Diverticular disease represents the most common disease affecting the colon in the Western world. Most cases remain asymptomatic, but some others will have symptoms or develop complications. The aims of treatment in symptomatic uncomplicated diverticular disease are to prevent complications and reduce the frequency and intensity of symptoms. Fibre, probiotics, mesalazine, rifaximin and their combinations seem to be usually an effective therapy. In the uncomplicated diverticulitis, outpatient management is considered the optimal approach in the majority of patients, and oral antibiotics remain the mainstay of treatment. Admission to hospital and intravenous antibiotic are recommended only when the patient is unable to intake food orally, affected by severe comorbidity or does not improve. However, inpatient management and intravenous antibiotics are necessary in complicated diverticulitis. The role of surgery is also changing. Most diverticulitis-associated abscesses can be treated with antibiotics and/or percutaneous drainage and emergency surgery is considered only in patients with acute peritonitis. Finally, patient related factors, and not the number of recurrences, play the most important role in selecting recipients of elective surgery to avoid recurrences.
La enfermedad diverticular es la enfermedad cólica más frecuente en el mundo Occidental. La mayoría de los pacientes permanecerán asintomáticos a lo largo de su vida, pero un porcentaje no despreciable presentarán síntomas o desarrollarán complicaciones. El objetivo del tratamiento en la enfermedad diverticular no complicada sintomática es prevenir las complicaciones y reducir la frecuencia e intensidad de los síntomas. La fibra, los probióticos, la mesalazina, la rifaximina y sus combinaciones parecen ser terapias eficaces. En la diverticulitis no complicada, el manejo extrahospitalario se considera actualmente el manejo óptimo, siendo los antibióticos administrados por vía oral la piedra angular del tratamiento. El ingreso hospitalario solo será necesario en pacientes con intolerancia oral, comorbilidad grave o ausencia de mejoría. Sin embargo, el manejo intrahospitalario es preciso en las diverticulitis complicadas. La mayoría de los abscesos podrán ser tratados con antibióticos y/o drenaje percutáneo, reservando la cirugía urgente para pacientes con peritonitis aguda. La indicación de cirugía electiva para prevención de recurrencias debe ser indidualizada y no basarse únicamente en el número de episodios previos de diverticulitis.
Diverticular disease of the colon represents the most common disease affecting the large bowel in the Western world. The prevalence of diverticular disease has increased over the past century throughout the world, probably because of changes in lifestyle, such as smoking, overweight, and over all physical inactivity and low fibre diet. The prevalence increases with age, ranging from approximately 5% in adults younger than 40 years of age to 50–70% among those 80 years of age or older; 80% of patients who present with diverticulitis are 50 or older. Diverticula can present in number from solitary to hundreds, they are typically 5–10mm in diameter, but can exceed 2cm in size. Diverticulosis occurs primarily in the sigmoid and descending colon in more than 90% of patients, but may be prevalent in varying degrees in the rest of the colon.1
There are several diverticular-related terms that will be used in this review. The presence of diverticula in the colon in the absence of overt inflammation is called diverticulosis or uncomplicated diverticular disease (UDD). It may be symptomatic or asymptomatic. The term “acute colonic diverticulitis” (ACD) is used to describe inflammation of the diverticula, which may or may not progress to complications (complicated ACD). There is also chronic diverticulitis, because of recurrent diverticulitis or because of the development of a segmental colitis associated with the diverticula. Summarily, the clinical spectrum of diverticular disease is wide.
