Acquired hepatocerebral degeneration (AHD) is an often debilitating neurological disorder characterized by a variety of movement disorders in the setting of chronic liver disease (CLD) and portosystemic shunt. AHD was first described by van Woerkem in 1914.1 Cirrhosis-related parkinsonism is the core manifestation in AHD and has been described to have a prevalence of 4.2%.2 Reports of myoclonus in patients with AHD are scarce3 and they always present along with other clinical manifestations, most often parkinsonism and cerebellar signs. Liver transplantation is presently the only therapeutic option that has been shown to ameliorate and even reverse neurological deterioration.4 Here, we describe the case of a 57-year-old woman with an unusual presentation of AHD who responded well to treatment with anti-ammonia therapy.
A 57-year-old woman was admitted to the emergency department because of altered mental status and abnormal upper-limbs movements. She had a history of primary biliary cirrhosis and reported no alcohol consumption. Viral markers for HIV, hepatitis B and C were all negative. She also had a history of esophageal varices and had a previous diagnosis of splenorenal shunt, which was detected by a contrast-enhanced abdominal computed tomography scan. Her regular medication included propranolol and ursodeoxycholic acid. In the past she has had several episodes of acute hepatic encephalopathy (AHE); these episodes have been successfully treated, in the outpatient setting, with oral L-ornithine-L-aspartate (LOLA) and oral lactulose.
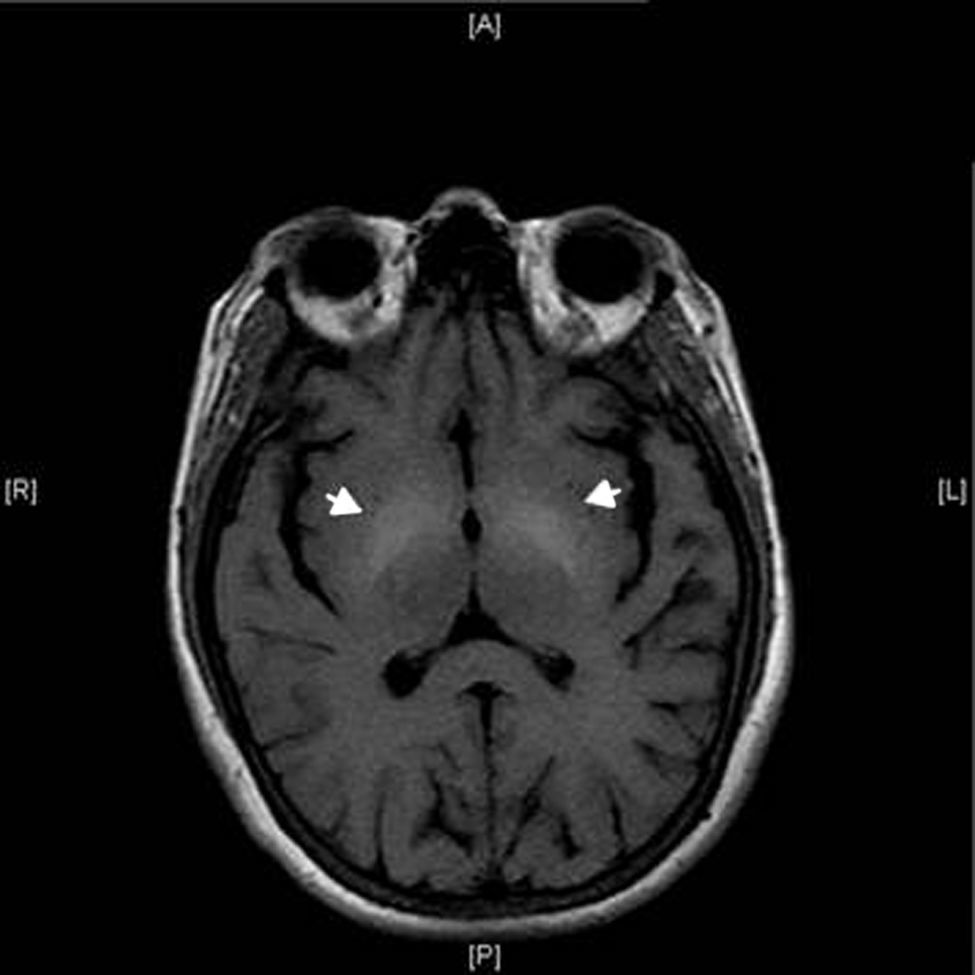
On admission our patient had an altered mental status. On physical exam, there was no jaundice, telangiectases, ascites, superficial collateral abdominal veins or hepatosplenomegaly. She had sudden, brief, shock-like jerks of her upper limbs consistent with myoclonus. Hyperreflexia was also found in the same limbs, and Babinski sign was elicited bilaterally. Kayser–Fleischer rings were not seen on ophthalmologic examination. She initially received lactose enemas as treatment for AHE, with improvement in her mental status. However, there was no change in her myoclonus. A brain magnetic resonance imaging (MRI) scan revealed bilateral symmetrical hyperintensities in lentiform nuclei on T1-weighted images (Fig. 1). She had a mild thrombocytopenia (148K/mm3) but neither anemia nor leukocytosis was found. She was euglycemic, and renal function was normal. Serum electrolytes and coagulation tests were non-contributory. She was not hypoalbuminemic but had a mild hyperbilirubinemia (total: 2.2mg/dL; conjugated: 0.6mg/dL); other liver function tests were as follow: serum glutamic–oxaloacetic transaminase 88U/L (10–42U/L), serum glutamate-pyruvate transaminase 42U/L (10–42U/L), alkaline phosphatase 134U/L (38–126U/L).
