Interferon-free regimens achieve sustained virologic response (SVR) rates of over 90%, have generally well-tolerated adverse effects and involve 12-week treatment durations for most patients with chronic hepatitis C, including naive or previously treated patients and patients with or without cirrhosis. However, some of the treatment options recommended by the guidelines require the addition of ribavirin (RBV) or extend the duration of treatment to increase efficacy. The use of RBV is a useful tool in those difficult-to-cure patients such as patients with decompensated or genotype-3-infected cirrhosis and those who have not achieved SVR after treatment with direct-acting antivirals (DAA). Overall, adding RBV to the different combinations causes adverse effects related to a decrease in haemoglobin and involves inconveniences such as its dosage, which requires patients to take several tablets twice daily. However, severe anaemia is rare and easily manageable with a dose reduction. In addition, RBV is teratogenic. In practice, because RBV is inexpensive and well tolerated when combined with an interferon-free regimen, it continues to be a useful tool to optimise the results of some HCV treatment regimens. RBV-free regimens eliminate RBV-related adverse effects related, resulting in better tolerability, improving patient adherence and quality of life and reducing the cost of treatment.
Los regímenes libres de interferón ofrecen tasas de respuesta virológica sostenida (RVS) por encima del 90%, efectos adversos generalmente bien tolerados y duraciones de tratamiento de 12 semanas para la mayoría de pacientes con hepatitis C crónica, incluyendo pacientes naive o previamente tratados y pacientes con o sin cirrosis. Sin embargo, algunas de las opciones de tratamiento recomendadas por las guías requieren la adición de ribavirina (RBV) o extender la duración del tratamiento para aumentar la eficacia. El uso de RBV es una herramienta útil en aquellos pacientes difíciles de curar como los pacientes con cirrosis descompensada o infectados por el genotipo 3 y aquellos que no han logrado una RVS después de un tratamiento con antivirales de acción directa (AAD). Globalmente, la adición de RBV a las diferentes combinaciones causa efectos adversos relacionados con una disminución de la hemoglobina y añade inconvenientes como su posología, por la que los pacientes deben tomar varios comprimidos dos veces al día. Sin embargo, la anaemia grave es rara y fácilmente manejable con una reducción de dosis. Además, la RBV es teratogénica. En la práctica, debido a que la RBV es barata y bien tolerada cuando se combina con un régimen sin interferón, sigue siendo una herramienta útil para optimizar los resultados de algunos regímenes de tratamiento contra el VHC. Los regímenes libres de RBV eliminan los efectos adversos relacionados con la misma, resultando en una mejor tolerabilidad, mejorando la adherencia y la calidad de vida del paciente y disminuyendo el coste del tratamiento.
Chronic hepatitis C virus (HCV) infection is the main cause of liver dysfunction and the development of hepatocellular carcinoma, and the main indication for liver transplantation in Spain. Epidemiological data and the natural history of the disease clearly suggest that all patients should be treated. Several studies have shown that the sustained virological response (SVR) (HCV RNA-negative at least 12–24 weeks after the end of treatment) is associated with a decrease in the morbidity and mortality associated with chronic HCV infection, and particularly benefits patients with advanced liver disease.1–3 2015 marked a turning point for HCV patients, since it saw the introduction of a varied therapeutic arsenal composed of numerous direct-acting antiviral agents (DAAs) capable of completely eliminating viral replication in more than 90% of cases after only 8, 12 or 24 weeks of treatment (depending on severity), with few adverse effects and good tolerability. Current treatment options are shown in Table 1, and include sofosbuvir (SOF), a uridine nucleotide analogue that inhibits the hepatitis C virus RNA-dependent RNA polymerase nonstructural protein 5B (NS5B), in combination with other compounds from different DAA families (ledipasvir [LDV], daclatasvir [DCV], simeprevir [SMV] or velpatasvir [VEL]), paritaprevir-ritonavir ([PTV/r] protease inhibitor) plus the NS5A inhibitor ombitasvir (OBV) and the non-nucleoside NS5B polymerase inhibitor dasabuvir (DSV), and the protease inhibitor grazoprevir (GZR) plus the NS5A inhibitor elbasvir (EBR), and also ribavirin (RBV).4–6 Combined therapy with RBV significantly improved response to pegylated interferon (Peg-IFN) by preventing relapse.7 RBV has also been an important component of treatment based on Peg-IFN in combination with first-generation protease inhibitors, which was shown to prevent relapse,8 although phase 2 clinical trials on IFN-free regimens based on DAAs have suggested that RBV is not always necessary.9,10 RBV-free regimens have a series of advantages, such as improved adherence, fewer undesirable effects, improved quality of life and lower treatment costs. Although RBV appears to have less toxicity when not combined with Peg-IFN, it is teratogenic and is associated with haemolytic anaemia and other undesirable effects, such as asthenia, pruritus, gastrointestinal discomfort, insomnia, headache, etc. Another drawback is its dosage schedule, which involves patients taking several tablets twice a day.