Studies on the natural history of the disease point out that a large majority of patients with diverticula (about 80%) will remain asymptomatic throughout their life. Of the 15–20% who develop symptoms, approximately 1/4 will eventually have an episode of symptomatic painful diverticular disease without inflammation, and up to 10–25% will have an episode of ACD. About 1–2% will require hospitalization and 0.5% surgery. Diverticula are responsible for the majority (24–42%) of episodes of lower gastrointestinal bleeding.2–4
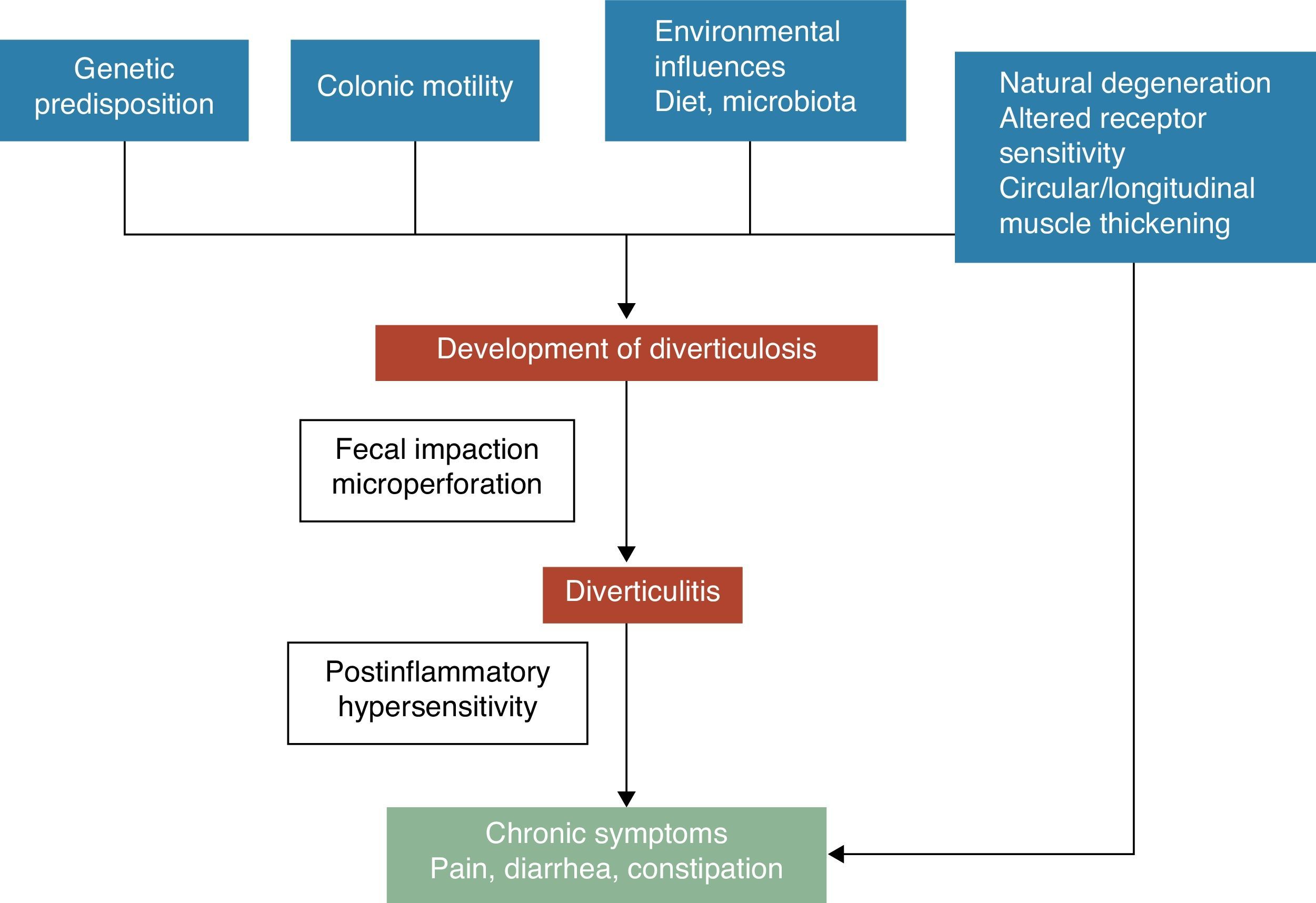
Physiopathology and symptom developmentA colonic diverticulum is a herniation of mucosa and submucosa, corresponding to a weak point where the vasa recti penetrate the tunica muscularis. The pathogenetic mechanisms of diverticular disease are still poorly understood, however it is generally recognized that these are probably related to complex interactions among diet, colonic microbiota, genetic factors, colonic motility and structure that result in their formation of colonic diverticula over time.5 See Fig. 1. In 1971, Painter and Burkitt published their famous hypothesis that diverticular disease was caused by excess pressure in the colon due to segmentation based on insufficient intake of dietary fibre. In response to increased intraluminal pressure, outpouchings may develop and protrude at areas of potential weakness.6 Stasis or obstruction in the narrow necked diverticulum may lead to bacterial overgrowth and local tissue ischaemia ultimately leading to perforation.7 Since then, numerous observational studies tried to demonstrate the possible effect of fibre on preventing diverticular disease. Most of them concluded that the risk of UDD was inversely associated with dietary fibre intake.8–10 Based on these evidences, a high fibre diet is recommended to prevent diverticular disease in most current guidelines and position papers.11–15 However, this hypothesis has been recently challenged since: (1) the inverse association of fibre intake and diverticulosis has been questioned in some recent epidemiological studies16,17 and (2) new pathogenic hypothesis such as the neuropathic and myopathic hypothesis are emerging.18–22 Other factors that have been associated with an increased risk of diverticular disease include physical inactivity, constipation, obesity, and smoking.23–27
Symptoms development in diverticular disease is probably related to complex interactions among genetic features, colon structure, intestinal motility low grade inflammation and postinflammatory hypersensitivity.