Based on MRI findings, we diagnosed AHD and ammonia-lowering therapy was continued to treat her myoclonus, since liver transplantation was not a practical possibility in our setting. The patient showed a mild improvement during her hospital stay. When her mental status normalized, after one week of treatment, she was discharged on oral lactulose (20g thrice daily), rifaximin (400mg twice daily) and LOLA (6g thrice daily). She was then evaluated weekly for tolerability and adherence. One month later she presented to the office with complete resolution of her myoclonus, and we decided to continue the same treatment. After 18 months of follow up, the patient continues with ammonia-lowering therapy and has not had any recurrence of movement disorders or encephalopathy.
CLD may be associated with a wide variety of motor and neuropsychiatric manifestations as a result of the diversion of portal blood flow to the systemic circulation, presumably through toxic effects of chemical substances that cross the blood–brain barrier. AHD is a chronic encephalopathy characterized by cognitive impairment, parkinsonism, and other movement disorders besides myoclonus, including ataxia, chorea, and dystonia.5,6 Pathophysiological mechanisms are poorly understood and many factors are probably involved. Ammonia and manganese are both candidate substances for chronic neurological dysfunction in liver diseases, and more recently it has been demonstrated that there is a pathologically decreased striatal dopamine D2 receptor availability and decreased dopamine transporter availability in the pathogenesis of cirrhosis-related parkinsonism.7 Brain MRI typically showed high intensity signal in the lenticular nuclei on T1-weighted images, which differentiates this entity from AHE.8
Although chronic AHD can present with almost any kind of movement disorder, myoclonus is an extremely rare symptom that has been very seldom reported.6 At least one other case has been described in the literature, involving a patient with Budd-Chiari syndrome and T1 hyperintensities in pallidum and substantia nigra.9 In CLD and portosystemic shunt, ammonia toxicity and manganese accumulation in the mitochondria of glial cells in the pallidum and other basal ganglia structures may lead to disruption of energy metabolism and the characteristic imaging findings.5,7,10 In general, dopaminergic treatment is ineffective, manganese chelation unavailable, and anti-ammonia therapy often without any substantial benefit, leaving liver transplantation as the most reasonable alternative.2–5,7 In our case, anti-ammonia treatment proved successful, suggesting that this therapy should be attempted in settings where liver transplantation is not possible.