This case report of a 78 year-old man demonstrates a case of bilateral optic neuropathy that does not completely fit the modern definition of Primary Open-Angle Glaucoma. In this case, visual field testing does not substantiate a diagnosis of glaucoma, and retrograde optic neuropathy seems to be a more likely source for the optic nerve presentation. Clinicians are reminded that incomplete clinical pictures merit further scrutiny.
Este caso clínico de un hombre de 78 años presenta una neuropatía óptica bilateral que no se ajusta completamente a la definición actual de glaucoma primario de ángulo abierto. En este caso, las pruebas del campo visual no corroboran un diagnóstico de glaucoma y aparentemente el origen más probable de esta presentación del nervio óptico sea la neuropatía óptica retrógrada. Se recuerda a los médicos que las imágenes clínicas incompletas merecen un examen más exhaustivo.
Masqueraders of glaucomatous optic neuropathy provide diagnostic challenges to clinicians, especially when the optic neuropathy is not accompanied by matching visual field defects as per current definitions of glaucoma.1 For this case, further investigation of bilateral optic neuropathy revealed only generalized cortical atrophy, which may serve to explain the observed clinical findings by means of a retrograde neuropathic process. Although traumatic bilateral retrograde optic neuropathy was reported as early as 1971,2 bilateral retrograde optic neuropathy associated with generalized cortical atrophy has not been reported in the literature to date.
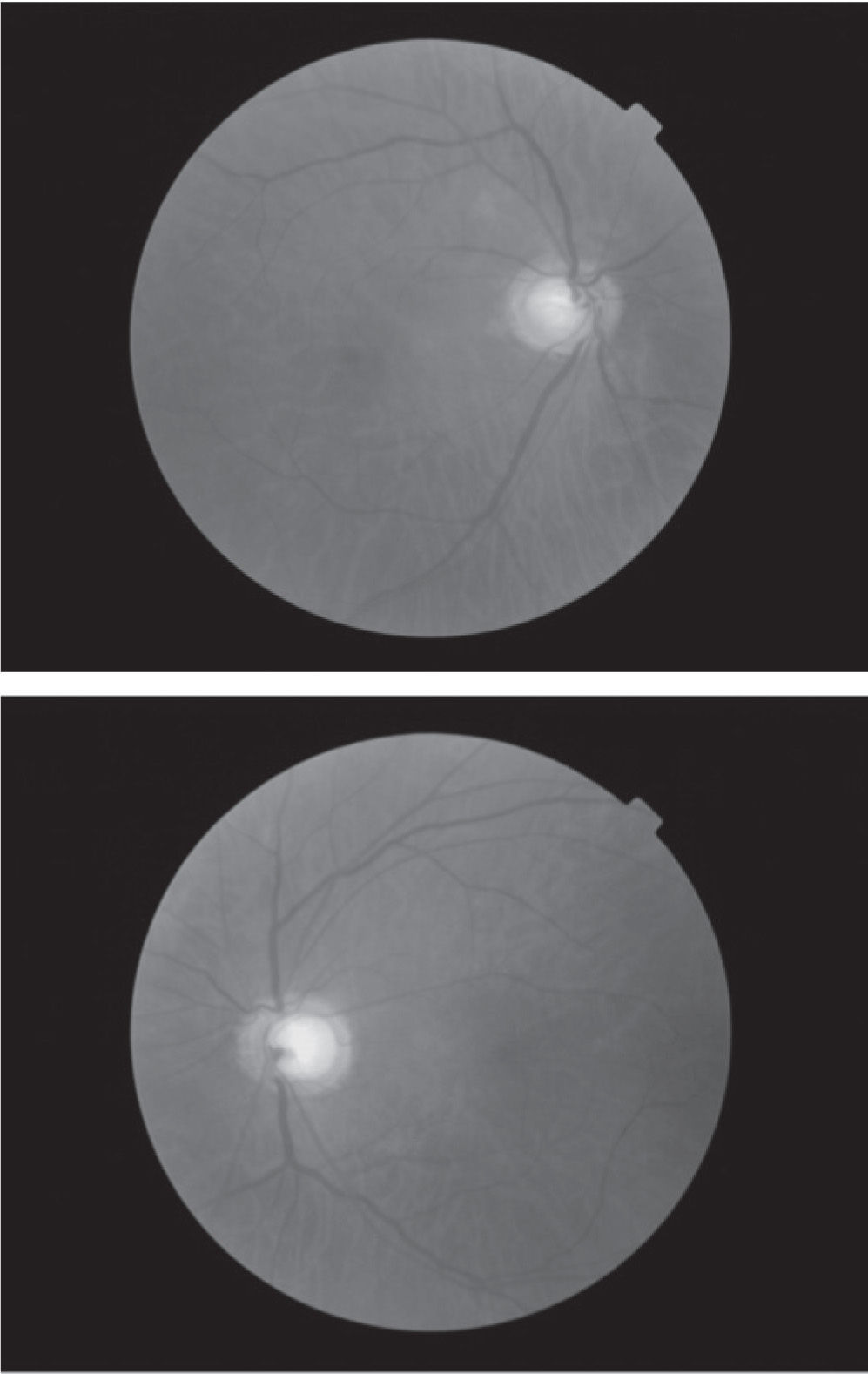
