Background/Objective: Cognitive-behavioral therapy (CBT) is one of the first-line treatments in the management of fibromyalgia (FM) and it has been applied with considerable success to treat the psychological processes associated with pain and insomnia. We hypothesized that treating sleep and pain jointly with new combined modalities of CBT may offer greater sleep-related benefits to patients. Method: Thirty-nine female patients with FM and insomnia were randomly allocated to receive CBT centered on pain (CBT-P) or combined CBT focused on pain and insomnia (CBT-C). Participants were assessed at baseline and post-treatment with the Pittsburgh Sleep Quality Index and an ambulatory polysomnography.Results: Participants who received CBT-P showed increases in time in bed and total sleep time and decreases in light sleep, but there was no improvement in perceived sleep quality. Participants who received combined CBT-C showed more meaningful improvements related to refreshing sleep (i.e., higher sleep efficiency and less time awake and longer time in Stage 4 sleep), and these changes were concordant with a significant improvement in self-perceived sleep quality. Conclusions: This study suggests that new CBT-C approaches can improve insomnia-related clinical aspects.
Antecedentes/Objetivo: La terapia cognitivo-conductual (TCC) es un tratamiento de primera línea para abordar la fibromialgia (FM) que se ha aplicado con cierto éxito para el tratamiento del dolor y el insomnio. Se hipotetiza que intervenir sobre el sueño y el dolor con una modalidad combinada de TCC (TCC-C) puede mejorar el sueño de estos pacientes. Método: Treinta y nueve mujeres con FM e insomnio fueron aleatorizadas para recibir TCC centrada en dolor (TCC-D) o TCC-C. Se evaluaron al inicio y en el post-tratamiento con el Índice de Calidad del Sueño de Pittsburgh y polisomnografía ambulatoria. Resultados: Las participantes en la TCC-D mostraron aumentos del tiempo en cama y del tiempo total de sueño, y un descenso del sueño ligero, pero no hubo una mejora en la calidad del sueño percibida. Las participantes en la TCC-C mostraron mejoras significativas relacionadas con el sueño reparador (mayor eficiencia del sueño, menos tiempo de vigilia y más tiempo en fase 4 del sueño), y estos cambios fueron congruentes con una mejora en la calidad del sueño percibida. Conclusiones: Este estudio sugiere que nuevos enfoques TCC-C en FM pueden mejorar aspectos clínicos relacionados con el insomnio.
Fibromyalgia (FM) is a chronic pain syndrome of unknown etiology that is has been described as a central pain syndrome in light of evidence supporting the involvement of dysfunctional sensory processing and central pain augmentation (Sluka & Clauw, 2016). In addition, recent research has identified a small fiber neuropathy in FM patients (Farhad & Oaklander, 2018). FM is a common condition in the general population, with an overall prevalence of 2.1%, mainly women (women/men ratio, 4:1) (Cabo-Meseguer, Cerdá-Olmedo, & Trillo-Mata, 2017). This chronic condition has a considerable impact on patients’ health-related quality of life (Gálvez-Sánchez, Montoro, Duschek, & Reyes del Paso, 2020) and leads to substantial healthcare-related costs that varies from €7813-9982 per patient, per year (Cabo-Meseguer et al., 2017).
Several studies analyzing sleep in patients with FM have reported worse sleep quality in such patients compared to the general population and patients with other rheumatic conditions (Choy, 2015). The analysis of sleep in FM patients using objective measures such as polysomnography (PSG) has revealed alterations in sleep architecture, microstructure and continuity such as a decrease in total sleep time (TST), delayed sleep onset, higher rapid eye movement sleep latency (REML), lower sleep efficiency (SE), greater sleep fragmentation, lower percentage of slow-wave sleep (SWS) as compared to control subjects. These alterations have shown significant relationships with clinical consequences of FM (e.g., self-reports of unrefreshing sleep, lower pain thresholds, higher fatigue, sleepiness, emotional distress and cognitive impairment) (Choy, 2015; Wu, Chang, Lee, Fang, & Tsai, 2017).
Sleep problems such as insomnia and pain are clinical manifestations that typically co-occur in FM (Choy, 2015). It is therefore highly important to clarify the influence of these clinical manifestations on the psychological status of FM patients. In line with the wealth of evidence supporting the modulating role of cognitive-affective factors in FM (Aguilera, Paz, Compañ, Medina, & Feixas, 2019; Lami, Martínez, Miró, Sánchez, & Guzmán, 2018), this syndrome is a prototypic chronic condition characterized by the existence of common cognitive-behavioral phenomena (i.e., symptom-related fear, catastrophizing thoughts, avoidance behaviors, inappropriate coping strategies, depressed mood or anxiety). These psychological phenomena may work like synergistic and interacting factors that contribute to patients’ general dysfunction and cognitive vulnerability for coping with threat due to clinical symptoms (Brandolim et al., 2018; Bryson, Read, Bush, & Edwards, 2015; MacDonald, Linton, & Jansson-Fröjmark, 2010; Pagnini et al., 2019). Cognitive-behavioral therapy (CBT) is a first-choice treatment in FM according to some evidence-based guidelines (Thieme, Mathys, & Turk, 2017). Considering the above-mentioned psychological factors involved in the relationships between pain and insomnia (MacDonald et al., 2010), FM patients could be potential beneficiaries of a CBT targeting both sleep problems and pain.
To date, most clinical trials of CBT in FM patients have involved conventional CBT focused on pain (CBT-P), which mainly targets disability, pain and related psychological factors but very little is known about sleep improvements after undergoing this kind of CBT-P (Bernardy, Klose, Welsch, & Häuser, 2018; McCrae et al., 2019; Tang, 2018). As regards CBT focused on insomnia (CBT-I), only five clinical trials have analyzed the efficacy of this therapy in FM patients. This therapy has shown encouraging improvements in insomnia-related outcomes, daily functioning, psychological well-being, pain catastrophizing, anxiety and depression, alertness and executive functioning compared to patients who received sleep hygiene advice or usual care (Edinger, Wohlgemuth, Krystal, & Rice, 2005; Martínez, Miró, Sánchez et al., 2014; McCrae et al., 2019; Miró et al., 2011; Sánchez et al., 2012).
