The identification of subepithelial lesions is a relatively frequent finding at endoscopy however their natural history is not well known. Our aim was to analyze the role of endoscopic ultrasound (EUS) in the diagnostic approach of subepithelial lesions of the upper gastrointestinal tract.
MethodsRetrospective study which included 324 patients undergoing upper radial EUS for evaluation of subepithelial lesions from 2008 to 2014. The EUS features, presumptive diagnosis and management decision were analyzed.
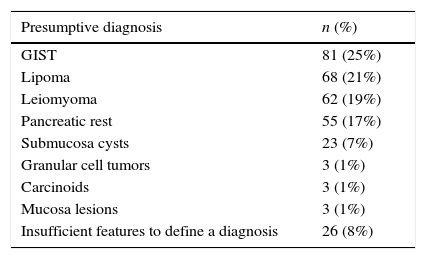
Results324 patients included, 60% with gastric subepithelial lesions, 28% oesophageal and 12% from the duodenum. Based on EUS features the presumptive diagnosis was: 25% gastrointestinal stromal tumor, 21% lipoma, 19% leiomyoma, 17% pancreatic rest, 7% submucosa cysts, 1% granular cell tumors, 1% carcinoids, 1% mucosa lesions and 8% not defined. After EUS the suggested approach was no follow-up in 45%, follow-up with re-examination with EUS in 35% and additional tissue sampling or endoscopic/surgical resection in 20%. The latter was based on EUS features of risk at the diagnosis (53%), such as size ≥2cm, hypoechogenicity, heterogeneity, lobulation, calcifications, cystic component and regional adenopathies; impossibility to define a presumptive diagnosis (39%) or EUS features change at follow-up (8%). The combination of multiple features correlated with a higher probability of this recommended strategy (p<0.001), in 100% when 4 or more features were present. Among the 33 patients who underwent fine needle aspiration, in 66% the result was inconclusive. During follow-up, none of the patients who were managed with surveillance radial EUS presented complications.
ConclusionEUS is the method of choice in the study of subepithelial lesions of the upper gastrointestinal tract, in most cases defining a diagnosis. The need for a definitive diagnosis or therapeutic approaches can be based on ultrasound risk features, presented, in the majority, at presentation. This study shows that EUS is capable of safely and accurately define those subepithelial lesions that can be managed only with surveillance ultrasound while waiting for better results with fine needle aspiration.
As lesões subepiteliais (LS) são achados frequentes, particularmente no trato digestivo alto. Incluem um grande número de entidades, algumas com potencial maligno, cuja história natural não é totalmente conhecida e o adequado manejo controverso. O nosso objetivo foi analisar o papel da ultrassonografia endoscópica (EUS) na abordagem diagnóstica das LS do trato digestivo alto.
MaterialEstudo retrospetivo de doentes consecutivos submetidos a EUS alta para estudo diagnóstico de LS entre 2008-2014. Analisadas as características ultrassonográficas e a orientação definida.
ResultadosIncluídos 324 doentes, 60% com LS gástrica, 28% esofágica e 12% duodenal. O diagnóstico segundo as características ultrassonográficas foi: GIST 25%, lipoma 21%, leiomioma 19%, pâncreas ectópico 17%, quisto submucosa 7%, tumor células granulares 1%, carcinoide 1%, lesões da mucosa 1% e em 8% indefinido. A orientação proposta após EUS foi em 35% de vigilância e em 20% intervenção diagnóstica/terapêutica (punção aspirativa agulha fina - PAAF ou ressecção cirúrgica/endoscópica). Esta última por características EUS de agressividade no diagnóstico (53%), diagnóstico indefinido em EUS (39%) ou alterações de tamanho em EUS subsequentes (8%). As características EUS associadas significativamente à decisão de PAAF/ressecção foram: tamanho, hipoecogenicidade, heterogeneidade, bordos irregulares, calcificações, componente quístico e adenopatias. A associação de várias características associou-se a maior percentagem de doentes submetidos a esta abordagem (p<0,001), em 100% quando 4 ou mais critérios. Nos 33 doentes submetidos a PAAF, em 66% o diagnóstico foi inconclusivo. Em todas as LSE orientadas para vigilância não se verificaram intercorrências neste período.
ConclusãoA EUS é o método de eleição no estudo das LS do trato digestivo alto, na maioria definindo um diagnóstico. A abordagem diagnóstica definitiva ou terapêutica, pode ser baseada na associação de características ultrassonográficas de agressividade, apresentadas na maioria logo no diagnóstico inicial. Foi demonstrada segurança nas LSE orientadas para vigilância e a necessidade de aguardar melhores resultados com PAAF.