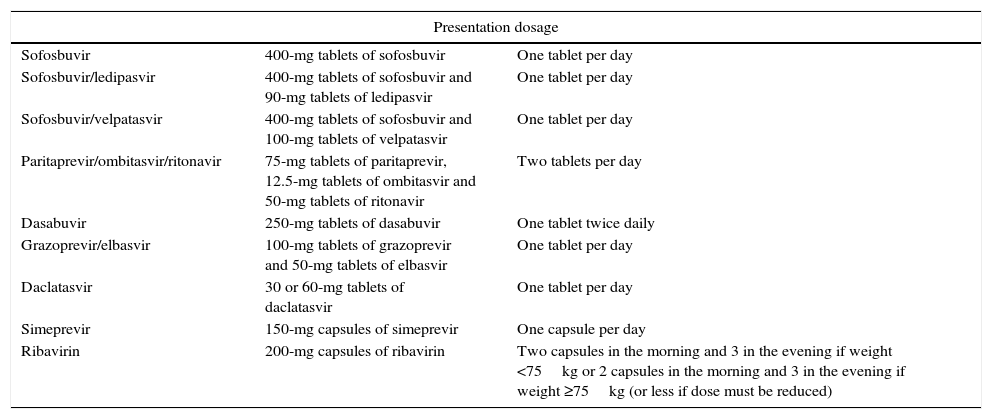
Treatments with direct-acting antivirals approved in Europe for HCV.
| Presentation dosage | ||
|---|---|---|
| Sofosbuvir | 400-mg tablets of sofosbuvir | One tablet per day |
| Sofosbuvir/ledipasvir | 400-mg tablets of sofosbuvir and 90-mg tablets of ledipasvir | One tablet per day |
| Sofosbuvir/velpatasvir | 400-mg tablets of sofosbuvir and 100-mg tablets of velpatasvir | One tablet per day |
| Paritaprevir/ombitasvir/ritonavir | 75-mg tablets of paritaprevir, 12.5-mg tablets of ombitasvir and 50-mg tablets of ritonavir | Two tablets per day |
| Dasabuvir | 250-mg tablets of dasabuvir | One tablet twice daily |
| Grazoprevir/elbasvir | 100-mg tablets of grazoprevir and 50-mg tablets of elbasvir | One tablet per day |
| Daclatasvir | 30 or 60-mg tablets of daclatasvir | One tablet per day |
| Simeprevir | 150-mg capsules of simeprevir | One capsule per day |
| Ribavirin | 200-mg capsules of ribavirin | Two capsules in the morning and 3 in the evening if weight <75kg or 2 capsules in the morning and 3 in the evening if weight ≥75kg (or less if dose must be reduced) |
Experience in the use of DAAs has allowed clinicians to shorten treatment and eliminate RBV in the majority of patients. However, RBV is still the key to maximising SVR rates in certain patients, including those with genotype 3 infection, decompensated cirrhosis, or in those who have not achieved SVR after treatment with DAAs. The aim of this review, therefore, is to identify the patient groups in which the use of RBV is recommended, in accordance with the different IFN-free regimens currently approved, by highlighting difficult-to-treat patient populations in which RBV continues to play an important role.
Role of ribavirin according to severity of liver disease, genotype and previous treatmentAs can be seen in Tables 2 and 3, RBV-free therapeutic combinations are already available for patients with genotype 1, genotype 2, genotype 4 and genotypes 5 or 6 infection. We will briefly summarise the treatment regimens recommended by the guidelines to which RBV must be added to optimise outcomes. The recommended treatment for HCV genotype 3 patients will be discussed further on. As will be seen below, the addition of RBV to certain therapeutic combinations is associated with increased efficacy and/or shorter treatment duration.
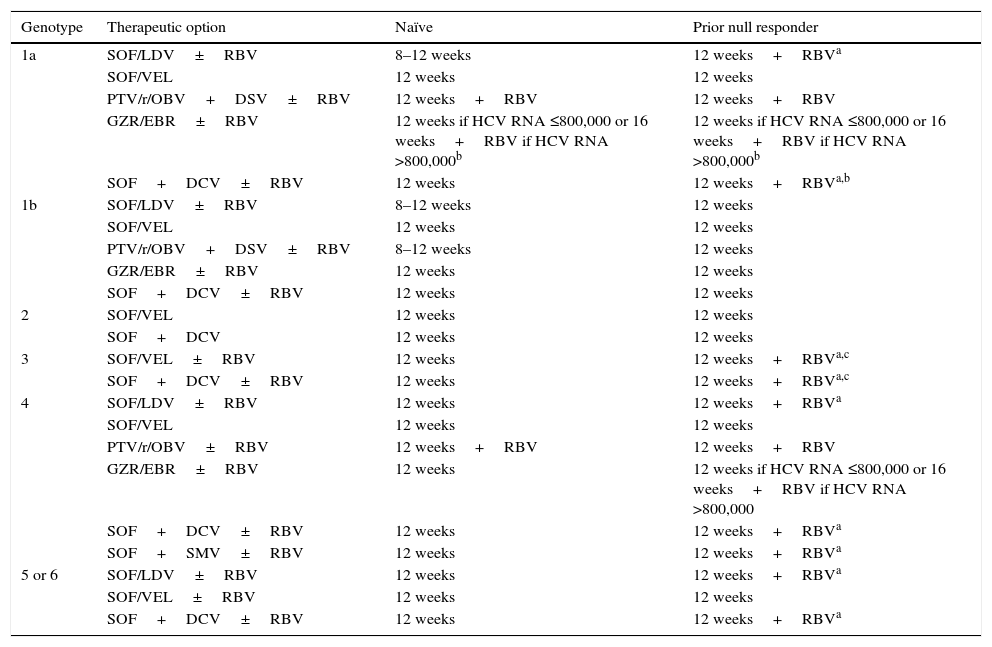
Recommended treatment options in patients without cirrhosis.