To the best of our knowledge, the unique clinical trial carried out applying CBT-C among FM patients has been developed by our research group (Lami, Martínez, Miró, Sánchez, Prados et al., 2018). This study compared the efficacy of CBT-C vs. CBT-P vs. usual medical care in a sample of 126 patients considering self-reports as outcome measures (sleep quality, pain-related variables, fatigue, functioning, and emotional distress). Congruently with clinical trials providing CBT-C in patients with chronic pain conditions and suffering from insomnia (Pigeon et al., 2012; Tang, Goodchild, & Salkovskis, 2012; Vitiello et al., 2013), Lami's study found that CBT-C yielded significant benefits in perceived sleep quality between pre- and post-treatment that were not observed in patients receiving CBT-P or usual medical care. Additionally, participants in CBT-C and CBT-P reported significant improvements concerning other relevant clinical variables.
PSG is increasingly being used to explore the impact of psychological treatments on sleep. Combining objective measures of sleep quality together with the subjective assessment might complement the comprehension of the treatment effects through the complex insomnia-related phenomena. Beyond the patients sleep quality perception, sleep objective measures assessed by PSG may provide sound information about processes in sleep homeostasis at baseline and post-treatment in CBT (Krystal & Edinger, 2008). This would make it possible to refine treatment components as a function of their efficacy in improving sleep physiology and improve patient selection criteria. In addition, misperception of sleep duration and quality appears to be common in FM patients (Okifuji & Hare, 2011). It therefore seems essential for controlled randomized trials of CBT interventions to include objective sleep measures along with self-reported measures to better understand the psychological processes related to distorted beliefs and worries about sleep in the FM population.
The aim of the present study was to compare the efficacy of CBT-P and CBT-C with regard to sleep quality in FM patients suffering from insomnia. Objective and subjective measures of sleep were used. We developed the following hypotheses: (1) CBT-P and CBT-C will lead to significant clinical improvements in self-reported sleep quality and PSG parameters related to sleep architecture; and (2) CBT-C will be better than CBT-P in improving sleep architecture and perceived sleep quality measured with a questionnaire.
MethodDesignA randomized controlled trial was performed. We used a between-subjects experimental design with repeated measures (Montero & León, 2007). The study was guided by the CONSORT statement (Moher et al., 2010).
Participants and procedureWomen with FM aged between 24 and 62 years were recruited from the Rheumatology Service and Pain Unit of Virgen de las Nieves University Hospital and from AGRAFIM (a local FM association), both in Granada, Spain. Selected patients were referred to the Clinical Psychology Unit of our university, where the clinical assessment and treatment took place. Patients were eligible for participation if they met the following inclusion criteria: (1) having been diagnosed with FM according to the American College of Rheumatology criteria (Wolfe et al., 1990); (2) having significant self-reported insomnia according to well-established diagnostic criteria, i.e., when the chief complaint is nonrestorative sleep or difficulty in initiating or maintaining sleep and the complaint continues for at least one month (the disturbance must occur at least three times a week for a month) (American Psychiatric Association, 2000); and (3) following a stable medication regime over the past month. Exclusion criteria were: (1) being pregnant; (2) having major medical conditions (e.g., inflammatory rheumatic diseases, uncontrolled endocrine disorders, cancer) including a clinical history of significant head injury or neurological disorder; (3) having a severe psychopathology such as major depression with suicide ideation, schizophrenia, personality disorder (American Psychiatric Association, 2000); (4) suffering from other sleep disorders that better explained insomnia (e.g., having an apnea-hypopnea index of 15 or more, having a periodic limb movement-related arousal index of 15 or more, or other previously diagnosed significant sleep disorders); (5) having severe dependence on hypnotic drugs, suggested by the use of a hypnotic agent in a higher than recommended dosage or repeated episodes of rebound insomnia on withdrawal; and (6) being enrolled in another physical or psychological treatment during the study period.