The association between uncomplicated diverticular disease (UDD) and symptoms is uncertain. There is some evidence to suggest that painful diverticular disease may be a condition related to inflammation and its effects on neuromuscular function in the colon.22–28 The presence of a chronic, low-grade intestinal inflammation would induce a sensory-motor dysfunction, leading to symptom development and/or persistence. Changes in intestinal micro flora could be one of the putative mechanisms responsible for low-grade inflammation. Bacterial overgrowth aided by the faecal stasis inside the diverticula could contribute to chronic low-grade inflammation that sensitizes both intrinsic primary efferent and extrinsic primary afferent neurons. These alterations could lead to smooth muscle hypertrophy, and increased sensitivity to abdominal distension, and finally, to symptom development.3,22 See Fig. 1.
As we mentioned above, most people with colonic diverticulosis remain asymptomatic, but eventually can develop complications as ACD or diverticula bleeding. In this article we focus on ACD. The clinical manifestation of this event will depend on a number of factors, including the size of the perforation, the level of extracolonic contamination, and the body's ability to contain this contamination.29,30
Management of diverticular diseaseManagement of uncomplicated diverticular disease (UDD)In patients with asymptomatic UDD,2 a high fibre diet may be recommended because of its possible prophylactic benefit in preventing symptomatic UDD and complications. There is no evidence that other drugs are useful in these patients.
There is more evidence on the benefit of treatment in symptomatic UDD. The most frequent symptom is abdominal pain, which may be exacerbated by eating and eased by defecation or the passage of flatus. Other symptoms are nausea, diarrhoea, constipation and bloating. Over 61% of patients with symptomatic UDD who are not taking any therapeutic measure to prevent recurrence of symptoms will become symptomatic within 1 year, and about 4% will develop complications.31
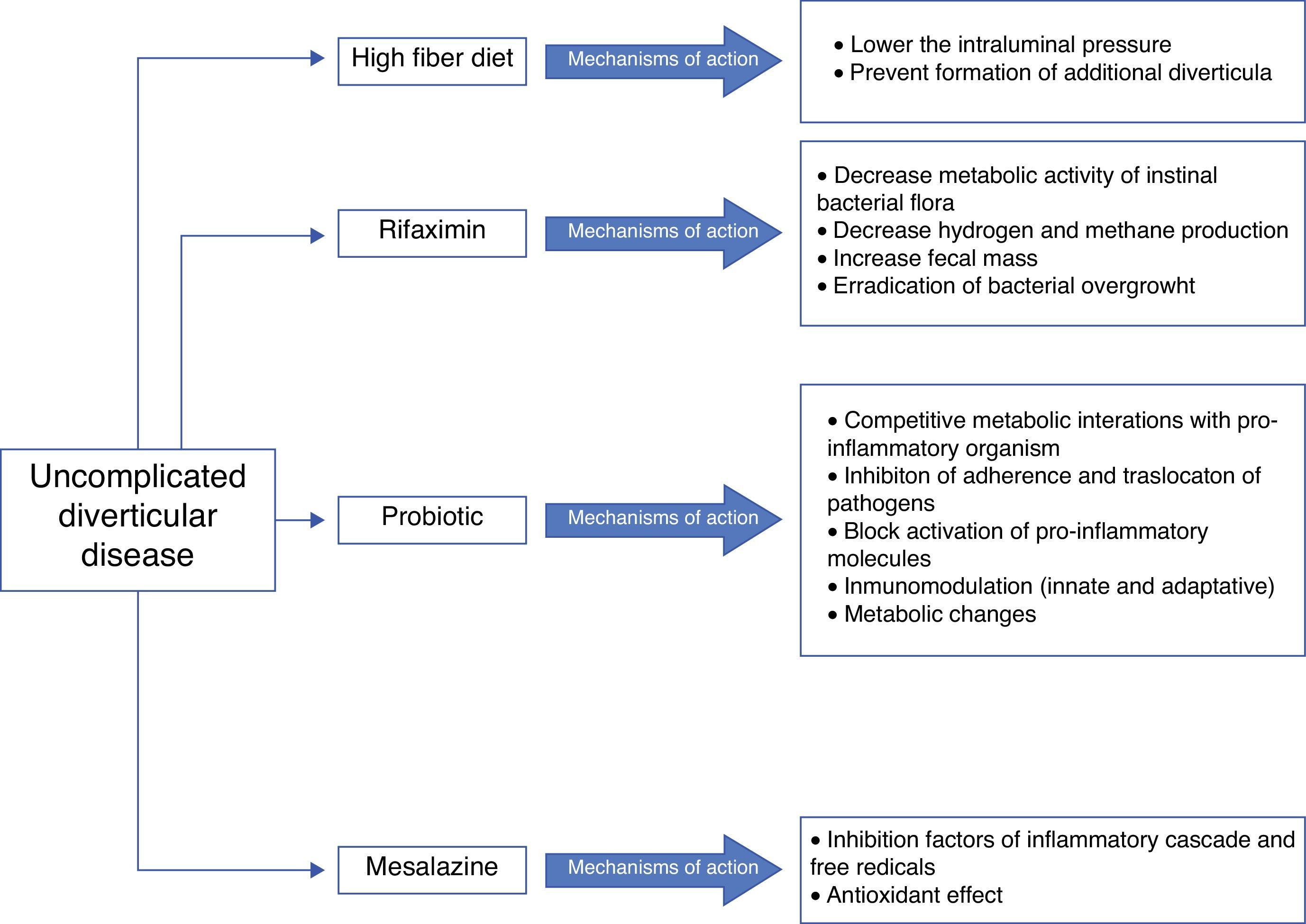
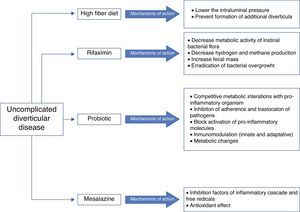
Five agents have been proposed for treatment (see Fig. 2):
A) High fiber diet or bulking agentsSeveral randomized controlled trials (RCT) and other interventional studies evaluate the effect of fibre in symptomatic UDD, but with inconsistent results.32–37 In any rate, fibre is recommended in the prevention and treatment of symptomatic UDD, as well as in the prevention of ACD by most of the current guidelines and position papers.11–15
