To evaluate the efficacy of daily cleaning with 4% chlorhexidine-impregnated sponges in decreasing contamination of blood cultures in critically-ill patients.
Material and methodsProspective, quasi-experimental, longitudinal, single-centre trial. During 24 months (April 2013 to March 2015), we analysed 237 patients who fulfilled the inclusion criteria, divided into 2 groups: one underwent daily cleaning with common soap (control group, n=108), and the other with chlorhexidine (intervention group, n=109). Demographic variables, pathology group, severity scores, ICU and hospital length of stay and mortality, and time passed since cleaning to blood culture extraction were included.
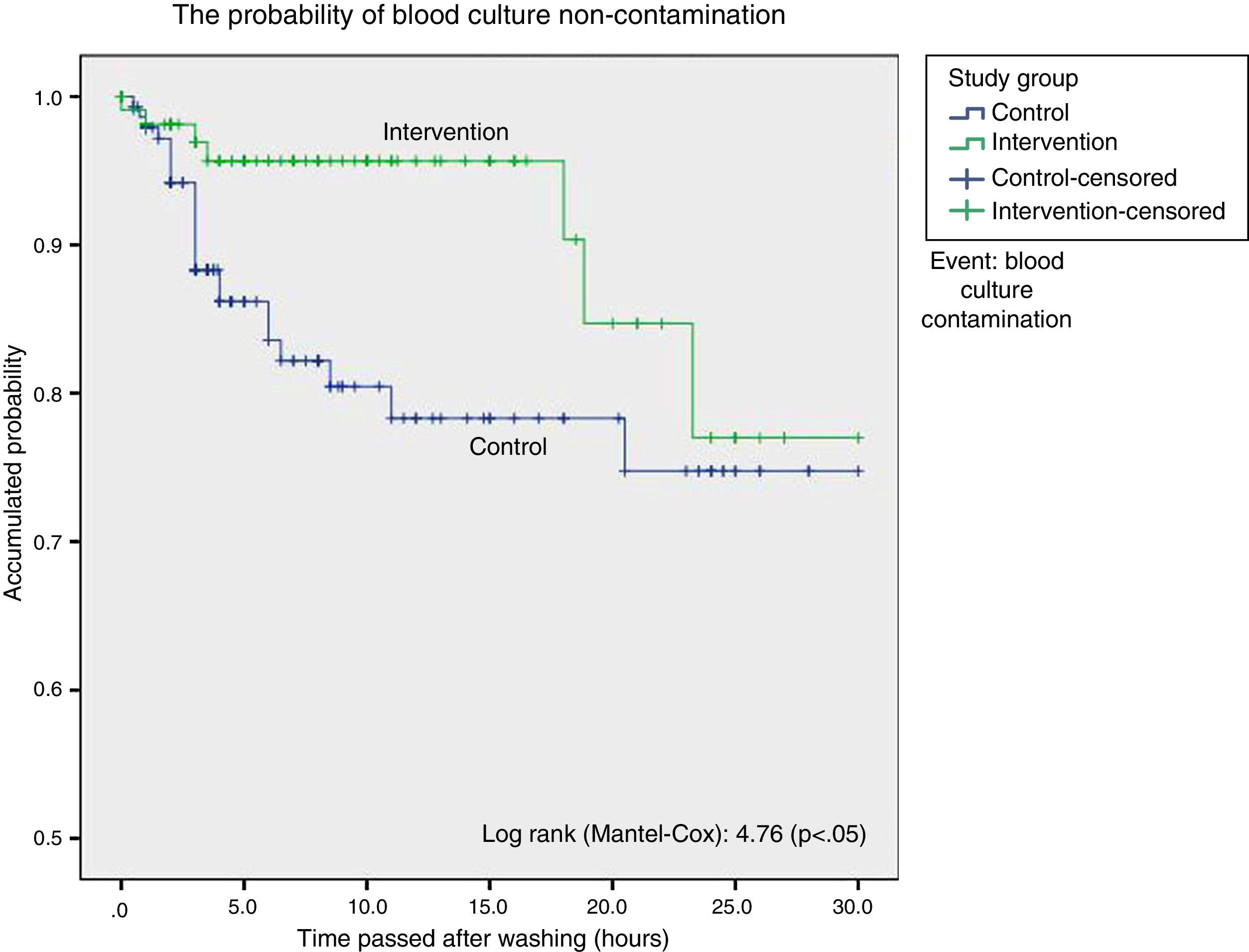
ResultsStatistical analysis showed a higher proportion of contaminated blood cultures during the control group period in contrast with the intervention group period (15 vs. 6.3%), with a significant difference: 9.23% (CI 95%: 1.34–16.7%), with an odds ratio of 2.73 (CI 95%: 1.13–6.63). Surveillance analysis showed lower probability of blood culture contamination within the 18h following daily cleaning. Cleaning without chlorhexidine increased contamination of blood cultures (HR: 3.05; CI 95%: 1.14–8.12).