| Genotype | Therapeutic option | Naïve | Prior null responder |
|---|---|---|---|
| 1a | SOF/LDV±RBV | 8–12 weeks | 12 weeks+RBVa |
| SOF/VEL | 12 weeks | 12 weeks | |
| PTV/r/OBV+DSV±RBV | 12 weeks+RBV | 12 weeks+RBV | |
| GZR/EBR±RBV | 12 weeks if HCV RNA ≤800,000 or 16 weeks+RBV if HCV RNA >800,000b | 12 weeks if HCV RNA ≤800,000 or 16 weeks+RBV if HCV RNA >800,000b | |
| SOF+DCV±RBV | 12 weeks | 12 weeks+RBVa,b | |
| 1b | SOF/LDV±RBV | 8–12 weeks | 12 weeks |
| SOF/VEL | 12 weeks | 12 weeks | |
| PTV/r/OBV+DSV±RBV | 8–12 weeks | 12 weeks | |
| GZR/EBR±RBV | 12 weeks | 12 weeks | |
| SOF+DCV±RBV | 12 weeks | 12 weeks | |
| 2 | SOF/VEL | 12 weeks | 12 weeks |
| SOF+DCV | 12 weeks | 12 weeks | |
| 3 | SOF/VEL±RBV | 12 weeks | 12 weeks+RBVa,c |
| SOF+DCV±RBV | 12 weeks | 12 weeks+RBVa,c | |
| 4 | SOF/LDV±RBV | 12 weeks | 12 weeks+RBVa |
| SOF/VEL | 12 weeks | 12 weeks | |
| PTV/r/OBV±RBV | 12 weeks+RBV | 12 weeks+RBV | |
| GZR/EBR±RBV | 12 weeks | 12 weeks if HCV RNA ≤800,000 or 16 weeks+RBV if HCV RNA >800,000 | |
| SOF+DCV±RBV | 12 weeks | 12 weeks+RBVa | |
| SOF+SMV±RBV | 12 weeks | 12 weeks+RBVa | |
| 5 or 6 | SOF/LDV±RBV | 12 weeks | 12 weeks+RBVa |
| SOF/VEL±RBV | 12 weeks | 12 weeks | |
| SOF+DCV±RBV | 12 weeks | 12 weeks+RBVa |
DCV: daclatasvir; GZR/EBR: grazoprevir/elbasvir; PTV/r/OBV+DSV: paritaprevir/ritonavir/ombitasvir+dasabuvir; RBV: ribavirin; SMV: simeprevir; SOF: sofosbuvir; SOF/LDV: sofosbuvir/ledipasvir; SOF/VEL: sofosbuvir/velpatasvir.
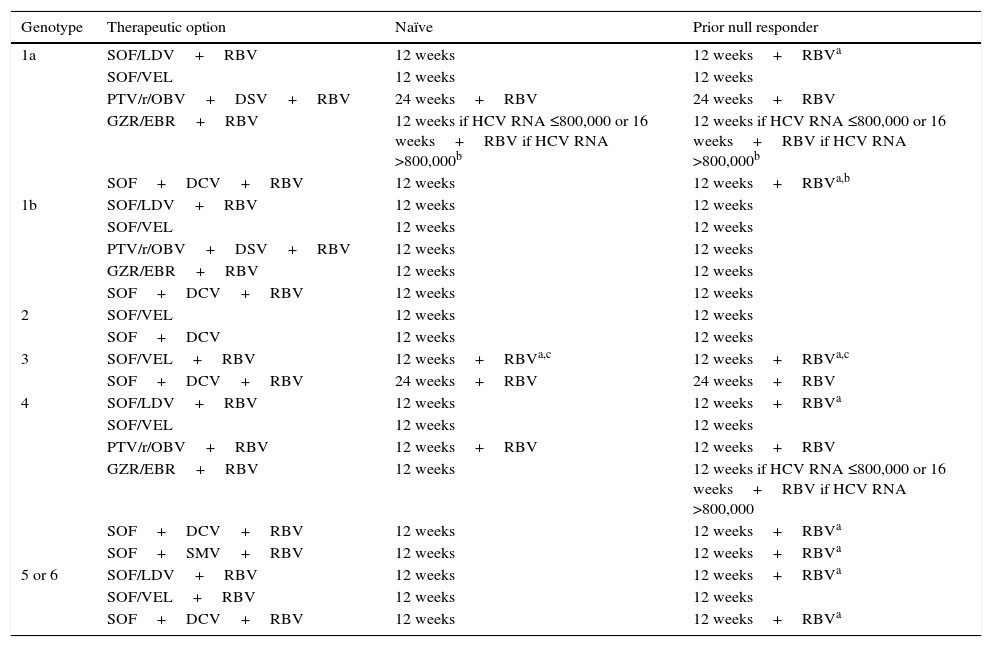
Recommended treatment options in patients with cirrhosis.