B) Antibiotic therapyThe rationale for the use of antibiotics in symptomatic UDD is not clearly established. Recent studies suggest that changes in gut microbiota (intestinal bacterial overgrowth) could contribute to symptoms development due to excessive production of bowel gas through carbohydrate fermentation. In order to avoid systematic effects, poorly absorbed antimicrobials that act against enteric pathogens but have minimal risk of systematic toxicity or side effects seem to be the most appropriate antibiotics. Rifaximin has been proposed.
RifaximinRifaximin is a non-systematic rifamycin analogue with a broad spectrum of activity in vitro. Rifaximin may decrease metabolic activity of bowel flora, increasing faecal mass, and may also eradicate bacterial overgrowth. This antibiotic has a high safety and high tolerability profile.33,38 Plasma level of rifaximin is minimum, therefore non-enteric pathogens are not exposed to selective pressure and the risk of bacterial resistance is low.39 Three open and two double blind RCTs40–44 have examined the effectiveness of cyclic administration of rifaximin and fibre in reducing symptoms compared with fibre alone. A systematic review and two meta-analysis have analysed these trials.44–46 They concluded that combined treatment is effective in obtaining symptom relief at 1 year in patients with UDD. 35% of patients treated with fibre alone were asymptomatic compared with 64% in groups of combined treatment. The number needed to treat was three for rifaximin vs. placebo to relieve symptom and nine to avoid complications. Summary, the best results have been obtained using a combination of soluble fibre, such as glucomannan, and rifaximin 1 week every month.
C) ProbioticsProbiotics are live microorganisms that can restore commensal gut flora that may have been altered in diverticular disease due to stasis and reduced colonic transit time.47 Unfortunately, there are few data available about its use in symptomatic UDD and most of studies are small and uncontrolled. The majority of them show symptoms improvement.48–50
Probiotics have also studied in combination with 5-aminosalicylate (5-ASA). Tursi and colleagues have conducted three RCTs comparing 5-ASA alone, probiotic alone or combination therapy.51–53 Both 5-ASA and probiotics appeared to be effective for the prevention of symptomatic UDD but their combination was better. A recent double-bind RCT published by this same scientific group concluded that both cyclic mesalazine and Lactobacillus casei subsp DG, particularly in combination, seem to be better than placebo for maintaining remission of symptomatic UDD.54 But, in summary, the poor study designs and small size of them do not allow definitive conclusions.
