Cost–effectiveness analysis of sofosbuvir combined with peginterferon alpha-2a and ribavirin (SOF/Peg-IFN/RBV) in early versus advanced fibrosis in previously untreated patients with chronic hepatitis C genotype 1 (CHC-GT1), from the perspective of the Spanish National Health System (NHS).
MethodsA Markov model was developed to compare lifetime costs and outcomes (life years gained [LYGs] and quality-adjusted life years [QALYs]) of 2 treatment strategies: SOF/Peg-IFN/RBV administered during early fibrosis (mild-moderate fibrosis; F2–F3) or advanced fibrosis (cirrhosis; F4). Efficacy (sustained virologic response), annual transition probabilities, disease management costs and utilities were obtained from the literature. Costs and outcomes were discounted annually at 3%. Direct costs were considered, expressed in Euros (€, 2014). Probabilistic sensitivity analysis (PSA) was also performed.
ResultsSOF/Peg-IFN/RBV therapy at F2–F3 was more effective (19.12 LYGs and 14.14 QALYs) compared to F4. In a cohort of 1000 patients, SOF/Peg-IFN/RBV prevented 66 cases of decompensated cirrhosis, 60 hepatocellular carcinomas and 4 liver transplantations compared with therapy in advanced fibrosis. The total lifetime cost of early therapy (€43,263) was less than the cost of treatment in the advanced stage (€49,018). Early therapy was a dominant strategy, more effective and less costly in all simulations. In the PSA analysis, administration of SOF/PEG-IFN/RBV at F2–F3 was dominant in all simulations.
ConclusionsStarting SOF/Peg-IFN/RBV therapy at F2–F3, compared with therapy at F4, reduced the incidence of liver disease complications and was associated with cost savings for the Spanish NHS in CHC-GT1 patients.
Análisis coste-efectividad de sofosbuvir con peginterferón/ribavirina (SOF/PEG-IFN/RBV) en pacientes con hepatitis C crónica genotipo 1 (HCC-GT1) no tratados previamente con diferentes grados de fibrosis, desde la perspectiva del Sistema Nacional de Salud (SNS).
MétodosModelo de Markov para estimar costes y resultados en salud (años de vida ganados [AVG] y años de vida ajustados por calidad [AVAC]), con una tasa de descuento del 3% anual de dos estrategias: SOF/PEG-IFN/RBV en fases precoces (fibrosis leve-moderada, F2-F3) o tardías (cirrosis compensada, F4). La eficacia (respuesta virológica sostenida), probabilidades anuales de transición, costes del manejo de la enfermedad y utilidades se obtuvieron de la literatura. Se consideraron costes directos expresados en € 2014. Se realizó un análisis de sensibilidad probabilístico (ASP).
ResultadosSOF/PEG-IFN/RBV en F2-F3 fue más efectiva (19,12 AVG y 14,14 AVAC) que en F4 (16,36 AVG y 9,27 AVAC). En 1.000 pacientes, SOF/PEG-IFN/RBV en F2-F3 podría evitar 66 casos de cirrosis descompensada, 60 de carcinoma hepatocelular y 4 trasplantes, en comparación con F4. El coste total de la terapia con SOF/PEG-IFN/RBV en F2-F3 (43.263€) fue menor que en F4 (49.018€). Administrar el tratamiento en F2-F3 frente a F4 representó una estrategia dominante (más efectiva y con menor coste). En el ASP, la administración de SOF/PEG-IFN/RBV en F2-F3 permaneció dominante en el 100% de las simulaciones.
ConclusionesLa administración de SOF/PEG-IFN/RBV en F2-F3, comparada con la terapia en F4, disminuyó la incidencia de complicaciones de la enfermedad hepática y se asoció con un ahorro en costes para el SNS en pacientes HCC-GT1.