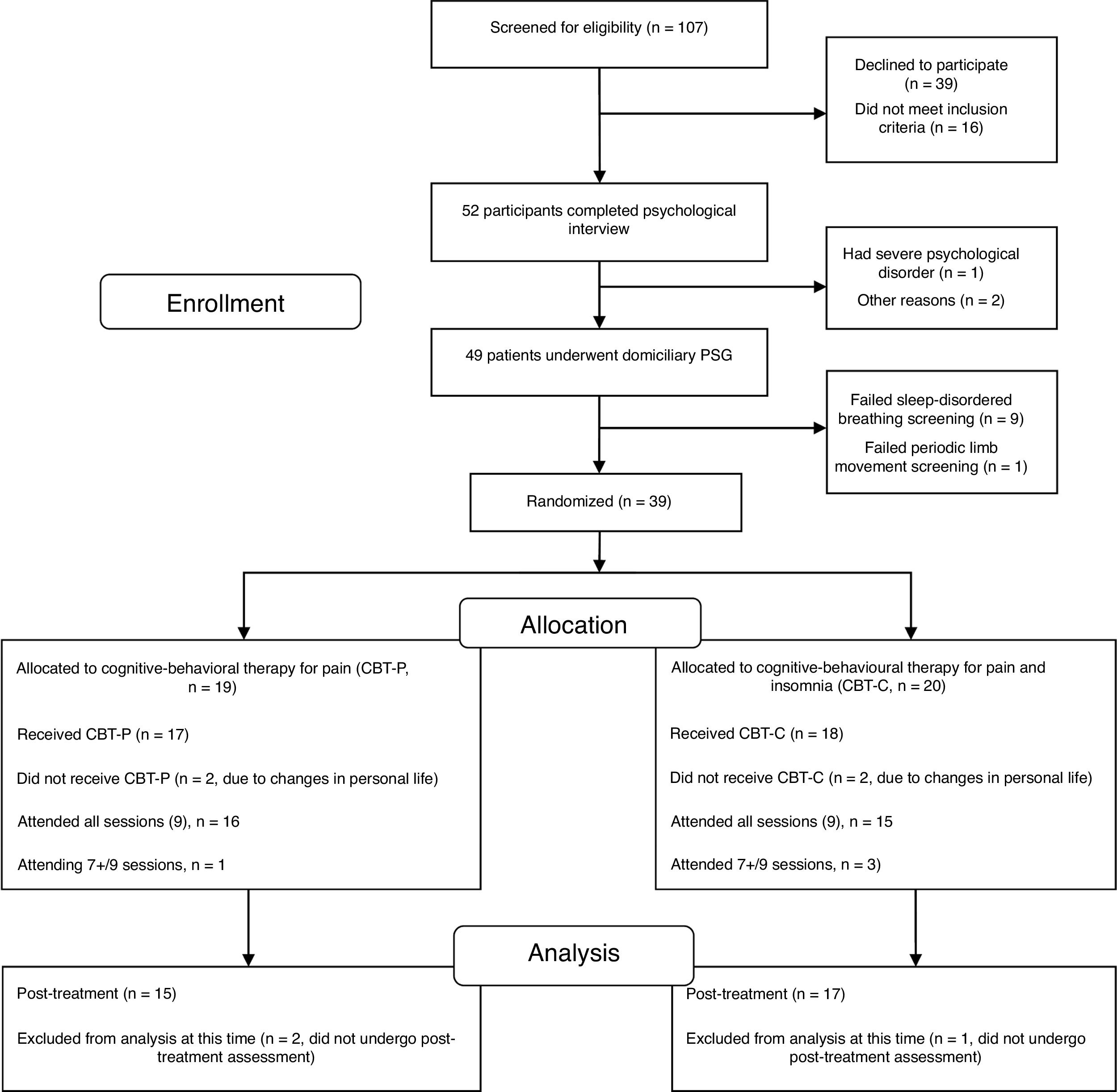
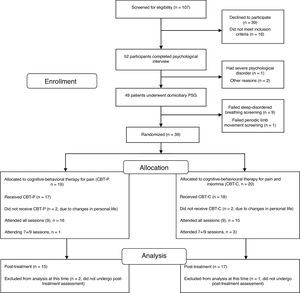
One hundred and seven candidates (FM women) who had received health care in the Rheumatology Service and Pain Unit in the past two years and current active members of the FM association were screened for eligibility via a brief telephone survey conducted by a psychologist. Eligible participants (52 FM women) underwent a medical examination by a rheumatologist and a psychological assessment by a psychologist in two sessions. The first session focused on a physical examination and collection of data on socio-demographic characteristics, onset and time course of symptoms, history of current insomnia and FM, past medical history, drug intake and psychological status. Three participants were ruled out during the interview because they did not meet the eligibility criteria for the study. After the interview, the remaining 49 FM women were given a set of questionnaires about pain, sleep, emotional distress and health status to be completed at home in one week. They received detailed instructions to complete the questionnaires and a psychologist was available over the telephone to answer any questions about completing the self-reported measures. The second session was devoted to completing additional data about clinical and psychological features and collecting the self-reported measures. FM participants who met the inclusion criteria and had undergone the evaluation sessions (n=49) were enrolled in a single night of ambulatory PSG within the next two weeks before therapy. Considering the inclusion criteria in the PSG analysis, 39 eligible participants were blindly randomized (1:1) to either the CBT-P (n=19) or the CBT-C (n=20) group using a computerized number generator. Of the 39 randomized participants, 15 in the CBT-P group and 17 in the CBT-C group completed the treatment and post-therapy assessment and were included in the analyses. A per protocol analysis was carried out in the present study, thus analysis was restricted to participants who finished the study. The participant flow diagram can be seen in Fig. 1.
Baseline and post-treatment assessments were performed between one and two weeks before and after therapy, respectively. Primary outcomes measures included perceived sleep quality measured by the Pittsburgh Sleep Quality Index (PSQI), and objective SE obtained by PSG. The remaining PSG variables were considered as secondary outcome measures.
This study was approved by the Ethics Committee of the University of Granada. All participants signed informed consent before engaging in the research protocol.
InstrumentsPerceived sleep quality was measured by PSQI. This instrument comprises 19 items that assess seven dimensions of sleep quality. Scores of these seven components are added to obtain a global score ranging from 0 to 21. Higher scores in this global measure indicate worse sleep quality. The PSQI has shown acceptable internal consistency (ranging between .67 and .81), sensitivity and specificity in the Spanish general population (Royuela & Macías, 1997).
Eligible participants were enrolled in a full-night PSG study at home that was recorded and digitized using the SomnoScreen TM plus (SomnoMedics, Germany). Gold electrodes were placed according to the International 10-20 system for electroencephalogram (EEG) recording. PSG signals included the following EEG channels: F3-A2, F4-A1, C3-A2, C4-A1, O1-A2, O2-A1, which were sampled at 128Hz; two electrooculogram channels; chin and bilateral anterior tibialis surface electromyography; two electrocardiographic leads; nasal and oral airflow (nasal pressure and thermistor); respiratory effort of thorax and abdomen (inductance plethysmography), body position; and finger oximetry. Two trained researchers blinded to group allocation hooked up the portable device and sensors used for PSG recording. The start and end of recordings were programmed according to each patient's sleep schedule to ensure higher ecological validity.