ConclusionsThe use of 4% chlorhexidine-impregnated sponges for daily cleaning of critically-ill patients decreases blood culture contamination incidence and its protection lasts for almost 18h.
Evaluar la eficacia de la higiene diaria con esponjas impregnadas con clorhexidina al 4% para disminuir la contaminación de hemocultivos en pacientes ingresados en UCI.
Material y métodoEstudio prospectivo, cuasi experimental, longitudinal, unicéntrico. Durante 24 meses (de abril de 2013 a marzo de 2015) fueron analizados 237 pacientes que cumplieron los criterios de inclusión, y fueron divididos en 2grupos: uno recibió higiene corporal diaria con jabón común (grupo control, n=118) y el otro con clorhexidina (grupo intervención, n=119). Fueron incluidas variables demográficas, tipo de enfermedad, nivel de gravedad, estancia y mortalidad en UCI y hospitalaria, y tiempo trascurrido (minutos) desde el baño hasta la extracción de los hemocultivos.
ResultadosEl análisis estadístico mostró una mayor proporción de contaminación de los hemocultivos durante el período control con respecto al de intervención (15,5 vs. 6,3%); con una diferencia significativa: 9,23% (IC 95%: 1,34-16,7%), odds ratio de 2,73 (IC 95%: 1,13-6,63). El análisis de supervivencia mostró una menor probabilidad de contaminación de los hemocultivos hasta las 18 h desde el baño. El baño sin clorhexidina aumentó el riesgo de contaminación de los hemocultivos (HR: 3,05; IC 95%: 1,14-8,12).
ConclusionesEl empleo de jabón con clorhexidina al 4% en la higiene diaria de los pacientes críticos disminuyó la incidencia de contaminaciones de hemocultivos y su efecto perduró al menos 18 h.
A high rate of false positives in critically-ill patient blood cultures leads to increased healthcare costs. The use of aseptic techniques to extract blood cultures gives rise to a reduction in the number of contaminations, which are often due to germs that colonise the skin. Nevertheless, it may sometimes be necessary to adopt additional measures if, in spite of compliance with blood culture extraction protocols the aim of maintaining a less than 3% level of contamination is unmet. Daily chlorhexidine bathing of patients in the ICU may reduce the possibility of blood culture contamination.
This study demonstrates the efficacy of daily chlorhexidine bathing in reducing the incidence of blood culture contamination in critically ill patients, and it may be especially useful in units with a high contamination rate.
Implications of the studyThe use of sponges with 4% chlorhexidine digluconate in the daily hygiene of critically ill patients helps to maintain a low rate of blood culture contamination. It is also a care quality indicator and leads to a reduction in healthcare costs due to diagnostic tests and unnecessary treatments.
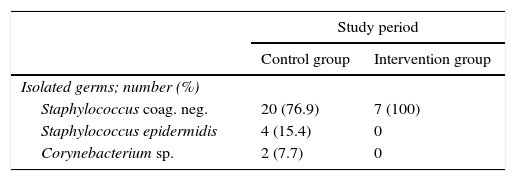
Bacteraemia is a severe condition with a high rate of morbimortality. Blood cultures are a very useful diagnostic and prognostic tool for doctors. Nevertheless, false positive results due to contamination of blood samples by patient skin flora organisms, chiefly coagulase negative staphylococcus,1 give rise to an increase in diagnostic tests and unnecessary treatment2 with the subsequent increase in healthcare costs.3 The most frequent causes of contamination are inadequate preparation of the skin and poor sample extraction technique.
