The mortality rate in acute variceal haemorrhage remains high (around 15%). Treatment is based on the combined use of vasoactive drugs, endoscopic band ligation, and prophylactic antibiotics. Effective resuscitation (haemostasis, volume management) is essential to prevent complications. Treatment failure is best managed by transjugular intrahepatic portosystemic shunt (TIPS). Balloon tamponade or specifically designed covered oesophageal stents can be used as a bridge to definitive therapy in unstable patients. Early, pre-emptive TIPS should be the first choice in patients at high risk of treatment failure (Child-Pugh B with active bleeding or Child-Pugh C<14). This article reviews the most recent advances in the management of variceal bleeding and discusses the recent recommendations of the Baveno VI consensus conference.
La hemorragia aguda por varices continúa presentando una alta tasa de mortalidad (alrededor de un 15%). Su tratamiento se basa en el uso combinado de fármacos vasoactivos y tratamiento endoscópico mediante ligadura, junto a profilaxis antibiótica. El uso de medidas de reanimacion efectivas (hemostasia, corrección de volumen) resulta esencial con objeto de prevenir las complicaciones. En caso de fallos del tratamiento, se debe realizar una derivación portosistémica percutánea intrahepática (DPPI o TIPS). En pacientes inestables, el taponamiento esofágico con balón o el empleo de prótesis (stent) esofágicas recubiertas pueden utilizarse como una medida transitoria hasta la realización del tratamiento definitivo. La utilización precoz de TIPS debe ser de primera elección en pacientes de alto riesgo de fallos del tratamiento (Child-Pugh B con sangrado activo o Child-Pugh C<14 puntos). En este artículo se revisan los avances más recientes en el manejo del sangrado por varices y se discute las últimas recomendaciones de la VI conferencia de consenso de Baveno.
Acute variceal bleeding (AVB) accounts for 70% of all upper gastrointestinal bleeding episodes in cirrhosis.1,2 Several advances in the treatment of these patients have been made in the last decades, mainly the introduction of endoscopic therapies (initially sclerotherapy and subsequently endoscopic variceal ligation and glue injection), pharmacological therapy (vasopressin, somatostatin and their analogues) and transjugular intrahepatic portal systemic shunt. The improvement in hemostatic treatments and in the general management has resulted in a major decrease in mortality, from around 40% in the 1980s3 to 15–20% in early 2000s.4 However, even in the last published series it remains above 15%5 which places this complication as one of the most serious of medical emergencies. Acute variceal bleeding is no longer the main cause of mortality in these patients. Nowadays most deaths are related to deterioration in liver or kidney function, or due to infections.4,5 Thus, therapy needs to focus not only in bleeding control, but also in protecting the liver, prevent acute kidney injury and preventing infections.6