D) 5-ASA: mesalazineMesalazine has anti-inflammatory and antioxidant effects. In 2010, Gatta et al.,55 published a Cochrane systematic review that evaluated the role of 5-ASA in patients with diverticular disease. Authors concluded that 5-ASA may be effective in the treatment of this disease and that everyday mesalazine was better than cyclic administration to prevent relapse. High quality well-designed RCTs are necessary to confirm their observations. In fact, the first placebo-controlled double-blind trial found mesalazine effective in obtaining pain relief in patients with acute UDD.56 Also, there are two interesting RCTs that showed a benefit for mesalazine compared with rifaximin in terms on preventing symptomatic recurrence and similar success in maintaining long-term remission compared with the probiotic Lactobacillus casei.53,57
E) Anticholinergic/antiespasmodic agentsThe hipermotility of the colon in diverticulosis suggests than antispasmodic agents such as dicyclomine and hyoscyamine might improve symptoms by decreasing muscular contraction. But, there are no RCTs that confirm this benefit.
F) Avoid NSAIDs treatmentSeveral controlled studies have shown that NSAIDs are a risk factor for the development of symptoms, ACD, perforation and bleeding.7,58–60 NSAID users have more risk to develop symptomatic diverticular diseases than non-users (RR: 1.5, CI 95%: 1.1–2.1).58 And in patients with complicated diverticular diseases there was a larger use of NSAIDs compared to controls without disease. It was postulated that this increased risk was due to mucosal damage resulting in impaired barrier function of the colonic mucosa allowing translocation of bacteria, which provoke inflammation.
G) Levels of vitamin DIt seems that the incidence of ACD has been associated with geographic and seasonal variation. Because of that, Maguire et al. conducted two interesting observational studies that showed that lower levels of vitamin D and low UV light exposure (UV exposure determines vitamin D status) are associated with significantly higher risk of ACD. More high quality studies are necessary before making a recommendation.61,62
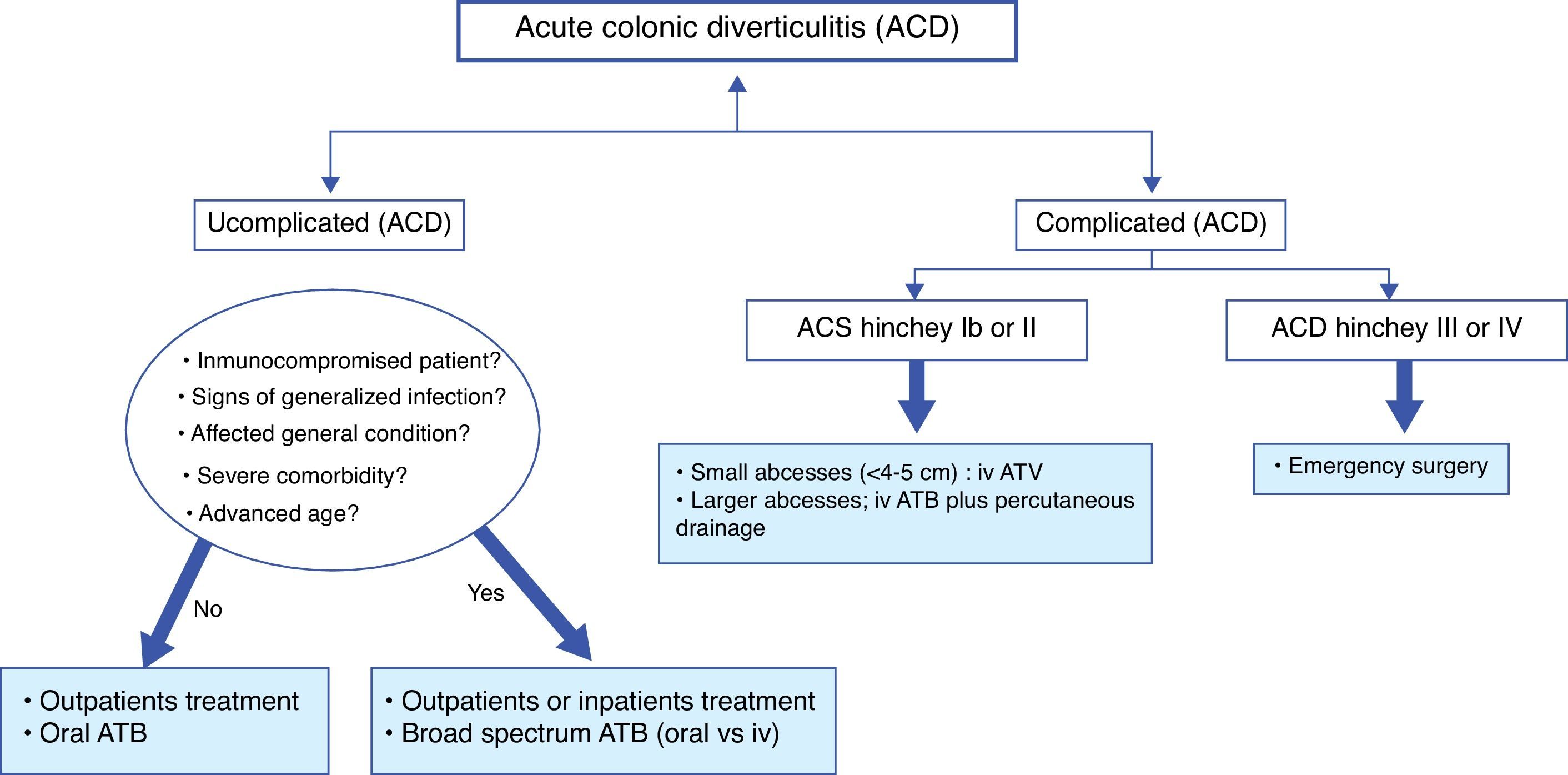
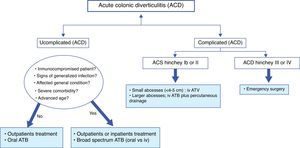
Treatment of acute colonic diverticulitis (ACD)Although most people with diverticulosis remain asymptomatic, it is estimated that about 10–25% of them will develop an episode of left ACD.63 Generally, clinical diagnosis is not sufficiently accurate and radiological techniques are indicated. In patients with mild symptoms (most) and without signs of complicated ACD, the combination of pain in lower left abdomen, the absence of vomiting and a C reactive protein >50mg/l, may be sufficient for the diagnosis.64,65 If imaging is indicated, probably, a conditional strategy with ultrasound as first line technique and followed by computerized tomography (CT), if ultrasound is inconclusive or doubtful, may represent the most effective approach. The number of CT exams may be reduced by 50%. In 1978, Hinchey et al. proposed a classification of ACD, which was modified later.66,67 It distinguishes five stages of ACD; stage 0, clinically mild diverticulitis, stage I (a: pericolic inflammation and b: abcess <5cm in the proximity of the primary inflammation), stage II, intraabdominal, pelvic or retroperitoneal abcess or abcess distant from the primary inflammation, stage III, generalized purulent peritonitis and stage IV, faecal peritonitis. See Fig. 3.
