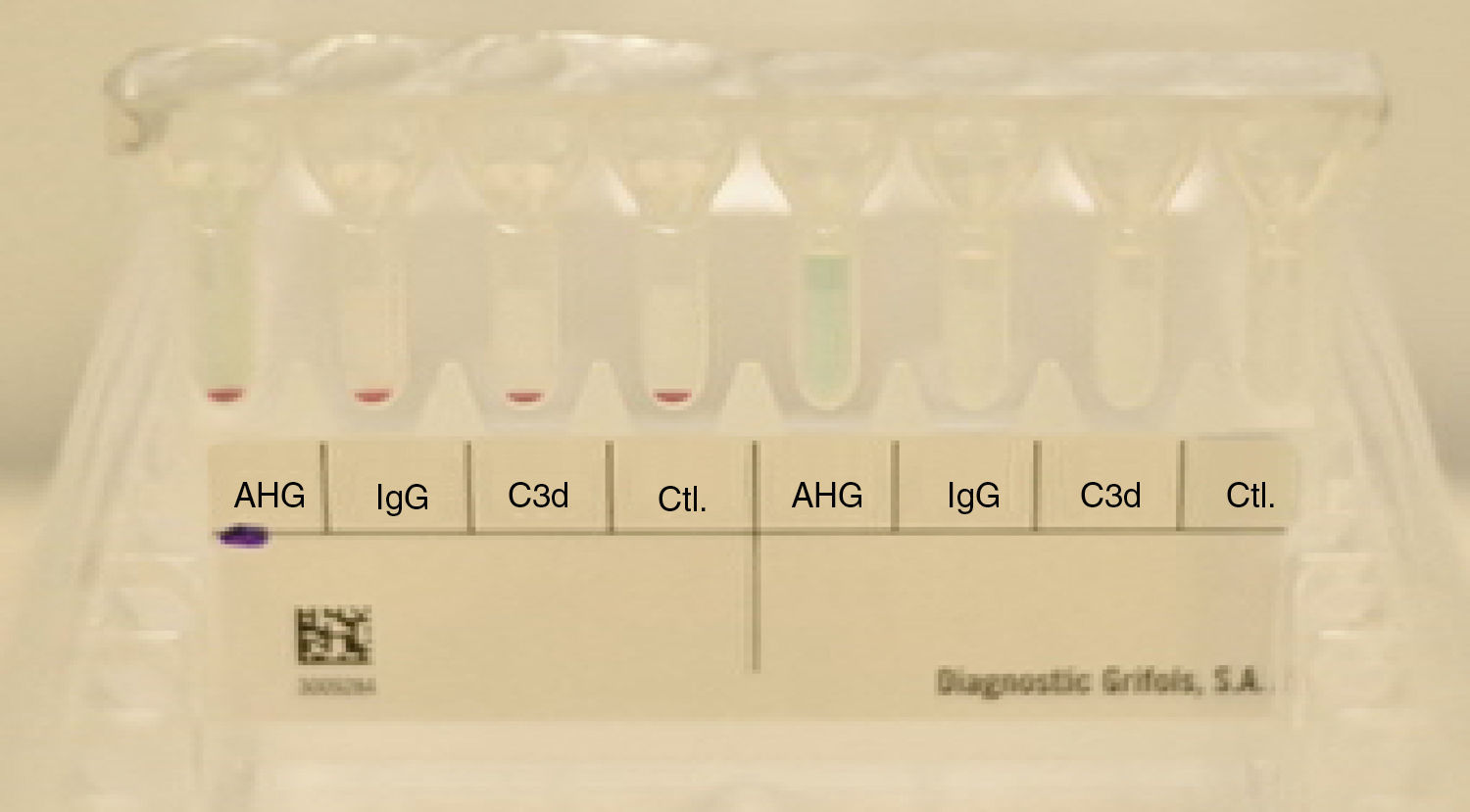
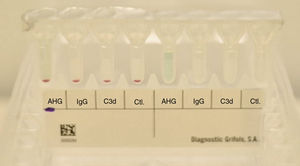
The patient's erythrocytes presented a negative direct anti-human globulin test (IgG and C3b), as well as a negative control. The presence of hemolysis in the patient's sample was not detected (see Fig. 3).