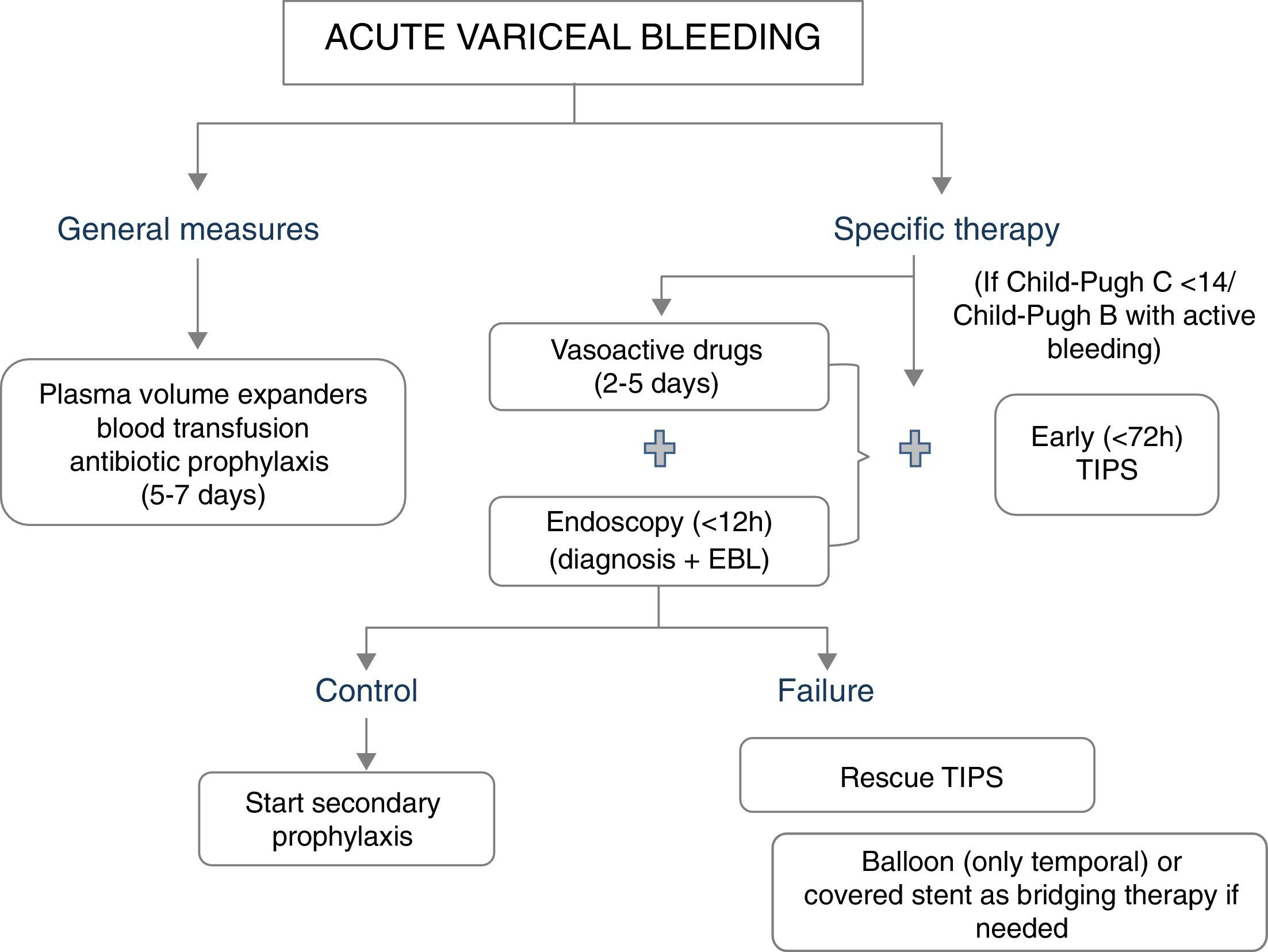
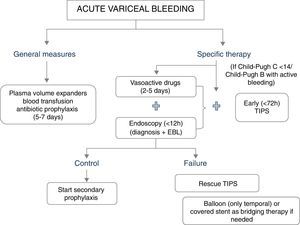
The major determinants of mortality are baseline liver and kidney function. Thus, not unexpectedly, the best predictor of mortality is the MELD score (which includes INR, Bilirubin and creatinine).5 A MELD score >19 points is associated with >20% mortality, whereas a MELD score <11 points is associated with a mortality risk <5%.5 This might serve to stratify a patient's care. Patients at high-risk should be managed in an intensive care setting by a team of experienced medical staff, including well-trained nurses, clinical hepatologists, endoscopists and interventional radiologists. Lack of these facilities requires immediate referral. A standardized set of orders can optimize adherence to guideline based care.7 Initial therapy should aim at hemodynamic resuscitation, initiation of vasoconstrictor therapy, antibiotics and endoscopic therapy (Fig. 1).
General managementThe general management of the bleeding patient is aimed at correcting hypovolemic shock (with judicious volume replacement and transfusion) and at preventing complications associated with gastrointestinal bleeding (bacterial infections, hepatic decompensation and renal failure).8
Blood volume replacement and transfusionBlood volume replacement should be initiated as soon as possible with plasma volume expanders, aiming to maintain the systolic blood pressure around 100mmHg. A rapid correction of hypovolemia is particularly important to reduce the risk of renal failure and impaired hepatic perfusion. Although in the past it was recommended to maintain these patients in relative hypovolemia (since reflex splanchnic vasoconstriction induced by hypovolemia would decrease portal pressure and in that way improve the control of bleeding), this was not evidence-based and it is probably harmful due to the risk of complications. In addition, it needs to be taken into account that nowadays haemostatic therapies are highly effective, and most deaths are not related to ongoing bleeding, but to the complications that could be associated with hypovolemia.
Blood transfusion strategy (which is differentiated from volume replacement), deserves special consideration. A recent large randomized trial in patients with acute upper gastrointestinal bleeding showed that a restrictive transfusion policy, using a Hb threshold for transfusion of 7g/dL, improves survival, as compared with a more liberal strategy (Hb threshold of 9g/dL).9 These results were also verified in the subgroup of patients with cirrhosis and acute variceal bleeding. It is important to note that all patients received adequate and similar volume resuscitation before blood transfusion, and that patients with rapid ongoing bleeding or with ischemic heart disease were excluded from the study.