Actions to reduce contamination are based on minimising skin bacteria by using disinfectants and optimising sample extraction protocols.
There are studies which show that washing with antiseptic solutions prior to surgery is effective in reducing the number of microorganisms on the skin,4 and that this also reduces the incidence of surgical wound infection in orthopaedic surgery.5–7
Patients in the ICU are especially liable to suffer infections caused by skin flora and nosocomial germs. This is due to the use of invasive devices, antibiotic therapy, immunodeficiencies and comorbidity. Preventative measures based on hand washing, the use of disposable materials and even patient isolation are fundamental to prevent crossed contamination by germs that often colonise the skin.
Chlorhexidine gluconate is a biguanide-type cationic antiseptic with broad spectrum action against several microorganisms including S. aureus and enterococcos species. It has prolonged residual activity8 and low toxicity.9
Quality standards propose a level of blood culture contamination below 3%.10 This margin is considered to be tolerable and, when it is surpassed in practice it shows that there is a problem. The strict application of protocols with an emphasis of the use of sterile techniques to extract blood cultures leads to a reduction in contamination.11 However, it sometimes may be necessary to adopt other additional measures such as changing the antiseptic used to decontaminate the skin or daily washing with antiseptic products. This will occur if in spite of careful compliance with blood culture extraction protocols no improvement is detected in the results.
This work aims to evaluate the efficacy of daily hygiene using 4% chlorhexidine digluconate soap-impregnated sponges to reduce blood culture contamination down to the level set by quality standards in ICU patients. A water-based 2% chlorhexidine antiseptic solution was used to decontaminate the skin for the extraction of blood cultures according to our protocol.
Material and methodsA quasi-experimental non-randomised prospective study with a pre-post design, longitudinal and in single hospital. Study period: 24 months from April 2013 to March 2015. It took place in a 12-bed medical-surgical intensive care unit in the Hospital Universitari Sant Joan de Reus.
Population selectionThe inclusion criteria were: age>18 years old, admission to the ICU>48h, and the exclusion criteria were: skin disruption >20% (for example, burns),12 pregnancy and allergy to chlorhexidine.
Sample sizeThe sample size was determined on the basis of our proportion of contaminated blood cultures prior to the study (16%) and the accepted quality standards (3%). To detect a reduction of 13% in the proportion of contaminated blood cultures (from 16% to 3%) with a type I error of 5% and a power of 80% 70 blood cultures per group were necessary. The Domenech–Granero macro ¡NSize for SPSS was used for calculations.
How the intervention was dividedThe study included 3 periods and 2 groups of patients:
- (a)
First period—control group. During this period patient hygiene was performed daily with warm water and CR-32 antiseptic-free dermatological soap (José Collado S.A., Barcelona, Spain). This period lasted for 11 months.
- (b)
Second period—rest. This period lasted for one month, and its function was to prevent the overlap between patients in both study groups. During this time the patients in the control group were discharged and no intervention group patients were included. Additionally, we reinforced training in blood culture hygiene and extraction protocols. During this period patient hygiene was undertaken using antiseptic-free soap.
- (c)
Third period—intervention group. During this period hygiene was undertaken using body sponges impregnated with 4% chlorhexidine digluconate (Dispomedic sponge C, CV Médica, Tarragona, Spain), applied on the patient's skin until the whole skin surface apart from the face was covered, to prevent contact with oral mucus membranes and eyes (following the manufacturer's recommendations). The solution was left to act during 3min., after which the skin was rinsed using water before being dried with clean towels. The hair was washed every 3 days. This period lasted for 11 months.