Treatment of uncomplicated ACD (Hinchey stage 0 or Ia)The majority of uncomplicated ACD can be safely treated conservatively with success rate between 70% and 100%.14 Outpatient treatment may allow important cost saving to the health systems. In cases of uncomplicated ACD, criteria for inpatient management are significant inflammation (include presence of fever or peritonitis), intolerance to oral fluids, age over 80–85 years, immunosuppression, or severe comorbidities. In most cases, a short hospital stay will be sufficient. There is no evidence that dietary restrictions influenced treatment outcomes, although most physicians usually recommend clear liquid diet.
One of the biggest recent changes in the management of uncomplicated ACD is the decreasing use of antibiotics. A recent Cochrane review, only a qualitative approach (with no meta-analysis), found that the best available data do no support its routine use.68 Antibiotics neither accelerate recovery nor prevent complications or recurrence. Therefore, the use of antibiotics in this case is questionable. Probably, they would be appropriate in patients with signs of generalized infection, signs of septicaemia or bacteraemia and in immunocompromised patients. Recommended regimens are based on clinical consensus. Various antibiotics may be used, ranging from ampicilin to third generation cephalosporins, as long as it is effective against gram positive, gram negative and anaerobic bacteria. The combination of ciprofloxacin and metronidazole is probably the most prescribed oral treatment. If this combination is poorly tolerated, ampicilin–sulphabactam may be a good choice. On the other hand, recent data have shown that there are no advantages of intravenous over oral antibiotics and of intravenous 4 days treatment over 7 days treatment.69–71 Usually, clinical improvement is observed within 3–4 days of treatment. Finally, admission to hospital with intravenous antibiotic is recommended when the patient is unable to intake food orally, is affected by severe comorbidity or does not improve with outpatient treatment.
Treatment of complicated ACD (Hinchey stage Ib to IV)ACD Hinchey Ib or II: abcessApproximately 15% of patients with ACD will develop an abscess.67 There is no high quality evidence about the most optimal management of ACD with abscess formation. Hospitalization is indicated. The size is an important determinant of successful treatment. In smaller abscesses (<4cm) is recommended conservative treatment with broad-spectrum antibiotics. It will be successful in up to 70%.72 When conservative treatment fails or in larger abscesses, percutaneous drainage should be performed. It is successful in up to 80%.73 Surgery will be a rescue treatment when previously mentioned treatments fail.