The correction of hemostasis is still a controversial issue in patients with cirrhosis and bleeding complications. It is widely recognized that prothrombin time or INR are not reliable indicators of coagulopathy and/or the risk of further bleeding. Currently, there is insufficient data to make recommendations about the role of platelet transfusions or fresh frozen plasma, since this has not been evaluated in randomized trials. There is now robust information about the potential role of recombinant activated factor VII (rFVIIa). This has been evaluated in two randomized controlled trials given in addition to standard therapy. The first one was conducted in a general population of patients with acute variceal bleeding.10 The second one targeted high risk patients, defined as patients with active bleeding at endoscopy and a Child-Pugh score ≥8 points.11 Both trials were negative, showing no relevant benefits of rVIIa. An individual patient meta-analysis of both studies12 showed a significantly lower rate of in treatment failure with rVIIa during the first 5 days after bleeding, and this effect was more marked in patients with a Child-Pugh score >8. Since there was no effect on mortality and the studies showed a potential risk for arterial thrombotic events, rFVII is currently not recommended in the management of acute variceal bleeding.
Bacterial infectionsIn the setting of AVB (in the absence of antibiotic prophylaxis), approximately 20% of patients are infected on the day of admission and up to 50% develop an infection during their hospital stay.13 Most infections develop within the first 5–7 days after the bleeding episode. The most commonly reported infections are bacteremia (19–56%), SBP (19–37%), urinary tract infections (12–34%) and pneumonia (12–19%).13–16 Bacterial infections increase AVB related mortality13,15,17 and in smaller studies, have been associated with the failure to control bleeding and increased re-bleeding.18,19
Systematic reviews and meta-analyses have demonstrated a clear reduction in the rates of bacterial infection, re-bleeding and mortality with antibiotic prophylaxis.13,15,20 Accordingly, antibiotic prophylaxis is instituted as early as possible on presentation of AVB and continued for 5–7 days in all patients.8,21 However, there is a well-known association between worsening liver disease severity and increasing risk of bacterial infection.16,21,22
Data from a recent observational study in 381 patients with AVB who either received or did not receive antibiotic prophylaxis supports the Child Pugh stratified increases in bacterial infection.23 After adjusting for a propensity score, the risk of infections in Child Pugh A patients not treated with antibiotics was negligible, suggesting that antibiotic prophylaxis could be avoided in these patients. However, in the Baveno VI consensus conference prospective multicenter trials were suggested before a formal recommendation could be made to avoid antibiotic prophylaxis in AVB patients with Child Pugh A disease.24
The choice of antibiotic deserves special consideration. Until recently, quinolones were recommended,8 however, the current rate of quinolone resistance is high. Individual patient risk characteristics and local antimicrobial susceptibility patterns must be considered when determining appropriate first-line AVB antimicrobial prophylaxis at each center. Intravenous ceftriaxone given at a dose of 1g intravenously every 24h25 is probably an acceptable approach in most settings.
Hepatic encephalopathyHepatic encephalopathy (HE) management in AVB should be based on recent AASLD/EASL guideline recommendations.26 Two recently published studies assessed the role of HE prophylaxis in AVB. The first study by Sharma et al. randomized 70 AVB patients to lactulose versus no lactulose with a primary outcome of the development of overt HE within 5 days.27 HE developed in 40% of the placebo group and 14% of the lactulose group. In the second study by Maharshi et al.28 120 patients with AVB were randomized to lactulose versus rifaximin with a primary outcome of overt HE within 5 days. There was no significant difference in the percentage of patients who developed HE (17% of the lactulose group and 15% of the rifaximin group), duration of hospitalization or mortality. Although both groups of investigators suggested that prophylactic anti-HE therapy be incorporated as part of standard, Baveno VI consensus concluded that there was insufficient data to recommend prophylaxis of hepatic encephalopathy in patients with AVB, though studies in high-risk patients are certainly needed.24
Acute kidney injury (AKI)Patients with AVB are predisposed to develop AKI predominantly as a result of intravascular volume depletion, bacterial infections and nephrotoxic medications. In the study by Cardenas et al., including 161 patients,29 renal failure was diagnosed in 11% of patients (increase in creatinine of ≥50% to a value >1.5mg/dL within the first 7 days following AVB), and was transient in 40% of cases. Mortality was 55% in the renal failure patients as compared to 3% in those without renal failure. Non-transient renal failure was associated with the highest mortality at 83%. Therefore, as with many other groups of patients with cirrhosis, AKI in AVB appears to be a robust predictor of mortality.