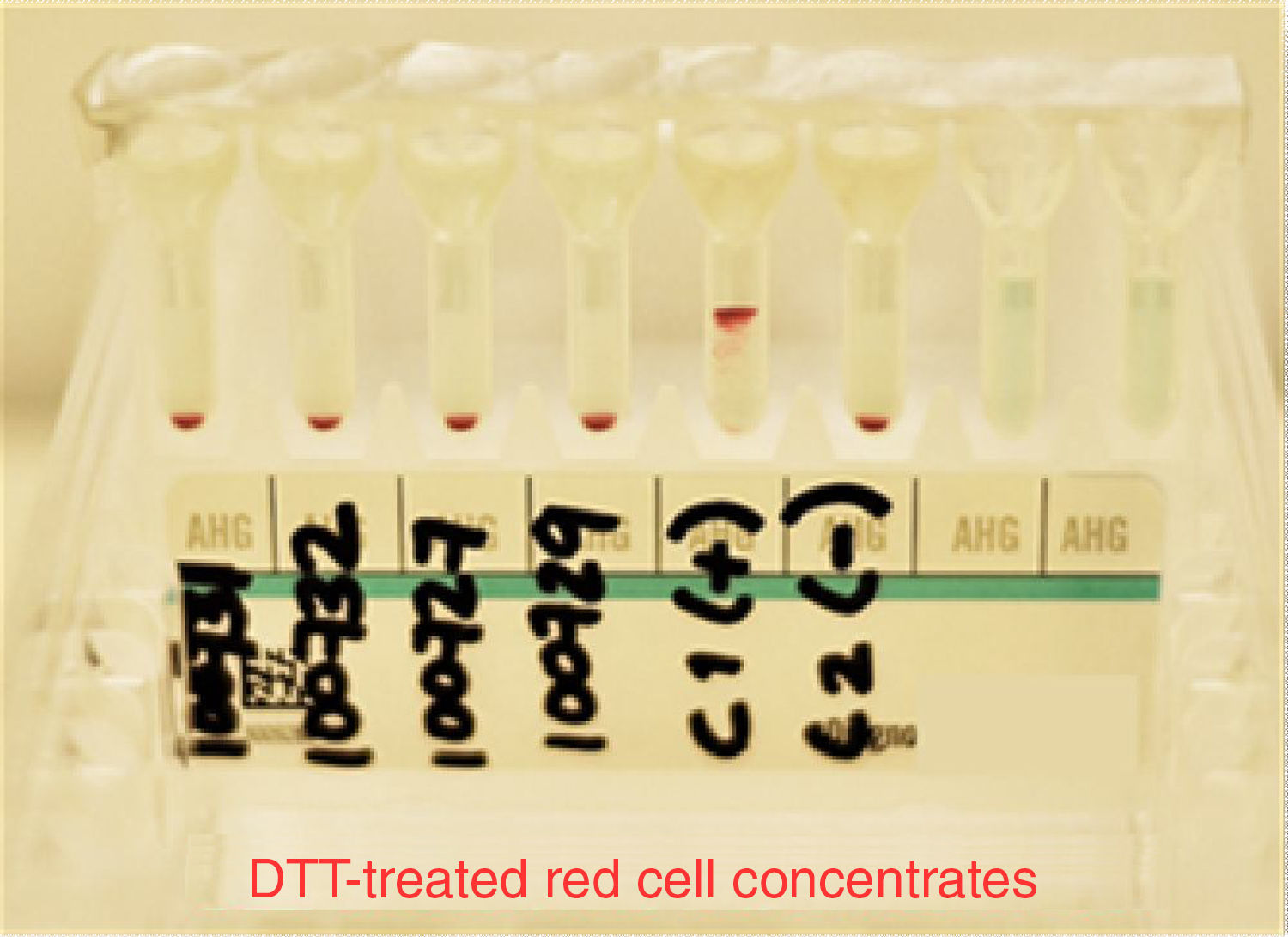
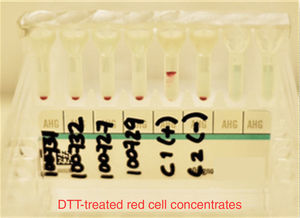
At the moment of conducting tests with the patient's plasma and the erythrocytes previously treated with DTT from the different donors, as well as those used in the antibody screening panel, we did not find reactivity in any of the studied samples. K+ and E+ control erythrocytes were used to verify that DTT denatured only the K antigen and preserved the E antigen; this was a part of the quality control of our study to determine that other antigens related to clinically significant antibodies were inactive (see Fig. 4).