Sleep stages were blindly scored offline with DOMINO Software (DOMINO 2.2.0., supplied with the SomnoScreen TM plus) by a researcher trained in PSG scoring (G.P.). The criteria of Rechtschaffen and Kales (1968) were followed, based on 30 s epochs for hypnograms. We analyzed key sleep architectural variables derived from the PSG analyses included total time in bed (TIB, the period of time between bedtime and awakening in the morning), TST, which is the period of time between sleep onset and sleep offset, excluding all wake stages, and SE, that is TST/TIB. The following variables were also analyzed and expressed as a percentage of TST: time spent in Stages 1, 2, 3 and 4 (S1, S2, S3 and S4, respectively), non-rapid eye movement sleep (NREM), rapid eye movement sleep (REM). In addition, wake time was defined as the percentage of time spent awake from bedtime to final wake up time. We also considered the following variables in minutes: REML and slow-wave sleep latency (SWSL).
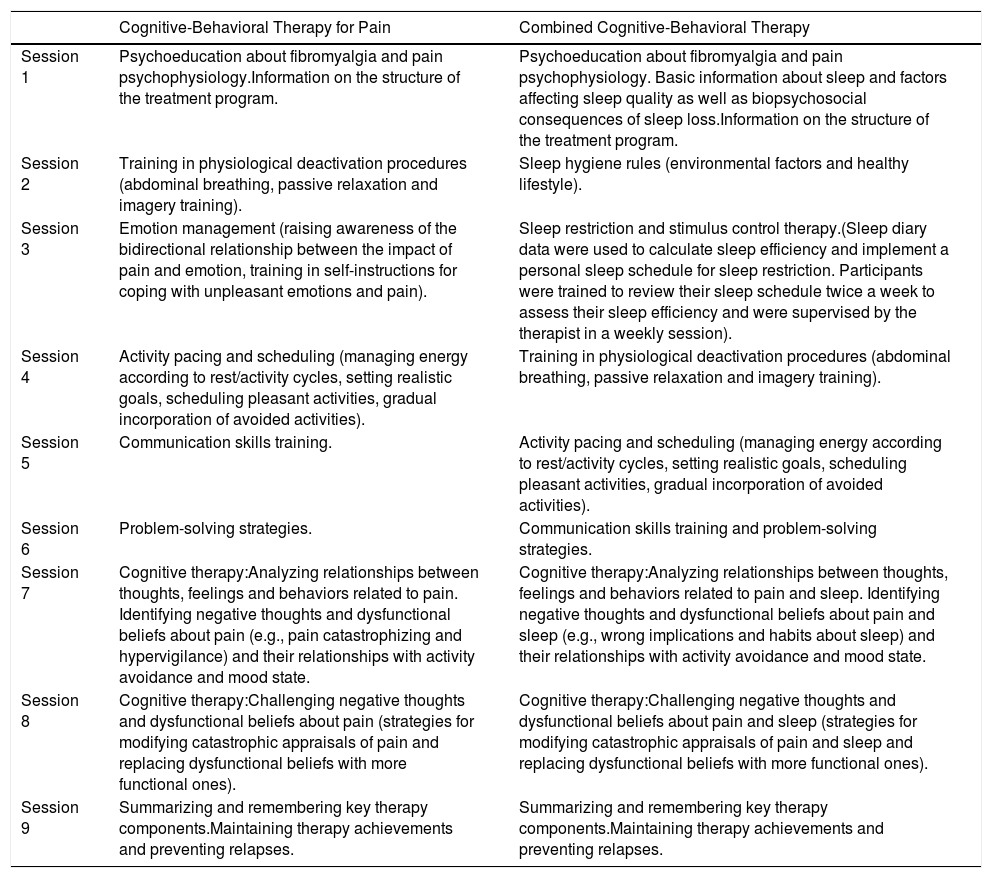
Intervention protocolStarting within one or two weeks after the baseline PSG and questionnaire assessment, participants in both therapy groups (CBT-P and CBT-C) received nine weekly sessions of therapy that lasted approximately 90minutes each (the contents of the CBT are shown on Table 1). The therapy was delivered by three female postdoctoral researchers in clinical psychology with expertise in CBT tailored to patients suffering from sleep disturbances and chronic pain conditions. E.M. was in charge of unique group in each treatment condition (i.e., CBT-P and CBT-C), the same applies for the other two therapists (i.e., M.P.M. and A.I.S.). This scheme of delivering therapy allowed us to control inherent personal features of each therapist when applying therapy. Treatment integrity was ensured through the use of a specific manual for each therapy modality that included detailed information about each session. These guides were created, designed and piloted by the three aforementioned researchers (i.e., E.M., M.P.M. and A.I.S.) under the umbrella of a research project with public funding. In addition, there were regular clinical meetings between the therapists and the research group, and video recordings to monitor the implementation of the intervention. Sessions were conducted in groups of five to seven participants. Self-reported measures were administered by a psychologist who was blinded to group assignment. FM women were instructed to avoid making changes in their usual medication regimen or enrolling in other non-pharmacological interventions during the study.
Components of Cognitive-Behavioral Therapy for Pain and Combined Cognitive-Behavioral Therapy.