The identification of a mass covered by normal-appearing mucosa is a relatively frequent finding at endoscopy, approximately 0.3% of all upper GI endoscopies.1–3 These masses, more correctly referred as subepithelial lesions (SL), can arise from within any layer of the gastrointestinal wall.1 They occur more frequently in the stomach, but are also common in the esophagus and duodenum.4 Common examples include gastrointestinal stromal tumor (GIST), leiomyoma, leiomyosarcoma, carcinoid, granular cell tumors, lipoma, pancreatic rest, schwannoma, etc.5 The majority of subepithelial lesions are benign at the time of diagnosis, with fewer than 15% found to be malignant at presentation. However many of these lesions have the potential for malignant transformation.6
The differential diagnosis of these lesions is broad and ranges from clinically insignificant to malignant conditions, which underlines the importance of an accurate diagnosis. Endoscopy alone is not reliable for the definitive diagnosis of subepithelial lesions and frequently they are incidentally detected, not explaining the indication for endoscopic examination.7
Histology is the “gold standard” to differentiate between the different types of subepithelial lesions, however this evaluation can only be obtained through invasive techniques such as endoscopic mucosal resection, fine-needle aspiration (EUS-FNA), or surgical resection.8 The role of less invasive techniques such as endoscopic ultrasound (EUS) remains unclear. EUS has the ability to differentiate extramural compression from intramural growth, determine layer of origin, provides information regarding echogenicity and vascularity, accurately size the lesion and evaluate regional lymphadenopathies.4,5 Some authors suggest that the diagnostic information on the subepithelial lesions provided by EUS helps in deciding whether a lesion should be removed or followed in situ.9,10
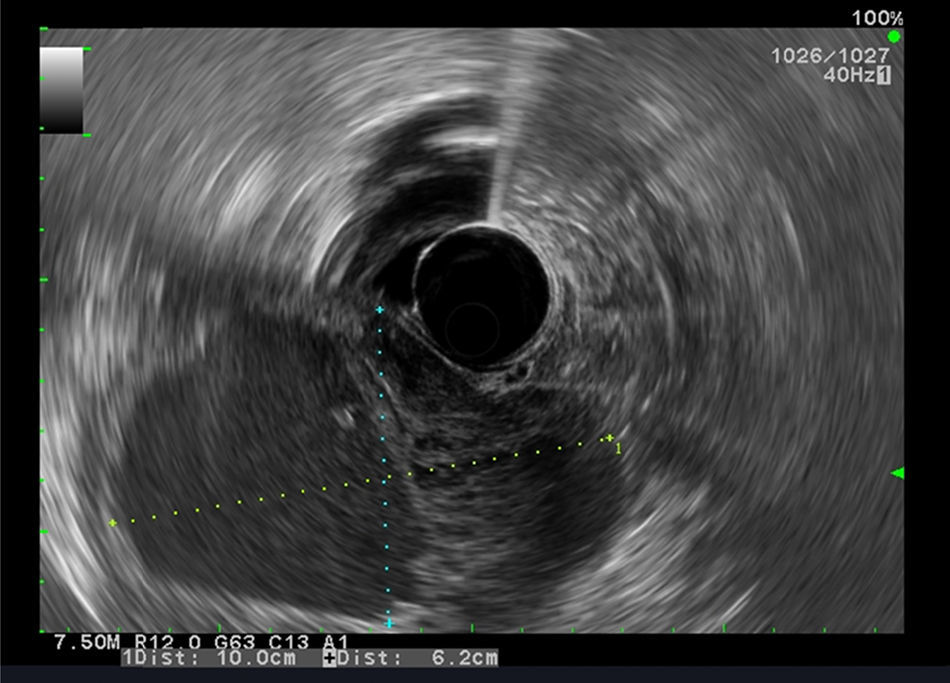
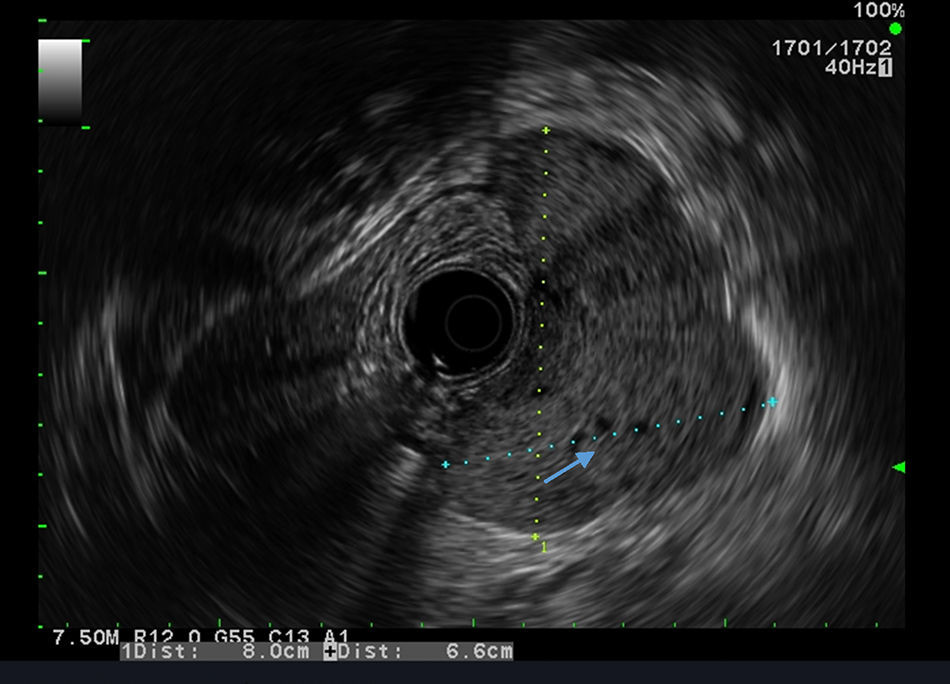
Our aim was to analyze the role of EUS in the diagnostic approach of subepithelial lesions of the upper gastrointestinal tract (Figs. 1 and 2).
Between January 2008 and October 2014, 324 consecutive patients were referred for EUS for further evaluation of a suspected subepithelial lesion discovered at upper gastrointestinal endoscopy. All cases of extrinsic compression were excluded and only the patients with confirmed SL were enrolled. For each patient the following information was recorded: demographics, EUS features, presumptive diagnosis assessed by EUS and management decision after EUS (either no follow-up, surveillance with EUS or additional tissue sampling with EUS-FNA or