Hepatitis C virus (HCV) infection affects around 160 million people worldwide,1 9 million of whom live in European countries.2 Chronic hepatitis C (CHC) is a disease that is asymptomatic in the early stages, but which can evolve to liver cirrhosis as it progresses. Up to 25% of patients with CHC develop cirrhosis,3 4% of whom progress annually to decompensated cirrhosis, with an associated risk of approximately 1.6% per year of developing hepatocellular carcinoma (HCC).3 HCV infection is the main indication for liver transplant, and is estimated to be responsible for 350,000 deaths annually.4
Genotype 1 (GT1) is the most common HCV genotype globally,5 and is responsible for 65.4–76% of cases in Spain.6,7 Current treatments recommended for patients with HCV GT1 infection are based on oral, direct-acting antiviral therapies free from interferon (IFN).8
However, these antivirals are not available in all countries, and even in countries where they are marketed, situations arise that make it difficult for patients to access treatment.9 In some settings, treatment is only reimbursed in patients with cirrhosis, so patients with mild fibrosis are treated with IFN-based regimens, or must wait until the disease progresses10 in order to meet healthcare system criteria for receiving subsidised IFN-free therapy.11
The criterion of sustained virologic response (SVR)—defined as absence of HCV RNA levels detectable in serum at the end of 12 weeks of treatment12—is widely accepted and recognised as indicative of therapeutic success.12,13
The clinical benefits associated with the SVR criterion are evident at several levels. Patients who achieve SVR have a life expectancy similar to that of the general population,14 since it is related with a substantial reduction in overall mortality.15,16 Furthermore, an SVR is associated with regression of liver fibrosis, even in patients with mild cirrhosis,17 with a subsequent reduction in the risk of developing HCC.18 However, this risk is not completely eliminated in patients with cirrhosis, even if they have responded satisfactorily to treatment.19–21
The efficacy of antiviral therapy in patients with advanced fibrosis or cirrhosis is significantly lower than in patients with mild fibrosis, resulting in a lower likelihood of achieving an SVR.22,23
Among the available IFN-based regimens, the combination of sofosbuvir (SOF) with peginterferon alpha-2a and ribavirin (Peg-IFN/RBV) in 12-week therapy in treatment-naive patients with HCV GT1 infection achieves high rates of SVR (90%), with no cases of virologic rebound or drug resistance having been observed.24 The SVR rates achieved with this regimen are higher in patients with mild or moderate fibrosis than in patients with cirrhosis, and the safety profile is associated with a slight increase in adverse effects in patients who present cirrhosis. It follows then that treatment in early stages of the disease could be related with improved quality of life and, consequently, potential cost-savings for healthcare systems.25
The aim of this study was to evaluate the cost–effectiveness ratio of the administration of SOF combined with Peg-IFN/RBV (SOF/Peg-IFN/RBV) in previously untreated patients with HCV GT1 infection with early versus advanced fibrosis, from the perspective of the Spanish National Health System (NHS).