| Cognitive-Behavioral Therapy for Pain | Combined Cognitive-Behavioral Therapy | |
|---|---|---|
| Session 1 | Psychoeducation about fibromyalgia and pain psychophysiology.Information on the structure of the treatment program. | Psychoeducation about fibromyalgia and pain psychophysiology. Basic information about sleep and factors affecting sleep quality as well as biopsychosocial consequences of sleep loss.Information on the structure of the treatment program. |
| Session 2 | Training in physiological deactivation procedures (abdominal breathing, passive relaxation and imagery training). | Sleep hygiene rules (environmental factors and healthy lifestyle). |
| Session 3 | Emotion management (raising awareness of the bidirectional relationship between the impact of pain and emotion, training in self-instructions for coping with unpleasant emotions and pain). | Sleep restriction and stimulus control therapy.(Sleep diary data were used to calculate sleep efficiency and implement a personal sleep schedule for sleep restriction. Participants were trained to review their sleep schedule twice a week to assess their sleep efficiency and were supervised by the therapist in a weekly session). |
| Session 4 | Activity pacing and scheduling (managing energy according to rest/activity cycles, setting realistic goals, scheduling pleasant activities, gradual incorporation of avoided activities). | Training in physiological deactivation procedures (abdominal breathing, passive relaxation and imagery training). |
| Session 5 | Communication skills training. | Activity pacing and scheduling (managing energy according to rest/activity cycles, setting realistic goals, scheduling pleasant activities, gradual incorporation of avoided activities). |
| Session 6 | Problem-solving strategies. | Communication skills training and problem-solving strategies. |
| Session 7 | Cognitive therapy:Analyzing relationships between thoughts, feelings and behaviors related to pain. Identifying negative thoughts and dysfunctional beliefs about pain (e.g., pain catastrophizing and hypervigilance) and their relationships with activity avoidance and mood state. | Cognitive therapy:Analyzing relationships between thoughts, feelings and behaviors related to pain and sleep. Identifying negative thoughts and dysfunctional beliefs about pain and sleep (e.g., wrong implications and habits about sleep) and their relationships with activity avoidance and mood state. |
| Session 8 | Cognitive therapy:Challenging negative thoughts and dysfunctional beliefs about pain (strategies for modifying catastrophic appraisals of pain and replacing dysfunctional beliefs with more functional ones). | Cognitive therapy:Challenging negative thoughts and dysfunctional beliefs about pain and sleep (strategies for modifying catastrophic appraisals of pain and sleep and replacing dysfunctional beliefs with more functional ones). |
| Session 9 | Summarizing and remembering key therapy components.Maintaining therapy achievements and preventing relapses. | Summarizing and remembering key therapy components.Maintaining therapy achievements and preventing relapses. |
CBT-P program. This therapy modality focused on psychoeducation on pain physiology, physiological deactivation, goal setting, increasing activity level and social skills, problem-solving and coping with dysfunctional beliefs and rumination related to pain, based on the pain-related fear and avoidance model (Vlaeyen, Crombez, & Linton, 2016).
CBT-C program. This therapy included many components of CBT-P (i.e., relaxation training, development of social skills, problem-solving and retraining the cognitive processes that maintain chronic pain). In addition, based on the strong evidence supporting the application of CBT models to insomnia in patients with chronic pain (Tang, 2018), this intervention was designed to target the factors that perpetuate sleep problems, considering Harvey's cognitive model of insomnia (Harvey, 2002) and the evidence-based practice parameters for the psychological treatment of the insomnia (Morin & Benca, 2017).
Data analysisAnalyses were performed using the SPSS-20.0 statistical package (SPSS, Inc., Chicago, IL, USA). Probabilities less than .05 were used as the level of significance. Data were summarized as the mean, standard deviation and percentages. Differences between both treatment groups in socio-demographic and clinical characteristics and outcome measures at baseline were compared using t-tests or Chi-square statistics. The effects of each treatment condition were analyzed by examining changes in PSG parameters and perceived sleep quality using 2 (Group; CBT-P vs. CBT-C)×2 (Time; Pre-treatment vs. Post-treatment) ANOVAs. Greenhouse-Geiser correction was computed. Additionally, unpaired and paired two-sample Student's t tests were computed. Effect sizes were calculated for statistical significance using partial η2 and Cohen's d. According to Cohen's guidelines (Cohen, 1998), d=0.20 is a small effect, 0.50 is a medium effect and 0.80 is a large effect; and η2=0.01 is a small effect, 0.06 is a medium effect, and 0.14 is a large effect.
Given an anticipated average effect size of Cohen's d=1.26 in sleep parameters (see review by Martínez, Miró, & Sánchez, 2014), a p-value<.05, and a desired statistical power of .90, the minimum required sample size for the study was 30 patients (n=15 subjects per group).
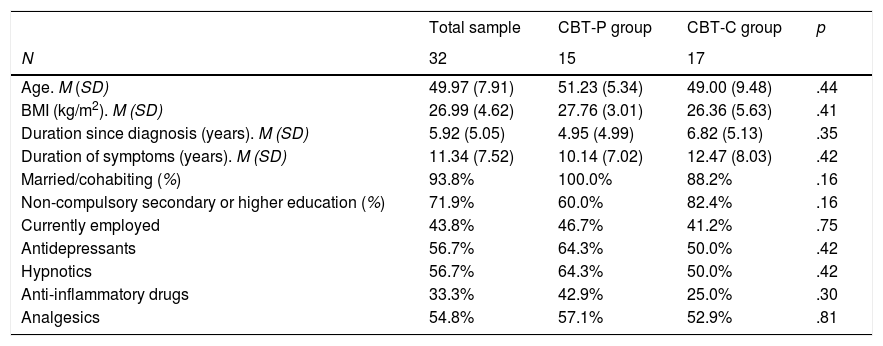
ResultsCharacteristics of participantsAt baseline, the CBT-P and CBT-C groups were statistically similar in all the socio-demographic and clinical variables measured (all p's>.16, see Table 2). The mean age of FM participants was 49.97 years (SD=7.91) and 93.8% of participants in both groups were married or cohabited with a partner. The percentage of participants with non-compulsory secondary or higher education was 71.9%. More than half of participants (58.8%) had an inactive work situation (i.e., unemployed, temporary disability or permanent disability). Concerning clinical aspects, the mean duration of FM symptoms was 11.34 years (SD=7.52) in the total sample, and approximately 50% of participants were taking a stable dose of antidepressants, hypnotics and analgesics. The rest of patient did not take any antidepressants or hypnotics and analgesics were rarely consumed in this group.