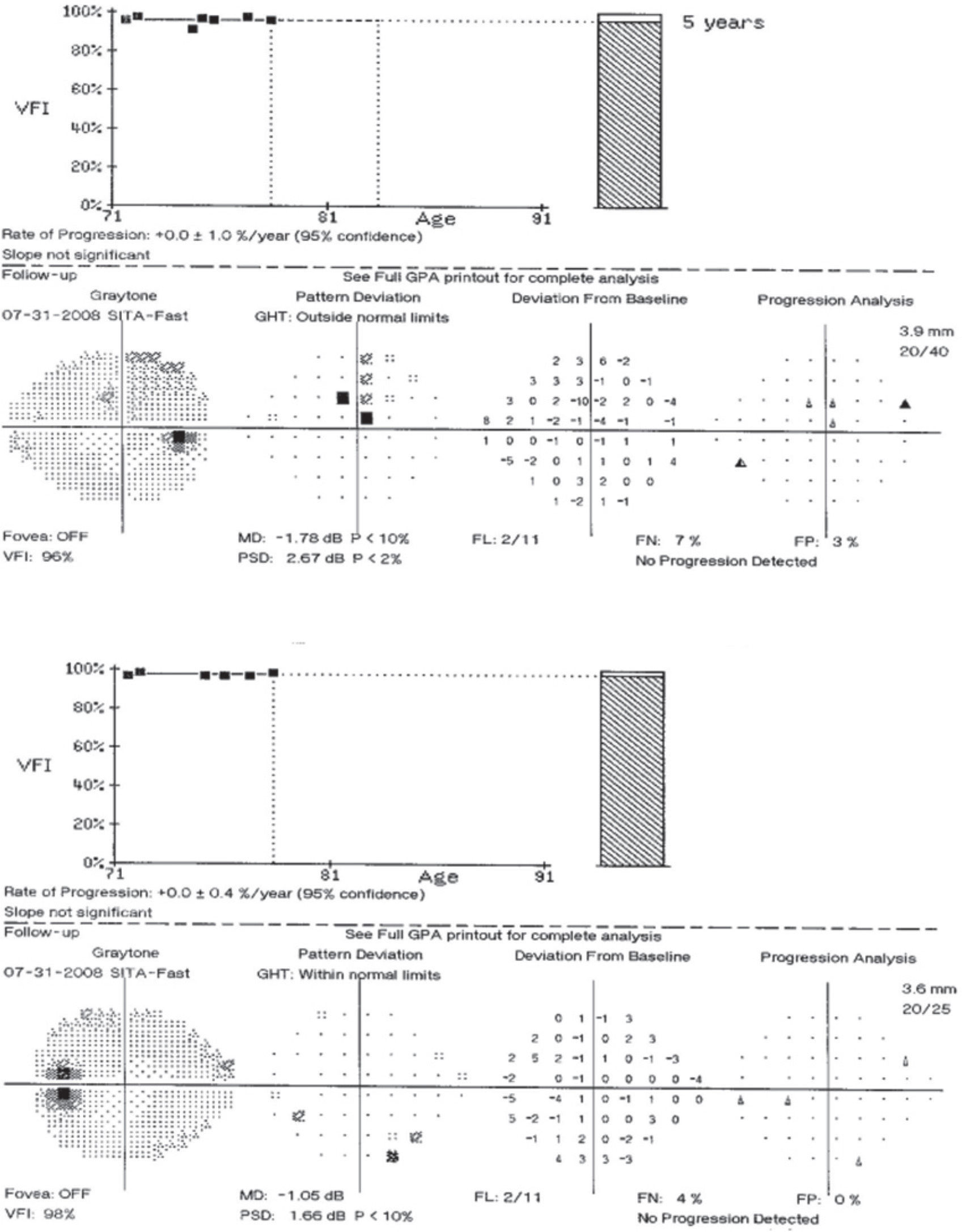
Case reportA 78 year-old asymptomatic Type 2 diabetic male has been followed for eight years with visual acuities remaining 6/6 OU and no diabetic retinopathy observed throughout the follow-up period. At the original evaluation in 2001, this patient was identified as a glaucoma suspect based on apparent bilateral optic neuropathy with enlarged optic cup-to-disc ratios of approximately 0.8 OU (Figure 1). Subsequent automated perimetry showed mild, scattered scotomata of varying depth, but which were unrepeatable on follow-up visual field testing, and which have demonstrated no progression during the follow-up period (Figure 2). Average tonometry for this patient is 11mmHg OU since initial evaluation (eleven readings over nine years). Physiological angles are unoccludable (40-r-D OU, Spaeth criteria) and there are no other ocular findings suggestive of secondary forms of glaucoma. In the absence of clinical data supporting a diagnosis for any form of glaucoma, further work-up was pursued for this patient with an atypical optic nerve presentation.
Brain magnetic resonance image (MRI) obtained in August 2008 was remarkable for generalized “moderate to severe” cerebral atrophy, mild cerebellar atrophy, and deep white matter ischemic changes attributable to small vessel disease. Carotid insufficiency was ruled out via duplex carotid ultrasonography, and RPR testing was non-reactive for this patient. Recent non-focal neurological examination and Global Assessment of Functioning (GAF) scores do not suggest other existent neurological or mental health issues at this time. As a result of these negative findings no additional medical treatment was instituted.
Based on the results of ancillary testing and recent years of stable ophthalmic follow-up, it is conjectured that this case demonstrates a bilateral retrograde optic neuropathy as a result of generalized, cortical, small vessel disease. Concurrent retinal arteriolar narrowing would also tend to support this supposition. Potential progression of findings remains to be seen.
DiscussionCurrent definitions of glaucoma focus on optic neuropathy with accompanying, corresponding, perimetric defects.1 Diagnostic challenges arise when optic neuropathy is not accompanied by matching visual field defects consistent with glaucomatous optic neuropathy or a history of visual symptoms suggestive of other acute optic neuropathies.
Following irreversible damage to a visual axon, neuronal death involves adjacent cells along the neural path in both directions from that site. In the eye, the neural path begins in the retina and extends posteriorly through the optic chiasm, terminating in the occipitus. Anterograde (Wallerian) degeneration occurs when the distal portion of the damaged neuron effects the death of the next axon “downstream” in the neural arc. This is occasionally observed ophthalmoscopically in the acquired optic nerve pallor that results from marked panretinal photocoagulation for complications of proliferative diabetic retinopathy3 or following some retinal detachment repairs. In these cases, an ocular event leads to the optic neuropathy.