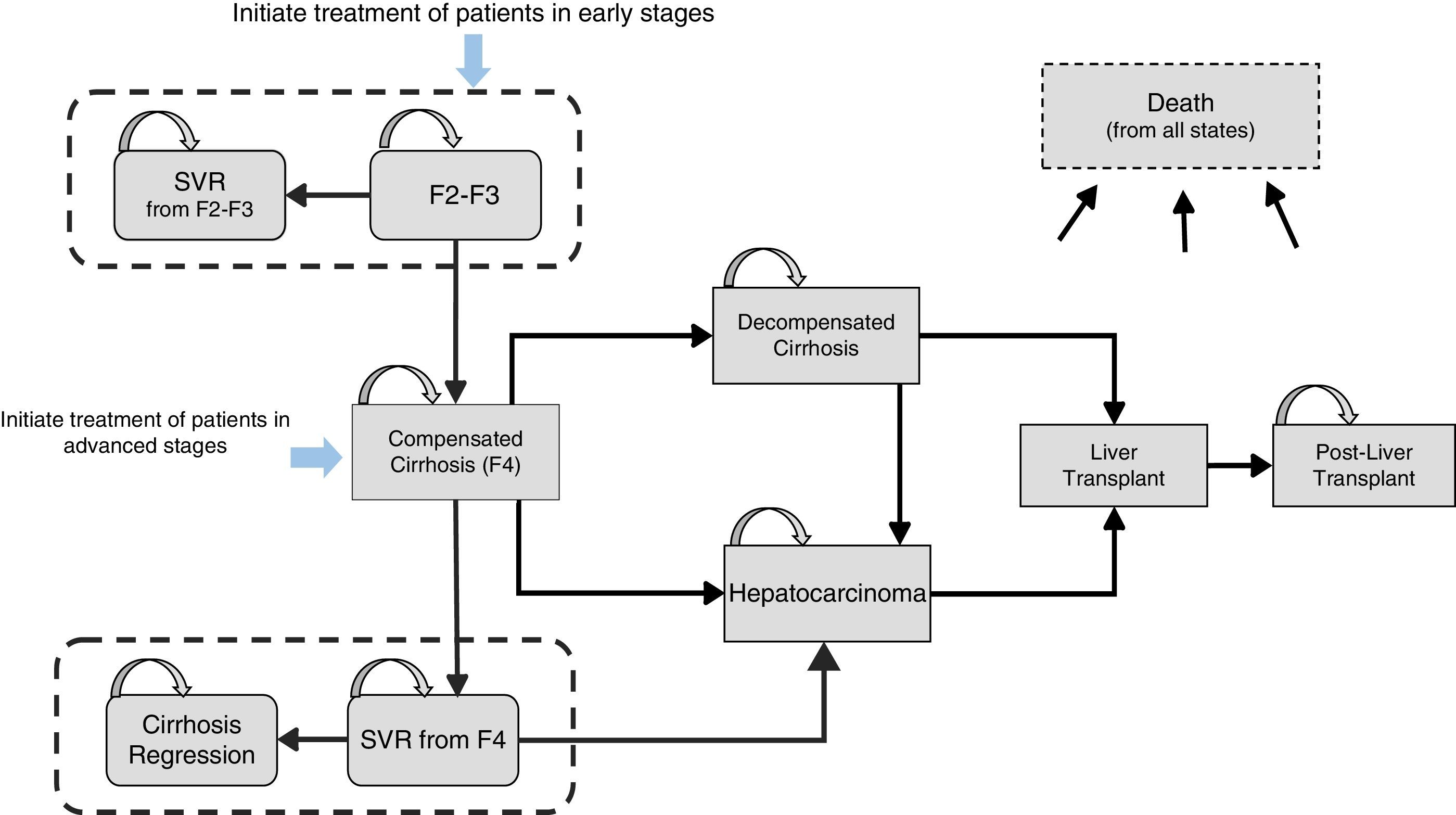
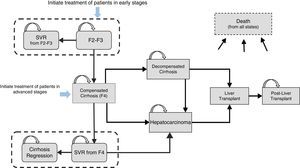
Materials and methodsDesign of the modelAn analytical decision-making model was designed with a Markov structure using Microsoft Excel 2013 to simulate the natural history of treatment-naive patients with CHC, GT1 (Fig. 1), and to evaluate the lifetime health costs and outcomes produced by two 12-week SOF/Peg-IFN/RBV treatment strategies in 2 different stages of liver fibrosis: (a) administration in early stages of the disease, represented by patients with mild-moderate fibrosis (METAVIR F2–F3, no cirrhosis), and (b) administration in advanced fibrosis (METAVIR F4, with compensated cirrhosis).
The model considered a long time frame, encompassing the patients’ entire life expectancy. Thus, the life years gained (LYG), quality-adjusted life years (QALYs) and total costs attributable to each of the 2 treatment strategies were estimated, from the perspective of the Spanish NHS.
Model structure and transition probabilitiesThe model, which includes 10 mutually exclusive health states, is based on annual dynamic probabilities that reflect disease progression. The reference probabilities were obtained from the literature,17,26–28 and were subsequently adjusted for each year based on age-adjusted rates for all-cause mortality and non-liver-related mortality (Table 1). The duration of the Markov cycles was 1 year, except for the first cycle, in which two 6-month periods were calculated in order to apply the efficacy reported in clinical trials more appropriately.24
Parameters used in the model.SVR, probabilities and utilities.
| Variable | Annual baseline value |
|---|---|
| SVR rate in stages F2–F324 | 0.913a |
| SVR rate in stage F424 | 0.808a |
| Reference annual transition probabilities | |
| F2–F3 to F426 | 0.073 |
| F4 | |
| To decompensated cirrhosis26 | 0.040b |
| To hepatocellular carcinoma26 | 0.015 |
| To death31 | 0.021 |
| SVR from stage F4 | |
| To cirrhosis regression17 | 0.169 |
| To hepatocellular carcinoma27,28 | 0.005 |
| Decompensated cirrhosis | |
| To hepatocellular carcinoma31 | 0.068 |
| To liver transplant28 | 0.023 |
| To death31 | 0.138 |
| Hepatocellular carcinoma | |
| To liver transplant28 | 0.040 |
| To death26 | 0.860 |
| Liver transplant to death26 | 0.210 |
| Post-liver transplant to death26 | 0.057 |
| Utility values by health states | |
| Stages F2–F3f | 0.72c |
| SVR from stages F2–F3f | 0.77 |
| F438 | 0.55 |
| SVR from stage F4f | 0.59d |
| Cirrhosis regressionf | 0.59d,e |
| Descompensated cirrhosis34 | 0.45 |
| Hepatocellular carcinoma34 | 0.45 |
| Liver transplant34 | 0.45 |
| Post-liver transplant34 | 0.67 |
Stages F2–F3: mild-moderate fibrosis; Stage F4: compensated cirrhosis; SVR: sustained virologic response.
The SVR rate was adjusted to patients with GT1 according to the fibrosis stage and total number of patients by genotypes.
The probability includes the sub-states considered in decompensated cirrhosis (ascites, hepatic encephalopathy, gastrointestinal bleeding due to portal hypertension and severe bacterial infection).