The International Ascites Club has published a consensus guideline that summarizes the principles for the prevention and treatment of AKI in cirrhosis.30 These recommendations can be applied to the AVB setting and include the removal of all potentially nephrotoxic drugs, adequate plasma volume expansion, prompt recognition and early treatment of bacterial infections and in selected patients, the early initiation of vasoconstrictor therapy.
Specific therapy for control of bleedingThe first-line hemostatic treatment of acute variceal bleeding is based on the combination of vasoactive drugs (somatostatin, octreotide or terlipressin) and endoscopic therapy (endoscopic variceal ligation). This is based on a meta-analysis comparing endoscopic treatment alone with endoscopic plus drug therapy31 which showed that the addition of vasoactive drugs to endoscopic therapy significantly improved the initial control of bleeding and rates of 5 day rebleeding. However, it must be noted that although treatment failure is strongly associated with increased 6-week mortality, the meta-analysis did not show an improved survival with combination therapy.31,32 Only one trial so far compared banding ligation plus drugs (low doses of terlipressin) with drugs alone. This trial included patients with inactive variceal bleeding at endoscopy and showed again that the combined therapy was superior to drug therapy alone in the reduction of very early rebleeding and treatment failure.33 Therefore, the current recommendation is to combine endoscopic and drug therapy in all patients with acute variceal bleeding, even if there is no active bleeding at the time of endoscopy.
Pharmacological therapyVasoactive drug therapy should be started as soon as possible (ideally during the transfer to hospital or at arrival to hospital), before endoscopy.24 A recent meta-analysis concluded that as compared to placebo/control, the use of a vasoactive agent was associated with a lower risk of mortality at seven days (relative risk 0.74; 95% CI 0.57–0.95), improved hemostasis (RR 1.21; 95% CI 1.13–1.30), lower transfusion requirements and shorter duration of hospitalization.34 The selection of the vasoactive drug depends on available local resources. Terlipressin, somatostatin or octreotide are acceptable options. The previously mentioned meta-analysis did not detect a significant difference in their efficacy.34 Furthermore, a very recent large (n=780) randomized trial with a non-inferiority design compared the efficacy of terlipressin, somatostatin and octreotide in combination with endoscopic therapy. The rates of treatment failure at five days were comparable with the three vasoactive agents.35
Regarding safety of these agents, data from the mentioned trial showed a similar adverse events profile without significant differences except for hyponatremia (defined as a drop of serum Na level ≥5mmEq from baseline to <130mEq/L). A total of 11.5% (30 of 261) patients in the telipressin group developed hyponatremia compared to 1.5% of patients received somatostin and 1.2% in octreotide group (p<0.001).35 This side effect had been reported earlier36 Baveno VI guidelines recommended to monitor sodium levels in patients on terlipressin treatment.24 Additionally, terlipressin is associated with cardiovascular side effects such as ischemia of extremities, cardiac arrhythmias, hypertension, left ventricular failure, myocardial ischemia and sudden death, which is not observed with somatostatin/octreotide. For this reason, it is contraindicated in patients with history of cardiovascular diseases.
The duration of vasoactive therapy is not well established, different regimens have been evaluated (from 8h until 6 days).37 Only one RCT directly compared two different treatment durations: terlipressin for 24 vs. 72h.38 This trial (n=134) showed that 24-h terlipressin treatment is non-inferior to 72-h terlipressin treatment in acute variceal bleeding after successful endoscopic band ligation. The current recommendations is to maintain the drug for up to 5 days, in combination with endoscopic therapy.24
Endoscopic therapyEndoscopy should be performed within 12h of admission, following hemodynamic resuscitation.24 In the absence of contraindications (long QT), an infusion of erythromycin prior to endoscopy is recommended. Data from a recent meta-analysis has shown that erythromycin (250mg IV 30–120min before endoscopy) reduces the need for second endoscopy, transfusion requirements and it may shorten the length of hospital stay,39 and therefore it should be given before endoscopy in the absence of contraindications.24
Current evidence supports EBL as the endoscopic therapy of choice for the initial control of bleeding as it is associated with less adverse events and less mortality than sclerotherapy.40 Additionally, sclerotherapy, but not EBL, may increase portal pressure.41
Rescue therapies: tamponade, esophageal stents, surgery and TIPSIn 10–20% of patients variceal bleeding is unresponsive to initial endoscopic and/or pharmacologic treatment.42 If bleeding is mild and the patient has a good liver function a second endoscopic therapy might be attempted.8 If this fails, or bleeding is severe, patients should be offered a salvage treatment, before their clinical status deteriorates further. Balloon tamponade achieves hemostasis in 60–90% of variceal bleedings but should only be used in the case of refractory bleeding, for a short period of time (less than 24h) as a temporal “bridge” until definite treatment is instituted.24,43 Bleeding recurs after deflation of the balloon in over half of the cases and serious complications are common (esophageal rupture, asphyxiation due migration proximal, aspiration), being fatal in 6–24% of cases.42 Recent case series suggest that the use of self-expanding esophageal covered stents might achieve haemostasis in most patients with refractory bleeding, with the advantage over tamponade of less severe complications despite longer periods of treatment.24,44–46 Only one RCT has been conducted so far with the esophageal stent. This was a multicentre trial conducted in Spain47 comparing esophageal covered stents with balloon tamponade for uncontrolled esophageal variceal bleeding. The primary endpoint was a composite including control of bleeding with absence of serious adverse events and survival at day 15. The trial was stopped at an interim analysis when half of the sample was included (n=28), due to extreme difficulties in recruitment within this very severe population. The primary endpoint was achieved in 66% of the cases in the esophageal stent group as compared to 20% in the balloon tamponade group. This result was significant (p=0.025), but did not fulfil the superiority criteria set for the interim analysis. The excellent results and safety profile of the stent will likely result in further trials in different indications, for example as initial therapy in high risk patients.