| Genotype | Therapeutic option | Naïve | Prior null responder |
|---|---|---|---|
| 1a | SOF/LDV+RBV | 12 weeks | 12 weeks+RBVa |
| SOF/VEL | 12 weeks | 12 weeks | |
| PTV/r/OBV+DSV+RBV | 24 weeks+RBV | 24 weeks+RBV | |
| GZR/EBR+RBV | 12 weeks if HCV RNA ≤800,000 or 16 weeks+RBV if HCV RNA >800,000b | 12 weeks if HCV RNA ≤800,000 or 16 weeks+RBV if HCV RNA >800,000b | |
| SOF+DCV+RBV | 12 weeks | 12 weeks+RBVa,b | |
| 1b | SOF/LDV+RBV | 12 weeks | 12 weeks |
| SOF/VEL | 12 weeks | 12 weeks | |
| PTV/r/OBV+DSV+RBV | 12 weeks | 12 weeks | |
| GZR/EBR+RBV | 12 weeks | 12 weeks | |
| SOF+DCV+RBV | 12 weeks | 12 weeks | |
| 2 | SOF/VEL | 12 weeks | 12 weeks |
| SOF+DCV | 12 weeks | 12 weeks | |
| 3 | SOF/VEL+RBV | 12 weeks+RBVa,c | 12 weeks+RBVa,c |
| SOF+DCV+RBV | 24 weeks+RBV | 24 weeks+RBV | |
| 4 | SOF/LDV+RBV | 12 weeks | 12 weeks+RBVa |
| SOF/VEL | 12 weeks | 12 weeks | |
| PTV/r/OBV+RBV | 12 weeks+RBV | 12 weeks+RBV | |
| GZR/EBR+RBV | 12 weeks | 12 weeks if HCV RNA ≤800,000 or 16 weeks+RBV if HCV RNA >800,000 | |
| SOF+DCV+RBV | 12 weeks | 12 weeks+RBVa | |
| SOF+SMV+RBV | 12 weeks | 12 weeks+RBVa | |
| 5 or 6 | SOF/LDV+RBV | 12 weeks | 12 weeks+RBVa |
| SOF/VEL+RBV | 12 weeks | 12 weeks | |
| SOF+DCV+RBV | 12 weeks | 12 weeks+RBVa |
DCV: daclatasvir; GZR/EBR: grazoprevir/elbasvir; PTV/r/OBV+DSV: paritaprevir/ritonavir/ombitasvir+dasabuvir; RBV: ribavirin; SMV: simeprevir; SOF: sofosbuvir; SOF/LDV: sofosbuvir/ledipasvir; SOF/VEL: sofosbuvir/velpatasvir.
The therapeutic combinations for non-cirrhotic patients that require RBV are shown in Table 2. The regimens for patients with compensated cirrhosis that contain RBV are shown in Table 3.
HCV genotype 1a patientsIn phase 3 trials evaluating the PTV/r/OBV+DSV therapeutic combination, it was observed that in non-cirrhotic, treatment-naive HCV genotype 1a patients, the virological failure rate was higher in the group not receiving RBV (7.8% vs 2.0%), and that the addition of RBV increased the SVR rate to 97%.11 In cirrhotic HCV genotype 1a patients with no response to previous treatment, the 24-week PTV/r/OBV+DSV plus RBV regimen achieved higher SVR rates higher than the 12-week regimen (94.2% vs 88.6%).12 When using PTV/r/OBV+DSV in prior null responders with cirrhosis, RBV must be added to the combination and the treatment must be extended to 24 weeks in order to increase SVR.
In phase 3 trials evaluating the combination of GZR/EBR in non-cirrhotic patients, and in the post hoc analysis of pooled data from the phase 2 and phase 3 trials,13–16 the SVR rate was similar in patients treated with or without RBV, with the exception of patients with genotype 1a infection and baseline NS5A resistance-associated substitutions (RASs) at positions 28, 30, 31 and 93, in whom prolonging the treatment to 16 weeks and adding RBV improved SVR.16 The same is true of the GZR/EBR combination in patients with cirrhosis. Therefore, if NS5A resistance testing is not performed, treatment-experienced and treatment-naive HCV genotype 1a patients with compensated cirrhosis and an HCV RNA level >800,000IU/ml (5.9log10IU/ml) should receive the combination of GZR and EBR with RBV for 16 weeks. In these patients, adding RBV and increasing the duration of treatment to 16 weeks overcomes the negative effect of baseline NS5A RASs on SVR.
The analysis of the pooled data from patients receiving the SOF/LDV combination in phase 2 and 3 clinical trials showed that the presence of baseline RASs that confer high-level resistance to LDV (>100-fold increase in EC50 in the replicon system: M28A/G/T, Q30E/G/H/K/R, L31 M/V, P32L/S, H58D and/or Y93C/H/N/S) was associated with a lower rate of SVR in non-cirrhotic genotype 1a patients with no response to a previous 12-week RBV-free regimen.17,18 The addition of RBV overcame the negative effect of baseline NS5A RASs on SVR. The findings of these clinical trials have been reproduced in various real-world studies conducted on different continents. The analysis of pooled data from cirrhotic genotype 1 patients treated with the combination of fixed-dose SOF/LDV with or without RBV included in different phase 2 and 3 studies, showed that neither treatment duration nor RBV had an impact on SVR in treatment-naive patients. However, in prior null responders, SOF/LDV with RBV for 12 weeks appears to be the most cost-effective option.19 This is also suggested by the results of the SIRIUS study, which compared SOF/LDV with RBV for 12 weeks with SOF/LDV for 24 weeks in cirrhotic patients with HCV genotype 1 infection who had not achieved SVR after previous treatment with first-generation protease inhibitors.20
The efficacy of SOF plus DCV for 12 or 24 weeks with or without RBV was evaluated in a large real-life cohort that included cirrhotic genotype 1 patients.21 Although this study was not controlled and the choice of treatment was at the discretion of the attending physician, the findings show that the addition of RBV could shorten the duration of treatment to 12 weeks in this population.
HCV genotype 4 patientsThe absence of RBV in the PTV/r/OBV combination has been associated with a lower SVR rate (91%). Failure was mainly related to virological reasons, which suggests that the use of RBV increases the probability of achieving SVR in this population.22
A randomised trial evaluating 12- or 16-week regimens of EBR/GZR with or without RBV in treatment-experienced patients, found SVR rates of under 95% in all groups except for patients on the 16-week regimen who received RBV.23
In patients without cirrhosis or in prior null responders with compensated cirrhosis, RBV should be added to the SOF/LDV, SOF plus DCV and SOF plus SMV combinations to increase the likelihood of achieving SVR.