The utility value in stages F2–F3 was estimated on the basis of the average utilities in stage F2 and stage F3.
The model estimated the health costs and benefits achieved in 2 hypothetical cohorts (patients treated with SOF/Peg-IFN/RBV in early stage and patients treated with SOF/Peg-IFN/RBV in advanced fibrosis) with the same baseline characteristics: 1000 treatment-naive patients with an average age of 52 years, with HCV GT1 infection.
Individual simulations were carried out for each of the 2 cohorts, assuming in both cases that patients completed a single 12-week treatment, without considering treatment discontinuation or interruption due to lack of response or adverse effects, or retreatment.
The efficacy rates (SVR) were obtained from the NEUTRINO study.24 This trial found rates of 92.3% and 79.6% for patients with and without cirrhosis, respectively. The SVR rate is available for each of the different genotypes (89.4% for GT1), but not separately for GT1 patients with or without cirrhosis. In the absence of this information, SVR rates were adjusted to estimate the transition probability required by the model.
The simulation of the cohort treated in early stages was initiated with the onset of stage F2–F3. Patients who achieved an SVR were considered cured from a clinical and virological point of view and transitioned to the “SVR from stages F2–F3” state. The simulation of the cohort treated in advanced fibrosis was initiated with all patients in stage F4. Patients with SVR were considered virologically cured, which meant transition to the “SVR from stage F4” state, while maintaining a certain risk of developing HCC29 (Fig. 1). In accordance with the published evidence, a certain proportion (61%)17 of cirrhotic patients who achieve an SVR after treatment can show clinical improvement, which, in the present model, is reflected in the transition probability of patients with SVR from stage F4 to the “cirrhosis regression” state.
Based on the results of other studies that have shown an association between SVR and cure,12,13,16,18,30 patients with SVR from stages F2–F3 and patients in cirrhosis regression were considered healthy patients, so the possibility of CHC-related complications was not considered during the rest of the simulation, and the life expectancy of the general population was applied to this group.
In contrast, patients with no SVR were considered treatment failures and continued to present a risk of disease progression to more advanced stages, such as compensated cirrhosis (CC), decompensated cirrhosis (DC) and HCC, based on the natural history of the disease (Fig. 1). Patients who presented DC or HCC were eligible for liver transplant. Liver transplant survivors transitioned to the “post-liver transplant” state, with the subsequent possibility of progression to death (Fig. 1).
Data for liver-related mortality for the DC, HCC, liver transplant and post-liver transplant states were estimated from various studies.26,31 Liver-related deaths were attributed to hepatic complications, excluding other causes of death. Furthermore, the model considered the possibility of death for a non-liver-related cause, the probabilities of which were estimated after considering data for all cause mortality32 and liver-related mortality,33 age-adjusted for the Spanish population.
UtilitiesIn order to include health-related quality of life in these types of patients, utility values obtained from the literature for the different health states were applied.34 The utilities are represented in an interval from 0 to 1, where 0 represents death and 1 a state of perfect health. The annual utility values used are shown in the data obtained using the EuroQoL 5 questionnaire (EQ-5D) in a representative sample of the United Kingdom population34 (Table 1).
CostsIn line with the focus of the analysis, the model considered only direct healthcare costs (pharmacological cost and cost of disease management in each health state).
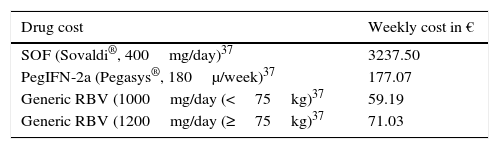
The pharmacological costs of the 12 weeks of treatment were calculated based on the recommended posology: 400mg of SOF daily, together with 1000mg or 1200mg of RBV according to the weight of the Spanish population (<75kg [43.8%] or ≥75kg [56.2%])35 and 1 weekly 180μg dose of Peg-IFN alpha-2a.24 The costs of SOF and Peg-IFN were estimated from published manufacturer's list prices, applying the deduction required by Spanish Royal Decree 8/2010.36 The least expensive RBV was selected for the cost of RBV. Drug prices were obtained from the Drug Catalogue of the Spanish Consejo General de Colegios Oficiales de Farmacéuticos (General Spanish Council of Pharmacists).37
The costs of disease management in each health state were obtained from the literature published for Spain, and were updated to 2014 values with the corresponding retail price index (RPI).40
All costs are expressed in Euros (€, 2014). Table 2 shows the unit costs used.