Finally, both TIPS and surgical shunts are extremely effective at controlling variceal bleeding (control rate approaches 95%), but due to worsening of liver function, encephalopathy and mortality remains high.48 TIPS is the treatment of choice, since most patients requiring rescue treatment have advanced liver disease with unacceptable surgical risk. It should be taken into account that in patients with Child-Pugh score over 13 points mortality with TIPS approaches 100%. This clearly indicates that some patients do not benefit from TIPS in this setting.
Role of TIPS: pre-emptive vs. rescue treatment.Two randomized controlled trials have assessed the beneficial effects of an early TIPS placement (within 72h of admission) in preventing re-bleeding and mortality in patients with AVB at high risk of treatment failure.49,50 The rationale of these trials was to prevent further deterioration in patients at high risk of rebleeding, since these patients have an already deteriorated liver function. In these trials high-risk patients were selected based on hepatic venous pressure gradient (HVPG) greater than 20mmHg49 or based on Child Pugh C 10–13 points or Child B with active variceal bleeding.50 Both trials showed significantly less treatment failure and lower mortality in patients treated with early-TIPS as compared with standard therapy.
Recent observational data confirm the efficacy of early-TIPS achieving bleeding control, but the effects on mortality are less clear-cut.51,52 Importantly none of these studies showed more adverse effects with TIPS, and the rates of encephalopathy were similar with TIPS and standard therapy.
Altogether, these results suggest that the management of variceal bleeding should be stratified according to patient risk, and that high-risk patients might benefit from more aggressive therapies such as an early, preentive-TIPS. Indeed, recent Baveno VI consensus recommended the use of TIPS in patients with Child-Pugh B cirrhosis and active bleeding and in patients with Child-Pugh C cirrhosis (<14 points). A further refinement on the criteria to select high-risk patients was suggested.24
Issues in designing new trials for variceal bleedingAcute variceal bleeding has been regarded as an area of high quality trials.53 This success is in large part attributable to standardization of trial design at successive Baveno consensus meetings.54 However, the definition of the primary end-point in trials in AVB has been source of controversy for many years. Most trials have used several definitions of “treatment failure” that were focused on the achievement of hemostasis.55 This concept was very relevant at a time when the efficacy of hemostatic therapies was much lower (∼60%) than it is today (∼85%). It has been repeatedly shown that treatment failure is strongly associated with increased mortality. However, a decrease treatment failure with drugs or endoscopic therapy has not been consistently associated with improved survival,31,32 which suggests that treatment failure and mortality might have a common determinant (likely the severity of liver dysfunction) that it not targeted by hemostatic treatments. Moreover, a recent FDA panel56 questioned the clinical relevance of treatment failure as a primary endpoint to assess the efficacy of drugs for variceal bleeding, mainly on the basis that improving treatment failure was not associated with a survival benefit. With a current mortality rate of 15–20% with standard therapy (that has not substantially decreased in the last decade), there is certainly room for improvement in mortality with new treatments. Baveno VI consensus proposed that new trials for AVB should have mortality as the primary end-point. Since liver function is the major determinant of mortality, new treatments would likely have to include strategies for protecting or improving liver function during acute bleeding.
Conflict of interestAuthors declare no conflict of interests for this article.