DiscussionThe use of anti-CD38 is a promising treatment for patients with multiple myeloma and is currently under study for other types of malignant neoplasms. The problem is that this medication interferes with the compatibility tests, thus complicating timely and safe delivery of blood components.
There is evidence that the anti-CD38 in the serum of patients treated with DARA bind with the CD38 antigen expressed in the erythrocytes utilized in the antibody tracking panel, as well as in the donors of the pre-transfusion cross-matching test, generating a pan-reactivity. This may mask the presence of clinically significant antibodies, especially in multiparous patients or in poly-transfused patients. This pan-reactivity can be reversed through the use of neutralizing agents, which re-establish irregular antibody identification and tracking tests. Anti-idiotype antibodies, as well as soluble recombinant CD38, have been evaluated in previous articles with positive results.
Of the possibilities available to eliminate this interference in the blood bank, we used the DDT technique. Its best feature is the fact that it is a redox agent which interrupts the protein's tertiary structure by irreversibly reducing disulfide links to free sulfhydryl. Without a tertiary structure, the antigens, which contain protein, can no longer fix antibodies, thus inhibiting serologic reactivity. A potential problem with this technique is that it also affects other blood group antigens. However, in everyday practice, it is only the K antigen that is clinically significant. Therefore, it is recommended for patients treated with DARA to only be transfused with K− erythrocyte concentrates. The possibility of losing reactivity to other agents like K and Yta are also present. Nevertheless, this turns out to be an extremely rare event. DDT does not deactivate the serological activity of most clinically significant and frequent antigens such as Rh, Duffy, Kidd and MNS systems. It is wise to determine the patient's phenotype or genotype before beginning treatment with DARA.
In Immunohematology, there is concern about the possible increase of this type of interference in routine blood transfusion tests, which may arise as a consequence of new monoclonal antibody therapies in patients with cancer.
In conclusion, our study demonstrated that the treatment of erythrocytes with DTT inactivates the CD38 antigen, eliminating reactivity with the serum of the patient treated with DARA. This simple and practical method allows studies to be performed without the interference the presence of alloantibodies can cause in transfusion reactions in patients receiving therapy based on this monoclonal antibody.
The problem of panreactivity that causes interference in the compatibility tests of patients treated with DARA can technically be solved with the treatment of erythrocytes with DTT from potential blood donors. This agent deactivates the CD38 antigen present in these cells. This panreactivity should not delay the delivery of blood products, which in emergency situations will be using identical ABO globular packets and Rh compatible red blood cells, according to the practices of the blood bank. This, forces the generation of recommendations and/or guides to treating doctors and staff of the blood banks that attend to these types of patients. It is important to consider that, to date, no hemolytic transfusion reactions have been observed in the approximately 2000 patients who have been treated with DARA.8 Recent research on transfusion safety in patients treated with DARA comprising about 76,000 cycles of drug application did not find a single adverse reaction to transfusion hemolysis.10–12
The joint work of the onco-hematologist and the blood bank must be present from the moment patients are admitted to the hospital. The procedures for transfusion applied to these types of patients should include the identification of the complete erythrocyte phenotype from the box with its blood group, and compatibility tests should be run to the best of our ability before the application of this medicine.
The DTT deactivation technique described above represents an alternative measure of transfusion safety in all cases. Our blood bank is a pioneer in our country in the adoption this recently described and internationally validated technology.13 However, in critical situations of transfusion emergency where the life of the patient is in danger, blood should only be transfused with ABO and RhD compatibility, without performing the compatibility tests according to the policies and procedures previously described in emergency transfusions.
Conflict of interestThe authors have no conflicts of interest to declare.