Drug costs and by health states.
| Drug cost | Weekly cost in € |
|---|---|
| SOF (Sovaldi®, 400mg/day)37 | 3237.50 |
| PegIFN-2a (Pegasys®, 180μ/week)37 | 177.07 |
| Generic RBV (1000mg/day (<75kg)37 | 59.19 |
| Generic RBV (1200mg/day (≥75kg)37 | 71.03 |
| Costs by health states | Annual cost in € |
|---|---|
| Stages F2–F338 | 241.92 |
| SVR from stages F2–F3a | 0.00 |
| Stage F439 | 449.32 |
| SVR from stage F4a | 449.32 |
| Cirrhosis regressiona | 0.00 |
| Descompensated cirrhosis39 | 1532.73 |
| Hepatocellular carcinoma39 | 7019.17 |
| Liver transplant39 | 143,647.97 |
| Post-liver transplant39 | 14,863.97 |
Stage F2–F3: mild-moderate fibrosis; Stage F4: compensated cirrhosis; PegIFN: peginterferon alpha-2a; RBV: ribavirin; SVR: sustained virologic response; SOF: sofosbuvir.
Health costs and outcomes were discounted annually at 3%.41
Sensitivity analysisUnivariate deterministic sensitivity analysis (DSA) and probabilistic sensitivity analysis (PSA) were carried out to evaluate the robustness of the model and the uncertainty of outcomes of the base case.
A range of variation of the parameters considered most relevant was applied in the DSA. The transition probabilities (probability of CC from F2 to F3, risk of HCC in patients with SVR from stages F2 to F3 and cirrhosis regression in patients with SVR from F4), the costs of disease management and the utility values were modified in a range ±25%. The SVR rates were modified with the upper and lower value of the 95% confidence interval (95% CI) reported in the NEUTRINO study.24 The effect of alternative annual discount rates was also explored (0% and 5%).
The PSA was performed using 5000 Monte Carlo simulations, applying the Dirichlet distribution to the transition probabilities. The utility values were modified using a beta distribution and the disease management costs with a gamma distribution.42
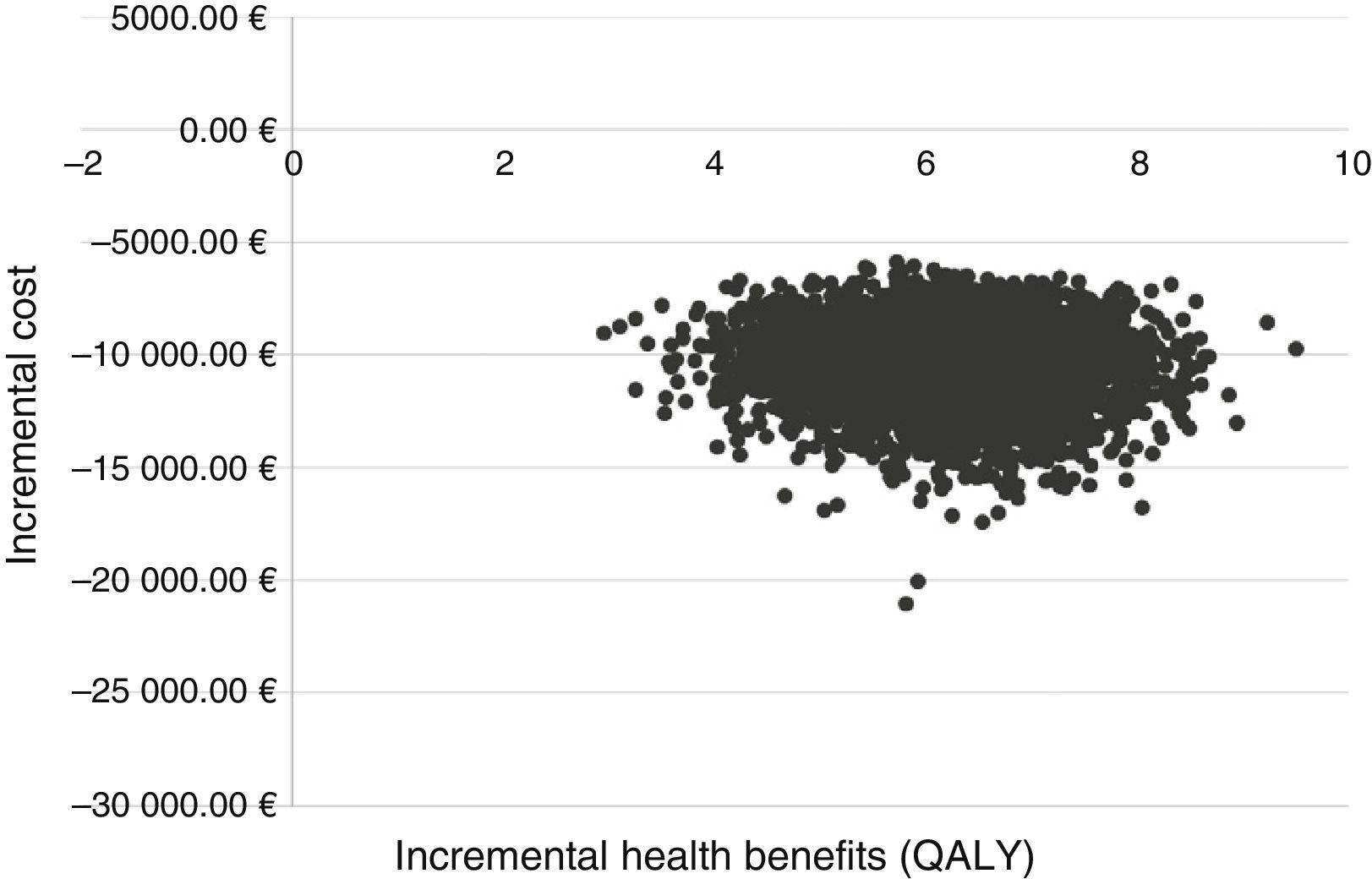
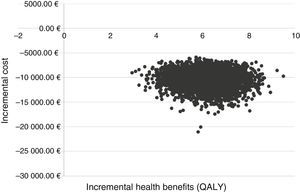
The incremental cost–effectiveness ratio (ICER) derived from the health costs and benefits of each of the 5000 simulations performed is shown graphically in a cost–effectiveness plan.43