Demographic and clinical features of FM participants.
| Total sample | CBT-P group | CBT-C group | p | |
|---|---|---|---|---|
| N | 32 | 15 | 17 | |
| Age. M (SD) | 49.97 (7.91) | 51.23 (5.34) | 49.00 (9.48) | .44 |
| BMI (kg/m2). M (SD) | 26.99 (4.62) | 27.76 (3.01) | 26.36 (5.63) | .41 |
| Duration since diagnosis (years). M (SD) | 5.92 (5.05) | 4.95 (4.99) | 6.82 (5.13) | .35 |
| Duration of symptoms (years). M (SD) | 11.34 (7.52) | 10.14 (7.02) | 12.47 (8.03) | .42 |
| Married/cohabiting (%) | 93.8% | 100.0% | 88.2% | .16 |
| Non-compulsory secondary or higher education (%) | 71.9% | 60.0% | 82.4% | .16 |
| Currently employed | 43.8% | 46.7% | 41.2% | .75 |
| Antidepressants | 56.7% | 64.3% | 50.0% | .42 |
| Hypnotics | 56.7% | 64.3% | 50.0% | .42 |
| Anti-inflammatory drugs | 33.3% | 42.9% | 25.0% | .30 |
| Analgesics | 54.8% | 57.1% | 52.9% | .81 |
Note. p values refer to Chi-square tests for dichotomous variables and t-tests for continuous variables. CBT-C=Cognitive-behavioral therapy for insomnia and pain; CBT-P=Cognitive-behavioral therapy for pain.
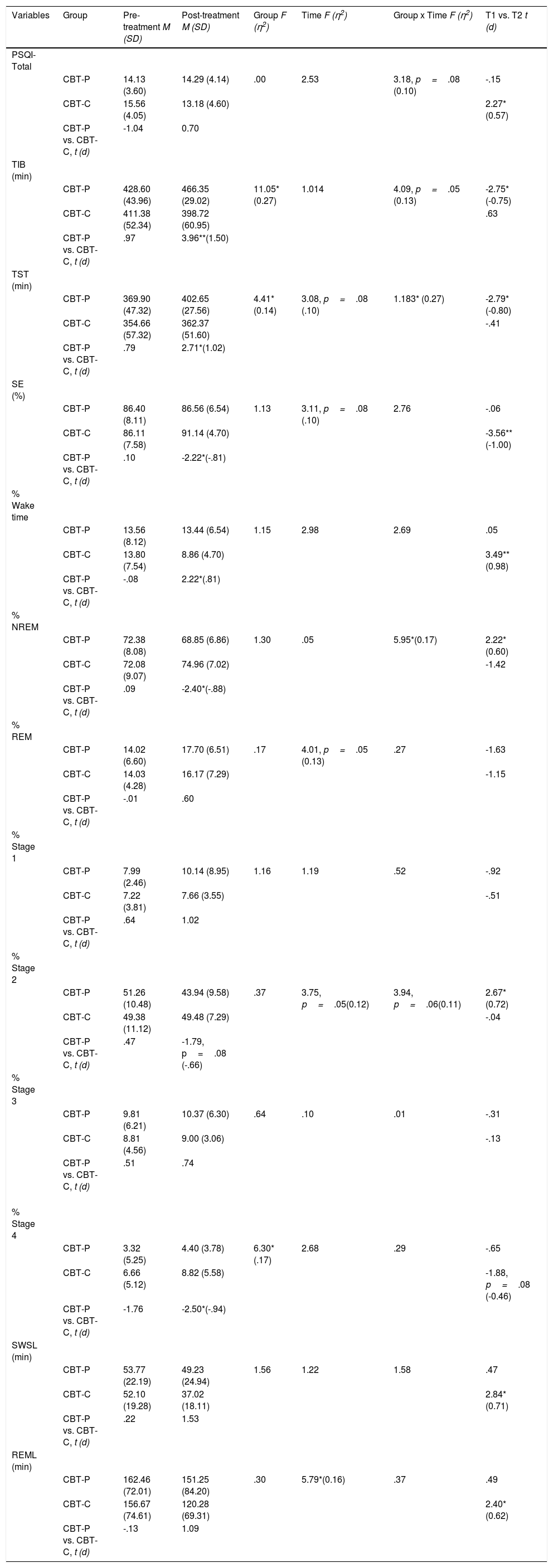
As can be seen on Table 3, ANOVA for the self-reported measure indicated effects close to significance of Group×Time in sleep quality (p=.08). The CBT-C group showed a small but significant decrease in the PSQI score from 15.56±4.05 to 13.18±4.60 (p<.05).
Changes in self-reported sleep quality and PSG parameters in the treatment groups.
| Variables | Group | Pre-treatment M (SD) | Post-treatment M (SD) | Group F (η2) | Time F (η2) | Group x Time F (η2) | T1 vs. T2 t (d) |
|---|---|---|---|---|---|---|---|
| PSQI-Total | |||||||
| CBT-P | 14.13 (3.60) | 14.29 (4.14) | .00 | 2.53 | 3.18, p=.08 (0.10) | -.15 | |
| CBT-C | 15.56 (4.05) | 13.18 (4.60) | 2.27* (0.57) | ||||
| CBT-P vs. CBT-C, t (d) | -1.04 | 0.70 | |||||
| TIB (min) | |||||||
| CBT-P | 428.60 (43.96) | 466.35 (29.02) | 11.05*(0.27) | 1.014 | 4.09, p=.05 (0.13) | -2.75*(-0.75) | |
| CBT-C | 411.38 (52.34) | 398.72 (60.95) | .63 | ||||
| CBT-P vs. CBT-C, t (d) | .97 | 3.96**(1.50) | |||||
| TST (min) | |||||||
| CBT-P | 369.90 (47.32) | 402.65 (27.56) | 4.41*(0.14) | 3.08, p=.08 (.10) | 1.183* (0.27) | -2.79*(-0.80) | |
| CBT-C | 354.66 (57.32) | 362.37 (51.60) | -.41 | ||||
| CBT-P vs. CBT-C, t (d) | .79 | 2.71*(1.02) | |||||
| SE (%) | |||||||
| CBT-P | 86.40 (8.11) | 86.56 (6.54) | 1.13 | 3.11, p=.08 (.10) | 2.76 | -.06 | |
| CBT-C | 86.11 (7.58) | 91.14 (4.70) | -3.56**(-1.00) | ||||
| CBT-P vs. CBT-C, t (d) | .10 | -2.22*(-.81) | |||||
| % Wake time | |||||||
| CBT-P | 13.56 (8.12) | 13.44 (6.54) | 1.15 | 2.98 | 2.69 | .05 | |
| CBT-C | 13.80 (7.54) | 8.86 (4.70) | 3.49**(0.98) | ||||
| CBT-P vs. CBT-C, t (d) | -.08 | 2.22*(.81) | |||||
| % NREM | |||||||
| CBT-P | 72.38 (8.08) | 68.85 (6.86) | 1.30 | .05 | 5.95*(0.17) | 2.22*(0.60) | |
| CBT-C | 72.08 (9.07) | 74.96 (7.02) | -1.42 | ||||
| CBT-P vs. CBT-C, t (d) | .09 | -2.40*(-.88) | |||||
| % REM | |||||||
| CBT-P | 14.02 (6.60) | 17.70 (6.51) | .17 | 4.01, p=.05 (0.13) | .27 | -1.63 | |
| CBT-C | 14.03 (4.28) | 16.17 (7.29) | -1.15 | ||||
| CBT-P vs. CBT-C, t (d) | -.01 | .60 | |||||
| % Stage 1 | |||||||
| CBT-P | 7.99 (2.46) | 10.14 (8.95) | 1.16 | 1.19 | .52 | -.92 | |
| CBT-C | 7.22 (3.81) | 7.66 (3.55) | -.51 | ||||
| CBT-P vs. CBT-C, t (d) | .64 | 1.02 | |||||
| % Stage 2 | |||||||
| CBT-P | 51.26 (10.48) | 43.94 (9.58) | .37 | 3.75, p=.05(0.12) | 3.94, p=.06(0.11) | 2.67*(0.72) | |