ACD Hinchey III or IV: purulent or faecal peritonitisPeritonitis is the most serious complication, with a mortality of 14%. Although there is no evidence, early surgery is considered standard therapy for these patients. The choice of operation is influenced by patient conditions, operative findings and the surgeon's experience. In critically ill patients with haemodynamic instability, Hartmann's procedure is recommended. However, in haemodynamically stable patients, primary anastomosis with or without proximal faecal diversion has to be considered a preferable choice.14,74
Traditionally, international guidelines recommend using endoscopy following an episode of ACD to exclude colorectal cancer. This recommendation is based only on expert opinions. Recent retrospective studies and a systematic review75–80 show that rate of cancer in these patients is rather low. Based on this recent evidence, the most effective strategy may be to refer to colonoscopy only those patients with persistently symptoms or those with suspicious CT findings. But more studies are necessary to a firm recommendation.
Management following an episode of ACDPrevention of recurrent ACDAfter one episode of ACD, about a third will have a second ACD, and after a second episode, a further third will have another attack.73,74 But the evidence to define the optimal treatment following an ACD episode in order to prevent a new episode is scarce.
A) High fiber dietOnce the acute episode has resolved, a high fibre diet is commonly recommended to reduce recurrences. But, RCTs on high fibre diets in patients with ACD has had inconsistent results. A recently published systematic review of high fibre diet could not include any study that investigates the role of fibre in prevention of recurrent ACD.26,81 Some foods (seeds, popcorn and nuts) are classically avoided because theoretically enter, block or irritate diverticulum. But Strate et al. in their large, prospective study found no association with an increased risk of ACD. Therefore the exclusion from the diet of these foods should be not recommended.82 Also, weight reduction and cessation of smoking can have a favourable influence on prevention of ACD.9,83
B) AntibioticsThere are three recent systematic reviews that assessed the role of cyclic rifaximin in preventing recurrence of ACD, but did not show a clear benefit.46,84,85 From a pathophysiologic point of view, a plausible explanation of ineffectiveness of rifaximin in preventing recurrences could be that a cyclic treatment may not control the colonic bacterial population during full month, because colonic bacterial population recovers within 7–14 days after the end of rifaximin. However, a recent Spanish open RCT has shown that cyclic rifaximin can improve symptoms and maintain periods of remission following ACD.86 Recurrences occurred in 10.4% of patients given rifaximin plus fibre vs. 19.3% of fibre alone. Moreover, patients first diagnosed since ≥1 year had a higher risk of exacerbation (OR 3.34, 95% CI: 0.01–12.18). But, further studies are needed since, at present time, no recommendations can be made. There is no evidence to support the use of other antibiotics in this setting.
C) ProbioticsSome open label studies have examined the role of probiotics in preventing recurrence of ACD. Giaccari et al., more than 20 years ago, evaluated the role of Lactobacillus sp. following rifaximin in 79 patients with post diverticulitis colon stenosis. 88% of patients remained asymptomatic for a period of 12 months.87 This observational study was the first that suggested a possible role of probiotics in this setting. A more recent study evaluated combined treatment; balsalazide and VSL#3 (an eight species probiotic mixture). After 12 months, 73% of subject on combination therapy were asymptomatic compared with 53% on probiotic monotherapy (p>0.05).52 In summary, probiotics seem to be effective in preventing recurrence of ACD, but well designed studies are lacking.
D) 5-ASASeveral double-blind and open RCTs have assessed the role of mesalazine in preventing recurrence of ACD. Unfortunately most of them have not found a benefit of mesalazine over placebo in preventing recurrence.88–90 Raskin et al. have recently published two interesting and identical phase 3 double-blind, placebo RCTs (PREVENT1 and PREVENT2) that also show that mesalazine is not superior to placebo in preventing recurrent ACD.91 Also the combined treatment, 5-ASA plus rifaximin, has been evaluated in several studies. Trivedi and Das reviewed data from five RCT and one open-labelled study, collectively involving over 600 patients, and concluded that combination seems to be superior to rifaximin alone for preventing recurrent ACD.92
E) SurgeryUntil a few years ago elective surgery was recommended after two attacks of uncomplicated ACD or one attack of complicated ACD to reduce morbidity and mortality by recurrence. But elective surgery also carries an increased risk of morbi-mortality.93 Because of that, it is important to weigh morbidity and mortality due to surgery against risk of complicated recurrences and severity of symptoms.