HCV genotype 5 or 6 patientsIn prior null responders with HCV genotypes 5 or 6, regardless of whether they are cirrhotic or not, the addition of RBV to SOF/LDV or SOF plus DCV also increases SVR.
Difficult-to-treat patients requiring ribavirinTreatment duration or the use of RBV continue to be useful tools in difficult-to-treat patients, such as those with genotype 3 infection, patients with decompensated cirrhosis, and those who have not achieved SVR after DAA therapy.
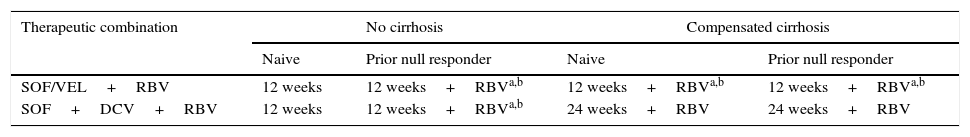
HCV genotype 3 patientsHCV genotype 3 patients treated with current therapeutic combinations present lower SVR and higher relapse rates. The treatment options recommended by various guidelines4,5 in these patients are shown in Table 4. Non-cirrhotic genotype 3 patients can be treated with SOF plus DCV for 12 weeks without RBV. However, this regimen is suboptimal in cirrhotic patients. Therefore, the use of RBV appears to be mandatory in cirrhotic genotype 3 patients treated for 12 weeks with SOF and DCV. In the ALLY-3 trial, evaluating the SOF plus DCV combination for 12 weeks in genotype 3 patients, the presence of cirrhosis was associated with a marked reduction in SVR (58–69%).24 In the ALLY-3+ study, the addition of RBV to the 12- or 16-week regimen given to patients with advanced liver disease increased SVR to 86%.25 No studies have yet compared the efficacy of SOF plus DCV with RBV for 12 weeks against SOF plus DCV with or without RBV for 24 weeks, so the optimal SOF plus DCV regimen and the need for RBV in cirrhotic genotype 3 patients has yet to be clarified. Furthermore, the SVR rates observed in patients with baseline NS5A RASs in the RBV arms were higher than those observed in the RBV-free arms. If NS5A resistance testing is not performed, prior null responders with HCV genotype 3 infection and no cirrhosis should be treated with the SOF plus DCV combination for 12 weeks with RBV. If NS5A resistance tests are performed, prior null responders with no cirrhosis and with NS5A-Y93H RAS should be treated with the SOF plus DCV combination for 12 weeks with RBV. Patients testing negative for NS5A-Y93H RAS should receive the SOF plus DCV combination for 12 weeks without RBV.5
Recommended treatment options in HCV genotype 3 patients with and without cirrhosis.
| Therapeutic combination | No cirrhosis | Compensated cirrhosis | ||
|---|---|---|---|---|
| Naive | Prior null responder | Naive | Prior null responder | |
| SOF/VEL+RBV | 12 weeks | 12 weeks+RBVa,b | 12 weeks+RBVa,b | 12 weeks+RBVa,b |
| SOF+DCV+RBV | 12 weeks | 12 weeks+RBVa,b | 24 weeks+RBV | 24 weeks+RBV |
DCV: daclatasvir; RBV: ribavirin; SOF: sofosbuvir; SOF/VEL: sofosbuvir/velpatasvir.
The phase 3 study, ASTRAL-3, which evaluated the SOF/VEL combination also reported lower rates of SVR in cirrhotic patients, in prior null responders, and in patients with NS5A RASs at baseline, particularly in position 93. In treatment naive cirrhotic patients, SOF/VEL achieved SVR rates of 93%, while in treatment experienced patients, SVR was 89%.26 The SVR rate was 97% in patients with no baseline NS5A RASs compared with 88% in those with baseline NS5A RASs (present in 16% of cases). Therefore, the addition of RBV can be considered in prior null responders with genotype 3 and no cirrhosis and in both prior null responders and treatment-naive patients with compensated cirrhosis.
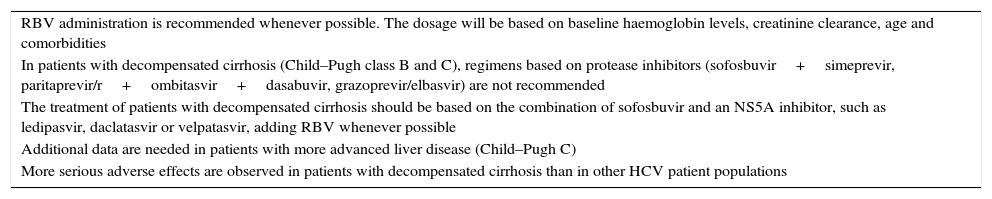
Patients with decompensated cirrhosisTreatment options are also limited in patients with decompensated liver disease; furthermore, more serious adverse effects are observed in patients with decompensated cirrhosis (10–52%) than in other HCV patient populations (<10%). In these patients, RBV administration is recommended whenever possible. In fact, relatively few patients with decompensated liver disease have been included in trials evaluating DAAs. In addition, the use of protease inhibitors is not recommended in Child–Pugh class B patients, and they are contraindicated in patients with decompensated cirrhosis Child–Pugh class C, because the metabolism changes occurring in these patients can cause toxicity. Protease inhibitors should not be used in patients with compensated cirrhosis with a history of decompensation, since cases of decompensation during treatment have been reported. Therefore, the treatment of patients with decompensated cirrhosis should be based on the combination of SOF and an NS5A inhibitor, such as LDV, DCV or VEL, adding RBV whenever possible (Table 5).