endoscopic or surgical resection). Based on other studies,11,12 the interval selected in our practice was 6 months and 1 year, and if the lesion is unchanged for 2 consecutive yearly follow-up examinations with EUS we extended the length between surveillance examination. The following EUS features were analyzed: site and size of the lesion, wall layer involved, echogenicity, heterogeneity, outer margins, presence of calcifications or cystic component and regional adenopathies.
In all patients careful EUS was performed using radial echoendoscopes at a scanning frequency of 5–10MHz (GF-UE160-AL5, Olympus ®; Tokyo, Japan; ultrasound system: Aloka ® ProSound Alpha 10). High-frequency catheter probes were not available. All procedures were performed on an outpatient basis, by one of two experienced endosonographers using intravenous propofol sedation. Written informed consent for EUS was obtained for all patients. All EUS-FNA were performed in two other hospitals by experienced endosonographers and there was no on-site cytopathologist. The statistical analysis was performed using SPSS software version 20.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were reported as number and percentage for categorical variables and median (Mdn) and interquartile range (IQR) for quantitative variables. As the sample did not follow a normal distribution, the Mann–Whitney test was used to test the differences between size lesions which were submitted or not to additional tissue sampling procedures. The relation between each ultrasonographic features and the decision to obtain biological material were investigated by chi-square analysis. EUS predictors of further therapeutic or diagnostic procedures were identified using a binary logistic regression analysis. Statistical significance was set at p<0.05.
3ResultsDuring the study period, 324 patients were included, 60% with gastric subepithelial lesions, 28% oesophageal and 12% from the duodenum. The mean follow-up after EUS was 41±21 months. Based on EUS features the presumptive diagnosis was: 25% GISTs, 21% lipoma, 19% leiomyoma, 17% pancreatic rest, 7% submucosa cysts, 1% granular cell tumors, 1% carcinoids, 1% mucosa lesions and 8% not defined (Table 1).
After EUS the suggested approach was no follow-up in 45% (namely lipomas, pancreatic rests and submucosal cysts), in 35% follow-up with re-examination with EUS and in 64 patients (20%) EUS-FNA or endoscopic/surgical resection (including mucosa lesions). The latter was based on EUS features of risk at the diagnosis (53%), impossibility to define a presumptive diagnosis (39%) or EUS features change in follow-up, namely significant size increase (8%, only 5 patients).