Recent data show that natural history of ACD is much more benign that thought in the past.73,94 The long term risk of relapse is more lower than previously believed, and the long term risks of subsequent emergency surgery (3–7%), death (<1%) and stoma formation (0–4%) are also quite low. The occurrence of multiple episodes did not increase the risk of major complications. In 2009, Pittet et al.95 showed that 16% of cases with first ACD were urgent operated compared to just a 6% in relapsing cases, and that the 30-day mortality for first episode was also higher compared to recurrent events (3% vs. 0%). As a matter of fact, the majority of patients presenting with complicated ACD lack a history of the disease.96 It is also proposed that recurrent ACD may protect against perforation, possibly due to adhesion formation caused by inflammation.95 Therefore, a policy of elective surgery after ACD does not decrease the likelihood of further surgery (up to 3%) and does not fully protect against recurrence. On the other hand, improved diagnostics and treatment modalities have reduced the morbi-mortality of complicated ACD. Because of these new data, The American Society of Colon and Rectum Surgeons in their most recent guideline recommend that elective sigmoid resection after recovery from ACD should be made on a case-by-case basis14 and consider that the number of previous episodes is not a good indicator for the selection of candidates to elective surgery. Physicians should consider the medical condition and age of the patient, the frequency and severity of the attack(s) and the presence of persistent symptoms after the acute episode.14
It is very difficult to anticipate which cases of ACD will relapse. CT graded severity of first episode of ACD seems to be a predictor of an adverse natural history. Left side ACD, >5cm of colon involved and a retroperitoneal abscess were predictors of recurrence and must be taken into account.97 There is no consensus regarding whether young age (<40–50 years) is an independent risk factor of ACD recurrence or complications.82 According to current evidence, age should not be considered as an indication for elective surgery as it does not seem to be related to a severe course of the disease after a medically treated ACD. But, taking into account that there is an increased incidence of ACD in younger patients, further studies are necessary to clarify this specific issue.
Special cases are the immunocompromised individuals. Cohort studies indicate that these patients had a high risk of complicated recurrent ACD (a 5-fold greater risk of perforation), and a high risk of emergency surgery.98 Therefore, a lower threshold for elective surgery may benefit them.14 But, Biondo et al. in their recent study show that immunocompromised patients had a significantly higher mortality rate but only in the first episode. Therefore, the controversy is present.99
Treatment of sequelaeA) FistulaA fistula occurs when a diverticular phlegmon or abscess ruptures into an adjacent organ. A fistula appears in fewer than 5% of patients with ACD. The most frequent are colovesical and colovaginal fistulas. Presumably, single-stage operative resection with fistula closure and primary anastomosis could be performed in most patients. Other fistulas as coloenteric or coluterine are rare.100,101
B) ObstructionRecurrent episodes of ACD, which may be subclinical, can produce chronic stricturing of the colon without ongoing inflammation. A high grade or complete obstruction can occur. First of treating obstruction, it is necessary to exclude a malignant aetiology. When neoplasm is sufficiently excluded and there is no ACD, endoscopic dilation or temporary decompression with metal stent may be therapeutic options.102 Later, a subsequent single stage resection without diversion can be realized.
Management of segmental colitis associated with diverticulosisIt is defined as a chronic inflammation of interdiverticular mucosa of a colonic segment involve. The rectum and the right colon are spared. It has become in a distinct clinical and pathological disorder and frequently presents with bloody stools. The pathogenesis is unknown. The spectrum of histological alterations ranges from mild non-specific inflammation to inflammatory bowel disease like changes. Because of that, the differential diagnosis is often difficult. Most patients recover completely in a few weeks or months. Some of them are initially treated with oral 5-ASA, but probably these drugs are not necessary because most cases spontaneously resolved.103
ConclusionsIn symptomatic UDD, the aims of treatment are to prevent complications and reduce symptoms. According to current evidence, fibre plus cyclic rifaximin or mesalazine plus probiotics seems to be the most effective therapies. In the ACD, antibiotics seem to remain the mainstay of treatment and an outpatient management is considered the optimal approach in the vast majority of patients with uncomplicated ACD. However, inpatient management and intravenous antibiotics are necessary in complicated ACD. Currently, the role of emergency surgery is changing. Most diverticulitis-associated abscesses can be treated with intravenous antibiotics and/or percutaneous drainage and emergency surgery will be considered standard treatment only in patients with peritonitis. Finally, elective surgery after recovery from ACD should be made on a case-by-case basis.
Authors’ contributionsGargallo CJ, Sopeña F and Lanas A contributed equally to the design, redaction drafting and reviewing process of this paper.
Conflict of interestDr. Carla J. Gargallo and Dr. Federico Sopeña do not report conflict of interest. Dr. Angel Lanas has been advisor to AlfaWasserman.