| CBT-C | 49.38 (11.12) | 49.48 (7.29) | -.04 | ||||
| CBT-P vs. CBT-C, t (d) | .47 | -1.79, p=.08 (-.66) | |||||
| % Stage 3 | |||||||
| CBT-P | 9.81 (6.21) | 10.37 (6.30) | .64 | .10 | .01 | -.31 | |
| CBT-C | 8.81 (4.56) | 9.00 (3.06) | -.13 | ||||
| CBT-P vs. CBT-C, t (d) | .51 | .74 | |||||
| % Stage 4 | |||||||
| CBT-P | 3.32 (5.25) | 4.40 (3.78) | 6.30*(.17) | 2.68 | .29 | -.65 | |
| CBT-C | 6.66 (5.12) | 8.82 (5.58) | -1.88, p=.08 (-0.46) | ||||
| CBT-P vs. CBT-C, t (d) | -1.76 | -2.50*(-.94) | |||||
| SWSL (min) | |||||||
| CBT-P | 53.77 (22.19) | 49.23 (24.94) | 1.56 | 1.22 | 1.58 | .47 | |
| CBT-C | 52.10 (19.28) | 37.02 (18.11) | 2.84*(0.71) | ||||
| CBT-P vs. CBT-C, t (d) | .22 | 1.53 | |||||
| REML (min) | |||||||
| CBT-P | 162.46 (72.01) | 151.25 (84.20) | .30 | 5.79*(0.16) | .37 | .49 | |
| CBT-C | 156.67 (74.61) | 120.28 (69.31) | 2.40*(0.62) | ||||
| CBT-P vs. CBT-C, t (d) | -.13 | 1.09 |
Note. CBT-C=Cognitive-behavioral therapy for insomnia and pain; CBT-P=Cognitive-behavioral therapy for pain; NREM=non-rapid eye movement sleep; PSQI-Total=global score in Pittsburgh Sleep Quality Index; REM=rapid eye movement sleep; REML=REM sleep latency; SE=sleep efficiency; SWSL=slow-wave sleep latency; T1=Pre-treatment; T2=Post-treatment; TIB=total time in bed; TST=total sleep time; Wake time=time spent awake from bedtime to final wake up time; *p<.05 **p<.01.
At baseline, the CBT-P and CBT-C groups did not show any statistically significant differences regarding PSG variables (all p's>.05). As shown in Table 3, ANOVAs for objective sleep variables showed a significant effect of Group in TIB, TST and percentage of S4. Significant effect of Time was found in REML and close to significant in TST, SE, percentage of REM and S2. Significant effect of Group×Time was observed in TST and percentage of NREM. In the case of TIB and S2, that kind of effect was marginally significant.
Participants in CBT-P condition showed a greater and moderate to large significant increase in TIB and TST from pre- to post-treatment according Cohen's d (-0.75 and -0.80, respectively). In addition, within-group comparisons showed significant changes through these parameters (p<.05; Table 3). In contrast, participants in the CBT-C group did not show any significant changes in either measure. With regard to SE, patients in the CBT-C condition showed statistically significant improvement in SE according to within-subject comparisons (i.e., SE increased from 86.11±7.58% to 91.14±4.70%; p<.01).
As regards the variables related to the hypnogram structure, we did not observe any significant effect according ANOVA in percentage of wake time in participants. However, within-group comparisons revealed a significant reduction in this variable through CBT-C group (i.e., wake time decreased from 13.80±7.54% to 8.86±4.70%; p<.01).
Significant effects of Group×Time were found in percentage of NREM and were related to a decrease from pre- to post-treatment in the CBT-P group (p<.05).
Light sleep significantly decreased from pre- to post-treatment in the CBT-P group but not in the CBT-C group. This change was due to a moderate reduction of S2 from 51.26±10.48% to 43.94±9.58% (p<.05), although ANOVA only showed significant effect close to significance of Time and Group×Time in this parameter.
Concerning deep sleep a significant effect of Group was found in S4, but we only observed a marginally significant within-subject improvement in CBT-C participants in this restorative sleep stage (p=.08).