ResultsBase caseTherapy with SOF/Peg-IFN/RBV in early stages (F2–F3) generated 19.12 LYG and 14.14 QALYs, while therapy in advanced fibrosis (F4) was associated with 16.36 LYG and 9.27 QALYs. In terms of survival, the strategy of starting therapy in HCV-positive patients in early stages was more effective than starting treatment in advanced fibrosis (Table 3).
Results of the base case.
| Therapy in early stage | Therapy in advanced fibrosis | Incremental difference (early stage vs. advanced fibrosis) | |
|---|---|---|---|
| LYG | 19.12 | 16.36 | 2.76 |
| QALY | 14.14 | 9.27 | 4.87 |
| Average total cost | €43,263.44 | €49,018.85 | –€5755.41 |
| Number of cases | Therapy in early stage | Therapy in advanced fibrosis | Cases prevented (early stage vs. advanced fibrosis) |
|---|---|---|---|
| Decompensated cirrhosis | 38 | 104 | 66 |
| Hepatocellular carcinoma | 17 | 77 | 60 |
| Liver transplant | 1 | 5 | 4 |
Therapy in early stage (F2–F3); Therapy in advanced fibrosis (F4).
QALY: quality-adjusted life years; LYG: life years gained.
In the hypothetical cohort of 1000 patients, SOF/Peg-IFN/RBV therapy in early stages would reduce the incidence of hepatic complications, resulting in the prevention of 66 cases of DC, 60 cases of HCC and 4 cases of liver transplant during the period analysed, compared with starting therapy in advanced fibrosis (Table 3).
The estimated total costs for early therapy were lower than the total cost of therapy in advanced fibrosis (€43,263.44 vs €49,018.85) (Table 3).
With greater effectiveness and lower costs, the strategy of starting therapy in early stages was a dominant treatment option with respect to starting therapy in advanced fibrosis.
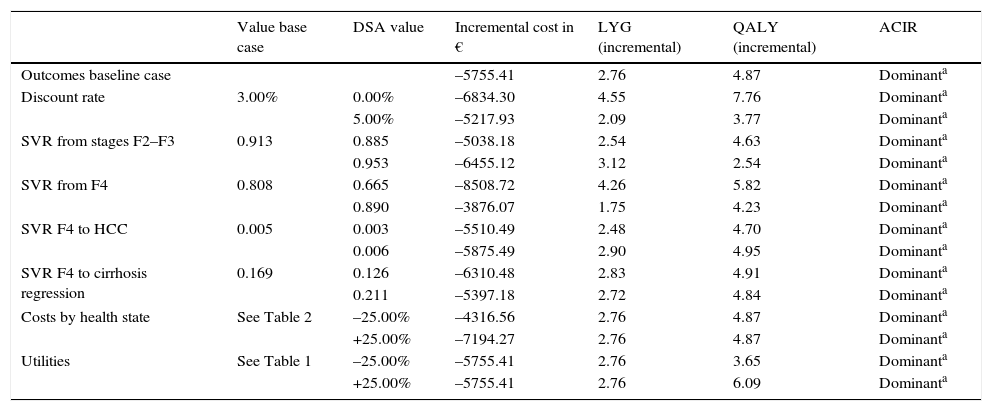
Sensitivity analysisIn the DSA, the parameters that most affected the outcomes were the SVR rates in each fibrosis stage, the discount rate and the utility values. When the SVR rates were modified by the same proportion in both groups, no differences were observed with respect to the outcomes of the base case, maintaining greater health benefits and savings when therapy was started in patients in early stages with respect to starting therapy in advanced fibrosis. The increase in the SVR rate of therapy, when administered in patients with advanced fibrosis, meant a reduction in the incremental differences of therapy in early stages with respect to administration in advanced fibrosis.