Retrograde degeneration occurs in the opposite direction, when a cortical event causes neuronal death “upstream” in the pathway. Retrobulbar optic nerve and chiasmal tumors can cause damage to ganglion cell axons and secondary optic atrophy in this manner. Although it is less clear whether cortical lesions can effect similar pathology, bilateral optic atrophy has been associated with occipital lesions.4 It is this process, which is believed to have caused the bilateral optic neuropathy in this patient. The antecedent, ischemic event is attributable to small vessel disease within the cortex, substantiated by MRI—the only underlying medical condition associated with the optic neuropathy in this case. Definitive substantiation of this relationship could only be made with sequential optic disk photos and MRI—say from age 50—unfortunately, previous exam data is not available for comparison for this patient, so the association remains conjectural at this time. Arteriosclerosis would not seem to account for this clinical presentation—at least based on reports from simian models.5
Based on the appearance of the optic nerves, this case must be considered a form of glaucoma until proven otherwise. However, that working diagnosis was disproven once the results of the visual fields were obtained. This patient manifests essentially full visual fields. The inclusion of both optic neuropathy and visual field loss is clearly stated in the inclusion criteria for both the Collaborative Normal-Tension Glaucoma Study6 and the Low-Pressure Glaucoma Treatment Study7. By definition, then, this cannot be a case of glaucoma. In sum visual field defects are required in order to make the diagnosis of glaucoma. This distinction is critical for this case.
(Regarding the terms “normal-tension” or “low-pressure” glaucoma, these are no longer widely-accepted terms when discussing cases of Primary Open-Angle Glaucoma. Both Harry Quigley—who has entirely discarded this term1—and Stephen Drance himself (principal investigator of the CNTGS)—who has described this distinction as “artificial”8 —have moved us beyond this historical, but arbitrary, distinction regarding IOP.)
Additionally, regarding nerve fiber layer (NFL) losses—an expected finding in cases of glaucoma: NFLscans for this particular patient did demonstrate thinning, but in a pattern inconsistent with the apparent symmetric rim thinning of the ONH appearances. There was marked inferior thinning only OD and marked superior thinning only OS, while the other quadrants were normal (this pattern was unchanged between Nov 2008 and May 2010). This presentation does not seem clinically consistent with this case. Regardless, the presence of NFL dropout could signify either anterograde or retrograde processes. The importance of NFL dropout in optic neuropathies other than glaucoma has yet to be determined.
Thus, the differential diagnosis for non-glaucomatous sources for bilateral disc cupping includes: congenital/hereditary optic neuropathies, demyelinating optic neuritis, enlargement of the intracranial carotid artery, and space-occupying lesions.9 To this list must be added, neurosyphilis (one of “the great imitators”), hypovolemia, and nutritional/toxic neuropathies. Patients with any of these conditions may show decreased vision or exhibit other visual symptoms as a result of the optic neuropathies, although early symptoms may be quite subtle. Negative testing ruled out each of the differential diagnosis possibilities for this patient.
At present time, there is no consensus regarding neuroimaging for patients with optic nerve cupping that resembles glaucomatous optic neuropathy, and the yield for previously-unknown, space-occupying lesions is low.9 Neuroimaging is suggested in patients with marked disc cupping in the presence of atypical visual symptoms: marked acuity loss, rapid vision loss, hemianopic field losses, mismatched cupping/vision loss, unexplained optic neuropathy, or pallor of the remaining neuroretinal rim.10 This case broadly falls into the “unexplained optic neuropathy” category—a topic on which the ophthalmic literature is frustratingly silent.
Generalized cortical atrophy manifesting as a secondary retrograde neuronal atrophy may be one presentation of bilateral optic neuropathy that can mimic glaucomatous optic neuropathy, but which can only be demonstrated via neuroimaging.
Conflict of interestsThe author states he has no conflict of interests.