As shown on Table 3, scarce significant effects were found in the ANOVA of SWSL and REML. Thus, only an effect of Time was observed in the latter. However, within-subject comparisons showed that latencies in SWS and REM onset exhibited a moderate reduction in the CBT-C condition (SWSL: 52.10±19.28% to 37.02±18.11%; p<.05 and REML: 156.67±74.61% to 120.28±69.31; p<.05). This improvement in sleep architecture was also observed in the CBT-P group, although it did not reach statistical significance.
DiscussionThis is the first study to compare the efficacy of a new approach of CBT-C with that of CBT-P in FM patients on PSG parameters. Although results related to effect of Group was limited in this study, in support of our hypothesis, we observed through the within-subject comparisons that CBT-C improved sleep quality across PSG parameters (i.e., higher SE, reduced wake time, increased S4, lower REM and SWS latencies) and self-reported sleep quality measured with the PSQI. In the CBT-P condition, a moderate reduction in light sleep was associated with a significant increase of TST and TIB.
On the basis of the results obtained in self-reported sleep quality and objective sleep measures in participants in the CBT-P and CBT-C conditions, it is not surprising to note that gains in sleep parameters from pre- to post-treatment in the CBT-P group were not concordant with better PSQI scores than those of CBT-C participants in self-reported sleep quality. Participants in this latter treatment condition reduced their percentage of wake time, SWSL and REML and increased their deep sleep without increasing TIB or TST. In line with these objective improvements associated with restorative sleep, SE was significantly higher after treatment, and patients in the CBT-C condition perceived their sleep as being more restorative. In addition, improvements in PSG parameters were smaller in CBT-P participants than in CBT-C participants. Although the former obtained higher TST than CBT-C participants and less light sleep from pre- to post-treatment, this improvement was associated with higher TIB and not with a significant change in SE. An increase in TIB without solid improvements in other parameters of sleep architecture can be considered as a debilitating factor in patients with insomnia, considering the working principles of sleep restriction, which is aimed at reducing time in bed to time spent asleep in order to increase sleep pressure and consolidate sleep (Morin & Benca, 2017).
Results in the present study are in keeping with those ones obtained by our research group (Lami, Martínez, Miró, Sánchez, Prados et al., 2018) in a randomized clinical trial carried out in a sample of women suffering from FM after applying the same CBT-C and CBT-P interventions. Thus, Lami reported significant improvements through several dimensions of PSQI in the CBT-C group but did not find sleep-related benefits in participants in the CBT-P condition. In addition, our results regarding sleep-related outcome measures fit well with three previous clinical trials conducted in a chronic pain population with comorbid insomnia. Tang et al. (2012) individually delivered a CBT-C in a sample of 10 patients with insomnia and chronic pain. These authors found that, compared to the control group, the CBT-C group showed significant and positive changes in insomnia symptoms and significant improvements and reduction in sleep onset latency and wake after sleep onset measured by sleep log. Pigeon et al. (2012) conducted a pilot clinical trial with 21 chronic pain patients with insomnia, who were randomized into four study arms: CBT-P (n=5), CBT-I (n=6), CBT-C (n=6), or a waiting-list control condition (n=4). As regards sleep-related measures, participants in the CBT-P and control groups did not show any significant gains; by contrast, participants in the CBT-I group showed better SE and a reduction in total wake time after treatment, measured across sleep diary values; CBT-C participants also exhibited this latter improvement (82.2min vs. 31.5min after treatment) but failed to show a significant SE increase. This is in contrast with participants’ SE gains observed in the CBT-C condition in our study and the aforementioned study conducted by Tang et al. (2012). The differences may be due to the low statistical power of Pigeon's study because of its very small sample size (i.e., six participants in the CBT-C group). In that study, however, participants in both CBT-I and CBT-C groups outperformed those in the CBT-P group in reducing insomnia severity. In another hand, the CBT-C conducted by Vitiello et al. (2013) among older adults with osteoarthritis pain and insomnia symptoms resulted in significant reductions in self-reported insomnia than either CBT-P or the education control. In contrast with our study, SE measured by actigraphy showed improvement in the CBT-P condition in the Vitiello's study.
Caution should be used when generalizing the results from this study due to of its limitations. The sample size was kept small for this pilot study and there were only few significant interaction and group effects. This is probably all down to a problem to statistical power in our study. Nevertheless, within group effects of the CBT-C group were larger so this informs us that indeed the combined approach may be better. We did not include a control group that received usual care or an attention-placebo condition, which makes it difficult to distinguish between the specific effects of each therapy modality. Only two-time points (baseline and post-treatment) were selected for assessing FM participants, so this study does not provide information on the temporal maintenance of the treatment effect. The small sample size in our study did not make it possible to stratify data in order to analyze plausible associations concerning confounding variables. In this regard, one of our main concerns was related to medication consumption. Although we ruled out participants with a severe dependence on psychotropic medications, the use of multiple drugs was an added complication in the study of PSG variables due to changes in sleep neurophysiology because of the side effects of such drugs. Future studies in larger samples of FM patients are needed to improve reliability and validity in the outcome data. In addition, these studies should implement long-term follow-up in order to test CBT benefits over the time on subjective and objective sleep measures. Finally, we have to consider the lack of an approach that emphasizes the fit of the patient and treatment within the context of individuals factors that impact the efficacy of psychotherapy (Beutler, Someah, Kimpara, & Miller, 2016).
ConclusionsWe found that CBT-C outperformed CBT-P regarding sleep objective measures: CBT-C participants gained higher SE, reduced their wake time and latency-related variables, and increased deep sleep. These improvements related to refreshing sleep were associated with a positive change in self-reported sleep quality in patients in the CBT-C group. The results of our study and previous encouraging findings on preliminary modalities of CBT-C support the need for large-scale randomized controlled trials in order to select the most powerful components of these therapies and enhance the validity of results obtained in these new CBT approaches.
FundingThis study was financially supported by the Spanish Ministry of Science and Innovation through project PSI2009-13765.
The authors thank all patients who took part in the study and F. ChouChou for his critical reading of the manuscript.