In the DSA performed with SVR rates reported in the NEUTRINO study (92.3% in F2–F3 patients and 79.6% in F4 patients),24 5.04 QALYs were obtained instead of the 4.87 QALYs in the base case, on using SVR rates estimated for patients with GT1 (91.3% and 80.8%, for early and advanced fibrosis, respectively).
SOF/Peg-IFN/RBV therapy in early stages was a dominant option (more effective and with lower associated cost) with respect to therapy in advanced fibrosis in all the DSA performed (Table 4).
Results of the deterministic sensitivity analysis (patients in early stage [F2–F3] vs. patients in advanced stages [F4]).
| Value base case | DSA value | Incremental cost in € | LYG (incremental) | QALY (incremental) | ACIR | |
|---|---|---|---|---|---|---|
| Outcomes baseline case | –5755.41 | 2.76 | 4.87 | Dominanta | ||
| Discount rate | 3.00% | 0.00% | –6834.30 | 4.55 | 7.76 | Dominanta |
| 5.00% | –5217.93 | 2.09 | 3.77 | Dominanta | ||
| SVR from stages F2–F3 | 0.913 | 0.885 | –5038.18 | 2.54 | 4.63 | Dominanta |
| 0.953 | –6455.12 | 3.12 | 2.54 | Dominanta | ||
| SVR from F4 | 0.808 | 0.665 | –8508.72 | 4.26 | 5.82 | Dominanta |
| 0.890 | –3876.07 | 1.75 | 4.23 | Dominanta | ||
| SVR F4 to HCC | 0.005 | 0.003 | –5510.49 | 2.48 | 4.70 | Dominanta |
| 0.006 | –5875.49 | 2.90 | 4.95 | Dominanta | ||
| SVR F4 to cirrhosis regression | 0.169 | 0.126 | –6310.48 | 2.83 | 4.91 | Dominanta |
| 0.211 | –5397.18 | 2.72 | 4.84 | Dominanta | ||
| Costs by health state | See Table 2 | –25.00% | –4316.56 | 2.76 | 4.87 | Dominanta |
| +25.00% | –7194.27 | 2.76 | 4.87 | Dominanta | ||
| Utilities | See Table 1 | –25.00% | –5755.41 | 2.76 | 3.65 | Dominanta |
| +25.00% | –5755.41 | 2.76 | 6.09 | Dominanta |
DSA: deterministic sensitivity analysis; QALY: quality-adjusted life years; LYG: life years gained; HCC: hepatocellular carcinoma; ICER: incremental cost–effectiveness ratio; SVR: sustained virologic response.
In the PSA, the treatment strategy with SOF/Peg-IFN/RBV in early stages was dominant versus treatment in advanced fibrosis in all the simulations (Fig. 2).
DiscussionAs far as the authors are aware, this study is the first cost–effectiveness analysis to evaluate decision-making strategies with regard to starting SOF/Peg-IFN/RBV therapy according to the grade of hepatic fibrosis in patients (early stages, F2–F3 vs advanced fibrosis, F4).
The results of the analysis of this study showed that starting treatment in early stages would be associated with a decrease in the incidence of developing DC, HCC and liver transplant. These findings are consistent with those reported in a previous study that suggested that the reduction in the incidence of developing hepatic complications is achieved more easily if patients are treated in the early stages of the disease.44
Furthermore, the results support the hypothesis that early treatment with respect to treatment in advanced stages of the disease is associated with a significant increase in life expectancy, as well as quality of life in patients with CHC, due to prevention of disease progression.25
HCV infection has a major economic impact due to the economic burden imposed by patients with CHC.15 In this respect, the present analysis suggests that SOF/Peg-IFN/RBV therapy in early stages could be less expensive than therapy in advanced fibrosis, generating major cost savings for the healthcare system.