Recommendations in patients with decompensated cirrhosis.
| RBV administration is recommended whenever possible. The dosage will be based on baseline haemoglobin levels, creatinine clearance, age and comorbidities |
| In patients with decompensated cirrhosis (Child–Pugh class B and C), regimens based on protease inhibitors (sofosbuvir+simeprevir, paritaprevir/r+ombitasvir+dasabuvir, grazoprevir/elbasvir) are not recommended |
| The treatment of patients with decompensated cirrhosis should be based on the combination of sofosbuvir and an NS5A inhibitor, such as ledipasvir, daclatasvir or velpatasvir, adding RBV whenever possible |
| Additional data are needed in patients with more advanced liver disease (Child–Pugh C) |
| More serious adverse effects are observed in patients with decompensated cirrhosis than in other HCV patient populations |
SOF/LDV for 12 weeks in 20 genotype 1 patients with Child–Pugh class B cirrhosis achieved an SVR rate of only 65%.27 In another study, SOF/LDV with RBV for 12 weeks was compared to SOF/LDV with RBV for 24 weeks in genotype 1 patients with Child–Pugh B and C cirrhosis. SVR rates ranged from 83 to 94%, with no differences observed in the rates obtained in patients treated for 24 weeks.28 Recently, data from an uncontrolled real-life study in patients with decompensated cirrhosis have shown a slight increase in the efficacy of this regimen administered for 12 weeks in the group receiving RBV compared to the group not receiving RBV. This suggests that RBV should be used in genotype 1 patients with decompensated cirrhosis.29 In summary, patients with decompensated cirrhosis receiving SOF/LDV should be treated for 24 weeks, while the addition of RBV allows treatment to be shortened to 12 weeks in this patient population.
The regimen of SOF plus DCV was evaluated in genotype 1 patients with decompensated cirrhosis enrolled in a phase 3 single-arm clinical trial. The initial RBV dose was 600mg daily, which was then stepped up to 1000mg daily, depending on haemoglobin levels and renal function. The overall SVR rate was 82%, and the treatment was more effective in patients with Child–Pugh A or B (>90%) than in those with Child–Pugh C (<60%), suggesting that SOF plus DCV and RBV for 12 weeks could be an optimal regimen for genotype 1 patients with decompensated cirrhosis Child–Pugh A or B.30 The combination of SOF and DCV with or without RBV (5 patients) was administered for 12 weeks in patients with genotype 1 and decompensated cirrhosis in an uncontrolled real-life cohort (45 patients). SVR rates were 82% and 60% in patients with and without RBV, respectively.31 In this study, however, the sample size was small and the choice of treatment was at the discretion of the attending physician, so the findings are insufficiently robust to support the use of RBV in these patients. In summary, the combination of SOF plus DCV and RBV for 12 weeks can be recommended in patients with genotype 1 with Child–Pugh class B compensated or decompensated cirrhosis. Additional data are needed in patients with more advanced liver disease (Child–Pugh C).
The phase 3 ASTRAL-4 study evaluated the efficacy of SOF/VEL with or without RBV for 12 weeks or SOF/VEL for 24 weeks in genotype 1–6 patients with decompensated cirrhosis (only Child–Pugh B). As in other studies using other DAA combinations, patients with HCV genotype 3 showed lower rates of SVR than patients infected with other HCV genotypes. SOF/VEL with RBV for 12 weeks was more effective than SOF/VEL alone for 12 or 24 weeks; however, RBV was associated with more treatment interruptions due to adverse effects. Decompensated cirrhotic patients with genotype 3 who received SOF/VEL plus RBV achieved an SVR of 85%, whereas SVR was only 50% in patients with genotype 3 who received SOF/VEL without RBV.32 This suggests that the contribution of RBV may be particularly important in patients infected with HCV genotype 3. Therefore, RBV should be used whenever possible in patients with genotype 3 and decompensated cirrhosis.