The lesion size had a median of 10.2 (IQR 7.5–13.8mm). Those which were submitted to additional tissue sampling procedures were larger (Mdn 16.9; IRQ 12.4–22.7) than those which did not require follow-up or that were surveyed with subsequent EUS (Mdn 9.2; IRQ 6.8–11.9), U=13.6; W=15.6; p<0.001.
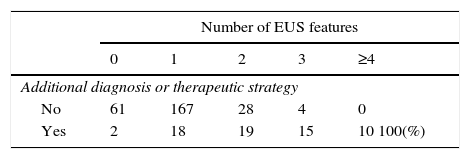
The EUS features considered of risk and used on the decision for additional diagnostic or therapeutic strategy were: large size (≥20mm) (p<0.001), hypoechogenicity (p<0.001), heterogeneity (p<0.001), lobulation (p<0.001), calcifications (p<0.001), cystic component (p<0.013) and regional adenopathies (p<0.038). The combination of multiple features correlated with a higher probability of this recommended strategy (p<0.001), in 100% when 4 or more features were present (Table 2).
Decision for additional diagnostic or therapeutic strategy based on the number of EUS features considered of risk (size, hypoechogenicity, heterogeneity, lobulation, calcifications, cystic component and regional adenopathies).
| Number of EUS features | |||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ≥4 | |
| Additional diagnosis or therapeutic strategy | |||||
| No | 61 | 167 | 28 | 4 | 0 |
| Yes | 2 | 18 | 19 | 15 | 10 100(%) |
p<0.001.
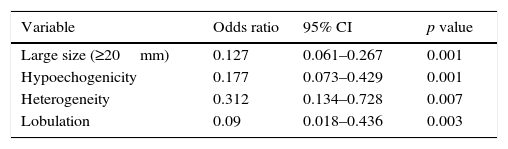
Logistic regression analysis identified as large size (≥20mm) (p<0.001), hypoechogenicity (p<0.001), heterogeneity (p<0.007), lobulation (p<0.003) as independent predictors of a therapeutic strategy (Table 3).
Binary logistic regression analysis – identification of independent predictors for additional diagnostic or therapeutic strategy.
| Variable | Odds ratio | 95% CI | p value |
|---|---|---|---|
| Large size (≥20mm) | 0.127 | 0.061–0.267 | 0.001 |
| Hypoechogenicity | 0.177 | 0.073–0.429 | 0.001 |
| Heterogeneity | 0.312 | 0.134–0.728 | 0.007 |
| Lobulation | 0.09 | 0.018–0.436 | 0.003 |
In 64 patients (20%) EUS-FNA or endoscopic/surgical resection was suggested after EUS. Of those 33 underwent EUS-FNA and 34 were submitted to endoscopic/surgical resection. Among the 33 patients who underwent fine needle aspiration, in 66% the result was inconclusive. Of those who were inconclusive 3 were submitted to surgical resection and the remaining were followed by EUS. For the last ones who were followed by EUS after inconclusive EUS-FNA none were in the group of patients who presented EUS features change during follow-up.
In those patients with inconclusive EUS-FNA the majority underwent fine needle aspiration in the setting of impossibility to define a presumptive diagnosis, without EUS features of risk as defined in the manuscript. In a minority of patients the EUS-FNA was performed twice however the result was always inconclusive and after discussion with the patients the decision was to follow with EUS. In this situation patients were followed with EUS more frequently, without complications or malignancy related to SL and none were lost to follow-up.
The histological diagnosis were: 26 GIST, 6 leiomyoma, 4 carcinoids, 4 brunner gland adenomas and 4 inflammatory fibroid polyps. All lesions submitted to surgical intervention were GISTs.
During follow-up (41±21 months) none of the patients who were managed with radial EUS for surveillance presented complications, namely malignant transformation. None of the patients were lost to follow-up.
For those patients with no follow-up (with EUS or endoscopy) an analysis of visits to the hospital or causes of dead was made and in none of the patients there were complications related to SL.
4DiscussionWhen a SL is suspected, after an upper gastrointestinal endoscopy, EUS is the second step in the evaluation of SL and adds valuable information to guide further management. EUS is the diagnostic test of choice to differentiate between intramural and extramural lesions, to assess the size, margins, layer of origin, echotexture of the lesion and presence of adjacent lymph nodes. Based on EUS a decision can be made to decide between no further exams, follow-up with EUS or additional diagnostic or therapeutic strategy with resection when the lesion is likely to be malignant.