The cost–effectiveness ratio of therapeutic strategies for CHC have been evaluated in various studies, in different populations (in treatment-naive and in previously treated patients) with different HCV genotypes and at different stages of the disease. However, few studies have evaluated the efficiency of SOF therapies according to the grade of fibrosis and/or the existence of advanced disease.11,31,45–50
The present analysis presents, however, substantial differences with respect to previously published cost–effectiveness analyses of SOF therapies, most of which compared therapeutic regimens with SOF versus other therapies for CHC. This study is the first to date to compare the clinical and economic impact of the decision to start combined triple therapy treatment with SOF/Peg-IFN/RBV according to the grade of hepatic fibrosis. The distinction between early stages, grouping stages METAVIR F2 and F3, and advanced fibrosis stages (METAVIR F4) was determined by the availability of clinical data reported in the NEUTRINO trial.24
In one of the cost–effective analyses available for Spain,46 various therapeutic options with SOF were evaluated, including triple therapy with SOF/Peg-IFN/RBV, in treatment-naive or pretreated patients, with different HCV genotypes. The analysis showed that the ICER of a 12-week regimen with SOF/Peg-IFN/RBV versus combined therapy was below the efficiency threshold considered by the authors (€40,000/QALY). The study included DSA, where the effect of a different distribution of grades of fibrosis (F2, F3 and F4) was examined, but failed to establish comparisons between the same therapy administered in cohorts with different grades of fibrosis.
Differences in methodology and design make it difficult to compare our model with other cost–effectiveness studies. In any event, our findings are consistent with other published evaluations as regards the reduction in cases of hepatic complications.51,52
The main study limitations are associated with the absence of utility data in the Spanish population in the scientific literature, which obliged us to make a number of assumptions. In particular, the absence of data specifically referring to the Spanish population meant that we had to use utility values obtained in a sample of HCV-positive patients in the United Kingdom.34 However, there is evidence that EQ-5D questionnaire values in Western Europe (Germany, Spain, Finland, the Netherlands, United Kingdom and Sweden) can be described by a common model.53 The DSA that was carried out by modifying the values of these parameters revealed that an increase or decrease in utilities in the QALYs gained with therapy in early stages or in advanced fibrosis has a major impact.
This analysis did not consider the possibility of treatment discontinuation or interruption due to lack of efficacy or onset of adverse effects, although data obtained in the NEUTRINO trial24 showed a small rate of discontinuation (2%) in all patients. In clinical practice, the discontinuation rate could be higher in patients with advanced stages of the disease than in those with early stages, which would have some impact on the pharmacological cost outcomes provided by the present model. This potential decrease in the pharmacological cost would not, however, compensate for the additional costs associated with the higher risk of these patients developing hepatic complications, given that the degree of liver disease progression is associated with increased use of healthcare resources, and thus higher costs.54
Despite the limitations described and the assumptions made, the results of the sensitivity analyses confirmed that the uncertainty associated with the parameters did not cause major deviations with respect to the outcomes obtained in the base case. This adds credence to the conclusion that starting treatment in early stages is more efficient than starting therapy in advanced fibrosis. In the DSAs, the individual variation in each of the parameters had little impact on outcomes. The PSA also confirmed the robustness of the outcomes of the base case.
It should be noted that in the last year, new IFN-free therapies have emerged that show even greater improvements in SVR rates. As a result, SOF/Peg-IFN/RBV therapy is not considered the treatment of choice in some patient subgroups. Nevertheless, this analysis focused on demonstrating the efficacy of treatment in early versus advanced fibrosis. The results obtained support the hypothesis that administration at early stages prevents disease progression and with it, development of liver complications and even liver transplant. These findings could be extrapolated to the new therapies available and be used to determine the best time to initiate treatment.
The present economic evaluation demonstrates the efficiency of starting SOF/Peg-IFN/RBV therapy in treatment-naive patients with HCV GT1 in early stages of the disease, compared with the administration of therapy in patients with advanced fibrosis. The decision to treat in early stages increased patient survival and prevented the development of cirrhosis and other hepatic complications.
Conflict of interestsMaria Buti is an advisor for Gilead Sciences, Bristol Myers Squibb, MSD and Novartis.
Raquel Domínguez-Hernández, Itziar Oyagüez and Miguel Ángel Casado are employees of Pharmacoeconomics & Outcomes Research Iberia, a consultancy firm specialising in the economic evaluation of healthcare interventions, which has received unconditional funding from Gilead Sciences.
Please cite this article as: Buti M, Domínguez-Hernández R, Oyagüez I, Casado MÁ. Análisis coste-efectividad de sofosbuvir, interferón pegilado y ribavirina en pacientes con hepatitis crónica por virus C: tratamiento precoz en fases iniciales de fibrosis vs. tratamiento tardío en fases avanzadas. Gastroenterol Hepatol. 2016;39:449–457.