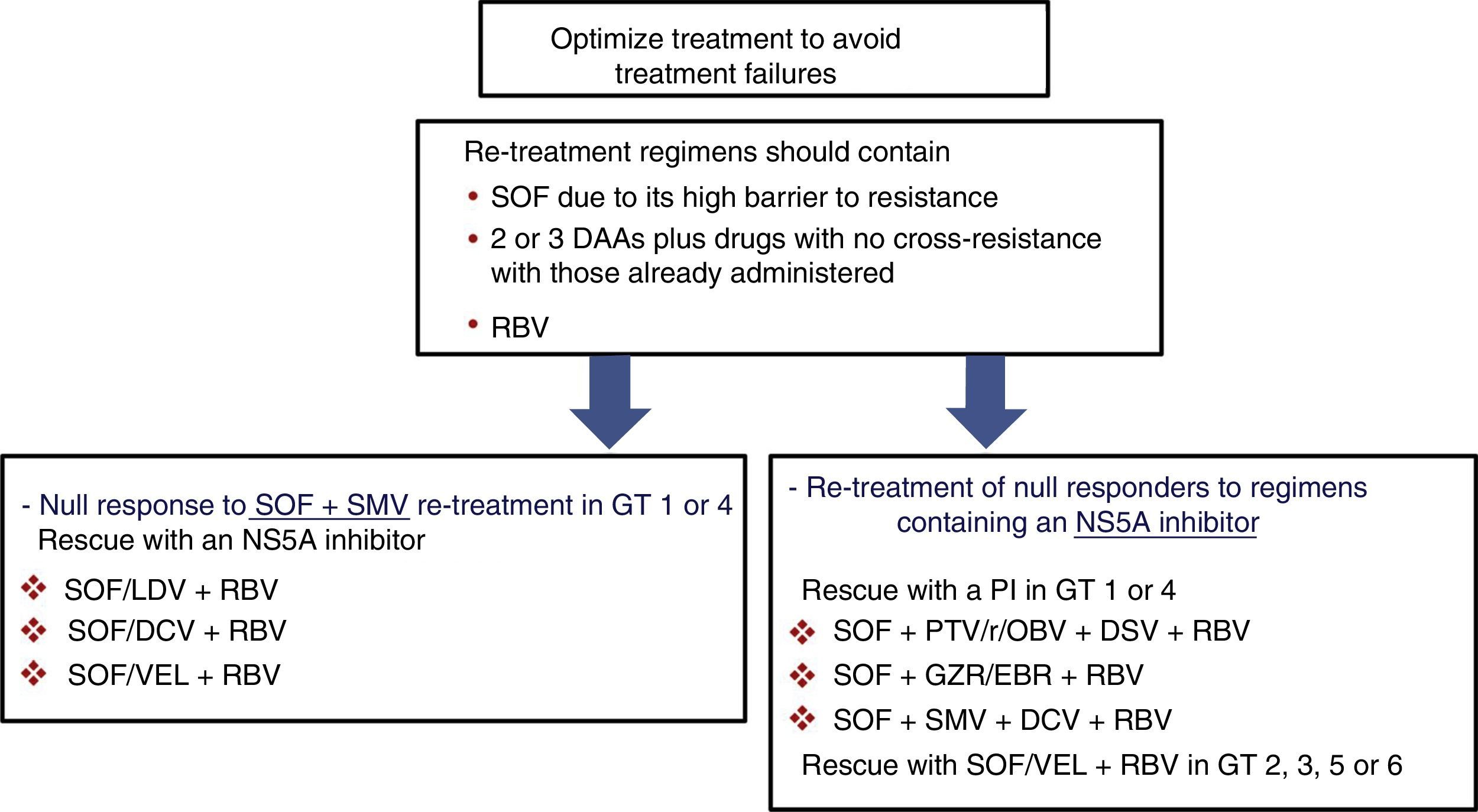
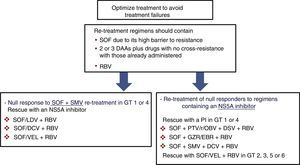
Prior null-responders to direct-acting antiviralsRASs that have been shown in vitro to confer reduced susceptibility to the corresponding drug class have been selected in patients who failed to achieve SVR after treatment with DAAs. These RASs and a number of alternative substitutions at the same positions can be present at the start of the new treatment in patients previously exposed to DAAs. For this reason, guidelines recommend using the results of resistance tests to guide treatment changes.4,5 As shown in Fig. 1, if resistance testing is unavailable, re-treatment strategies must necessarily include drugs that do not present cross-resistance, including RBV, and prolonging the treatment to 24 weeks, especially in patients with F3 fibrosis or cirrhosis. Furthermore, SOF-based regimens should be used in re-treatment of DAA null responders due to the fact that SOF displays a high barrier to resistance. HCV resistance-associated variants are exceptional after treatment with SOF, and, if present, will rapidly disappear after the end of treatment.33,34 In these patients, RBV will maximise the likelihood of achieving SVR.
Recommended re-treatment options in null responders to DAAs if resistance tests are not available. Treatment should last 12 or 24 weeks (24 weeks recommended in patients with F3 fibrosis or cirrhosis). DAA: direct-acting antivirals; DCV: daclatasvir; GT: genotype; GZR/EBR: grazoprevir/elbasvir; PI: protease inhibitor; PTV/r/OBV+DSV: paritaprevir/ritonavir/ombitasvir+dasabuvir; RBV: ribavirin; SMV: simeprevir; SOF: sofosbuvir; SOF/LDV: sofosbuvir/ledipasvir; SOF/VEL: sofosbuvir/velpatasvir.
RBV (1-beta-d-ribofuranosyl-1,2,4-triazole-3-carboxamide) is a synthetic guanosine analogue with antiviral activity. It was first synthesised in the 1970s, and has been shown to be active against many DNA and RNA viruses. RBV in monotherapy is not effective in the treatment of chronic hepatitis C, but RBV in combination with Peg-IFN is effective for this disease.
The antiviral effect of RBV is not fully understood. RBV appears to selectively inhibit the synthesis of viral DNA and RNA in infected host cells and increase the cytokine-mediated antiviral response. RBV undergoes intracellular phosphorylation to mono-, di-, and triphosphates. Once phosphorylated, RBV alters the cellular metabolism of purines by inosine monophosphate dehydrogenase (IMPDH) inhibition, which leads to depletion of guanosine triphosphate (GTP) that is essential for viral transcription and replication of RNA viruses. As a result, RBV inhibits viral RNA and protein synthesis by interfering with the ability of the virus to spread to other cells. RBV shows some host cell cytotoxicity when used at higher concentrations than those required to inhibit viral RNA synthesis. RBV also increases CD4 and CD8 T cell expression of antiviral cytokines, such as interleukin-2, tumour necrosis factor alpha and interferon-gamma.