During the first approach, hyperechoic and anechoic lesions should be differentiated from hypoechoic, isoechoic or mixed echogenic lesions. For hypoechoic, isoechoic or mixed SL, a specific diagnosis is required, because of their possible malignant potential.3
The results of our study indicate that EUS seems to be of great value in the initial evaluation of SL, allowing no further examinations in 45% patients without complications during follow-up.
Some authors13 suggest that the critical step during EUS examination is to recognize the hypoechoic intramural SL arising from the muscle layers, as they may represent GISTs. GISTs still present a particular challenge for EUS, because all of these lesions have malignant potential, according to the classification system proposed by the NIH Consensus Conference.14 Tissue diagnosis remains the gold standard to predict the risk of malignancy by evaluating number of mitosis/higher power field. Even though EUS-FNA may be feasible, the tissue adequacy has been reported to be as low as 20–57%.15,16
In our study the rate of failure to obtain an adequate material by EUS-FNA was high (66%), this can be explained by no on-site cytopathologist and for the type of lesions. For this matter, in a recent study,17 the frequency of failing to obtain an adequate material by EUS-FNA was significantly higher in patients with a lesion size below 25mm. In our cohort the mean lesion size that underwent EUS-FNA was 17mm and this may have contributed to the low rate of adequate material for diagnosis. In addition, due to the firm nature of some lesions, such as GISTs, which was our more frequent presumptive diagnosis, cytologic yield may be poor and overly blood stained material which can difficult interpretation.16
The possibility to obtain a tissue core biopsy, preserved tissue architecture and allowing a histologic examination, was tested with a Tru-Cut biopsy needle dedicated for EUS-guided fine needle biopsy (EUS-FNB). However the technique failed to reach widespread use due to technical difficulty and without a clear advantage as compared to EUS FNA.18 A new needle designed to obtain both cytology and histology, the ProCore ® needles has been developed and evaluated in few studies,19,20 however the most recent studies failed to demonstrate a significant difference between the ProCore ® and standard FNA needles for sample adequacy, diagnostic accuracy or acquisition of a core specimen.21
Furthermore few studies tried to assess the performance characteristics (sensitivity, specificity and accuracy) of EUS comparing with histopathology, with an accuracy found to be low (around 50%).1,5,22 However, it is important to reinforce that in these studies the decision to obtain tissue was sometimes driven by diagnostic uncertainty, with no presumptive diagnosis suggested, and because of that the true diagnostic accuracy of EUS cannot be determined based on these results.
Although previous publications questioned the accuracy of EUS to differentiate between benign and malignant lesions,22,23 in our experience the role of EUS to decide the management of SL lesions was actually high. In this study the combination of multiple EUS features correlated with a higher probability of definitive diagnosis strategy and/or resection strategy surgical or endoscopic, this aspect is supported by the literature. In one large multicenter study endosonographers were asked to assess whether the EUS changed management plans and they reported that EUS resulted in a major management change in 67% of patients with SL. In another view, EUS alone has been shown to have sensitivity and a specificity of 64% and 80%, respectively, in the differentiation of malignant and benign subepithelial lesions.23 Specifically for GISTs the simultaneous presence of 2 out of 3 EUS features (irregular extraluminal margins, cystic spaces, and lymph nodes with a malignant pattern) has been shown to have a positive predictive value of 100% for malignant or borderline GISTs,24 reinforcing that EUS alone may be helpful in selecting patients with higher risk of malignancy. In our study we used EUS features considered of risk, based on the literature,24–27 to decide for invasive maneuvers for additional diagnosis or therapeutic strategy. We concluded that the combination of multiple features correlated with a higher probability for this strategy (p<0.001), and we verified that this approach was conducted in all patients when 4 or more features were present.
A multicentre study13 of 51 patients has shown that the majority of upper gastrointestinal subepithelial lesions other than GISTs do not change in size or echogenicity after a median follow-up of 23 months. In our study the follow-up period was long, 41 months, and may be long enough to reassure the benign nature of the lesions followed up by EUS.
Our study includes a large number of participants and long-term follow-up data with fixed specified follow-up intervals, being the main limitation to be a retrospective study. We consider that large randomized controlled trials with cost-effectiveness analysis could help to definitely establish the best approach for this type of lesions.
In conclusion our study suggests that EUS is capable of safely and accurately define those SL that can be discharged. The need for a definitive diagnosis or therapeutic approach can be based on ultrasound features of severity, detected in the majority of patients at presentation. The large majority of SL managed with ultrasound surveillance did not change during follow-up which may suggest that larger length between examinations could be considered or even that surveillance strategy is not cost-effective.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.