RBV does not appear to be a potent inhibitor of RNA polymerase, since it only produces a slight decrease in viral load (<0.5log) and does not increase the first-phase slope of HCV RNA clearance. Its effects are related to second-phase acceleration, and depend on RBV-induced viral decline in patients in whom virus production is effectively blocked by IFN-free drug combinations through unknown mechanisms.35,36
Studies have shown that RBV exerts a transient and moderate, but significant, antiviral effect on a significant number of patients receiving RBV alone.35,37–39 Administration of RBV alone has been shown to decrease alanine transaminase (ALT) levels, an effect that is not related to the antiviral action of RBV.36–39 A number of mechanisms have been proposed, including direct inhibition of viral RNA polymerase. However, the modest antiviral effect of RBV alone in vivo makes this hypothesis unlikely. It has also been suggested that the antiviral activity of RBV is related to a depletion of intracellular GTP reserves caused by RBV-induced inhibition of the IMPDH enzyme. However, other potent IMPDH-specific inhibitors used alone or in combination with RBV or IFN do not exert a significant effect on HCV replication in patients with HCV infection, suggesting that IMPDH inhibition does not influence the antiviral activity of RBV.40 Finally, RBV appears to exert a mutagenic effect on HCV when incorporated into newly synthesised genomes, giving rise to mutations in the viral genome and causing an “error catastrophe” in replication (suicide mutations). This ultimately leads to the disappearance of infectious virions, thus preventing the virus from attacking healthy hepatocytes. In a recent study analysing the RBV-induced mutations using deep sequencing, the authors observed that RBV induces nucleotide transitions by exerting a mutagenic effect on HCV, leading them to suggest that this effect could be a relevant factor in the antiviral activity of the RBV.37 Nonetheless, RBV-induced mutagenesis does not explain the biochemical response. Finally, the hypothesis that RBV may act as a potentiator of IFN signalling by augmenting IFN-stimulated gene induction has not been demonstrated in vivo.39 In summary, the dissociation between RBV-induced antiviral and biochemical responses suggests that RBV can act through different mechanisms: a direct antiviral effect partially explained by its mutagenic properties, and an indirect biochemical effect through an unknown mechanism.
Adverse effects of ribavirinAlthough RBV appears to have less toxicity when not combined with Peg-IFN, it is teratogenic and is associated with haemolytic anaemia and other undesirable effects, such as asthenia, pruritus, gastrointestinal discomfort, insomnia, headache, etc. Therefore, RBV-free regimens have a series of advantages, such as fewer undesirable effects and improved quality of life.
Nonetheless, it is important to note that multiple trials with DAAs have shown that despite its association with increased anaemia, fatigue and insomnia, similar rates of serious adverse events and interruption of treatment are observed in patients treated with or without RBV.
Use of ribavirin: risk–benefit ratioThe decision to add RBV must be taken on a case-by-case basis after weighing up the risks and benefits: slight increase in efficacy and shorter duration of treatment versus worse safety profile and worse quality of life, mainly due to anaemia. The vast majority of patients are over-treated by adding RBV to their regimen, because it is impossible to select RBV candidates based on baseline factors. The severity of liver disease could be significant, since it has been suggested that there is a relationship between FibroScan values and treatment outcomes.41 Another parameter could be the presence of baseline NS5A RASs. Studies have shown that in cirrhotic patients receiving an SOF/LDV-based regimen, SVR rates in patients with baseline NS5A RASs in the arms containing RBV were higher than those observed in the RBV-free arms (patients with NS5A RASs), but similar to those reported in patients without baseline NS5A RASs treated with an RBV-free regimen.42 Unfortunately, resistance testing is not widely available, so it is not recommended in all cirrhotic patients. A more accurate estimation of the optimal RBV dose to use in DAA combinations would improve the risk–benefit ratio. Weight-based RBV dosing is used in Peg-IFN plus RBV regimens. Regimens containing SOF or 2 or 3 DAAs with low-dose RBV (600 or 800mg daily) may improve SVR rates over RBV-free regimens while minimising the risk of toxicity associated with weight-based RBV dosing. This option should be evaluated in different populations, but clinical trials with low-dose RBV are no longer possible due to the imminent release of second-generation DAAs administered without RBV. In practice, guidelines recommend starting treatment with a weight-based dose (1000 or 1200mg in patients <75kg or ≥75kg, respectively), and then adapting the dose according to haemoglobin levels and renal function. Finally, the use of RBV is problematic in different populations, such as patients on haemodialysis, patients with decompensated cirrhosis, or in pre- and post-transplant patients. In this context, RBV dosage must be individualised, for example, starting on 600mg RBV daily and then stepping up to 1000mg daily, depending on haemoglobin levels and renal function. A better understanding of the mechanism of action could lead to a more selective use of RBV in the era of DAAs.
ConclusionsCombined therapy with RBV significantly improved response to Peg-IFN by preventing relapse.7 RBV has also prevented relapse in regimens based on Peg-IFN plus first-generation protease inhibitors.8 Experience in the use of DAAs has allowed clinicians to shorten treatment and eliminate RBV in the majority of patients. Nevertheless, treatment duration or the use of RBV continue to be useful tools in difficult-to-treat patients, such as those with genotype 3 infection, patients with decompensated cirrhosis, and those who have not achieved SVR after DAA therapy. Either way, the imminent introduction of new drug combinations that will obviate the use of RBV, even in difficult-to-treat populations, will no doubt relegate RBV to a secondary role in hepatitis C antiviral therapy.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Morillas RM, Masnou H, Ardévol M, López D. Papel de la ribavirina en la terapia libre de interferón frente al virus de la hepatitis C. Gastroenterol Hepatol. 2017;40:699–708.