Prior to commencing periods 1 and 3 patient bathing technique was protocolised with the nursing and auxiliary staff. This technique emphasised sequential washing from clean zones to dirty ones. The stools of incontinent patients were washed off using soap and water or chlorhexidine, depending on the study group. The skin was hydrated using solutions that do not give rise to interference with the bactericide activity of chlorhexidine. Strict compliance with the hand hygiene protocol was recommended before and after contract with patients. Likewise, before periods 1 and 3 we implemented continuous training programs, emphasising the blood culture extraction and processing protocol (Appendix A: blood culture extraction and processing technique).
Data gathering- (a)
Basic level. On the admission to the ICU of each patient we recorded their age, sex, height, weight, disease type (medical, surgical or cardiological), degree of severity (APACHE II, SAPS II). All non-cardiological patients whose disease did not require surgery were considered to be medical.
- (b)
Follow-up. We recorded the date and time of each blood culture, the data and time of body washing, the location of blood culture extraction, days of admission in the ICU and hospital mortality. We also recorded the presence of central venous catheter, a bladder catheter, the need for mechanical ventilation, renal replacement therapy, antibiotic therapy and the presence of multiresistant germs (colonisation or infection).
Every day the nursing staff evaluated patient skin integrity using a SCORAD13 grading scale, a clinical tool used to evaluate the extension and severity of eczema in atypical dermatitis. If skin alterations were detected as a side effect of the chlorhexidine the patient's doctor or Dermatology Department proposed a therapeutic intervention.
Blood cultures were requested under medical criterion when there was a suspicion of infection and body temperature above 38°C. Blood cultures were always requested in sets of 2.
It is difficult to distinguish between contaminated blood cultures and a true infection without a “gold standard”. The initial independent evaluation was undertaken by the doctors in charge of the patient blood cultures had been extracted from. They were subsequently reviewed by 2 doctors involved in the study, based on the type of germ isolated, the number of jars with growth, the use of empirical antibodies following the extraction of blood cultures and the clinical evolution of the patient. All blood cultures were defined as cases of contamination when, after being extracted in the ICU on the order of a doctor due to the suspicion of infection, the Microbiology Laboratory reported the growth of germs in at least two blood cultures and these were interpreted by 2 doctors to be a caused by a germ with a low probability of being the cause of the suspected infection.
The study was simple blind: the patients did not know which study group they belonged to. The medical staff was aware of the product used in the daily hygiene of each patient taking part in the study.
Data analysisThe normal distribution of the groups was checked using the Shapiro–Wilk test.
The comparison between groups of continuous variables was undertaken using the Student t-test or the non-parametric Mann–Whitney test according to correspondence and the categorical variables test using χ2.
To graphically compare the time to appearance of blood culture contaminations following the daily bodily washing between group we made Kaplan–Meier curves and calculated the log-rank for each one. In survival analysis each contaminated blood culture was considered to be an event.
The difference between blood culture contamination incidence density rates between groups was determined using Cox regression controlled for confusion variables. We considered previous antibiotic treatment and the presence of multiresistant germs to be confusion variables. We also controlled regression according to level of severity and days of admission in the ICU, assuming that more severe patients and those with prolonged admission are at greater risk of colonisation.
We used the IBM SPSS 20 (Chicago, USA) statistics program for statistical analysis. Differences with a value of P<.05 were considered to be statistically significant.
Ethical aspectsThis study was carried out according to the ethical principles of the Helsinki Declaration and it was approved by the Hospital Ethics Committee, which considered that it was necessary to obtain informed consent. The patients or their legal representatives were asked to give their informed consent after the purpose and nature of the study had been explained. Checks were made to ensure that the patient or their family members had understood the risks and benefits of taking part. A simple answer was given to all of the questions that arose.
To ensure the confidentiality of the data of each individual, as a guarantee of the protection of personal information and to prevent divulgation of the same without their consent, alphanumerical identification codes were used to identify them computerised analysis. Access to these codes was restricted to the authors, and printed records were kept in a locked and protected place.
Results1061 patients were admitted during the period from April 2013 to March 2015; of these, 237 patients fulfilled the inclusion criteria and agreed to take part in the study. They were then distributed as follows: 118 in the control group and 119 in the intervention group.
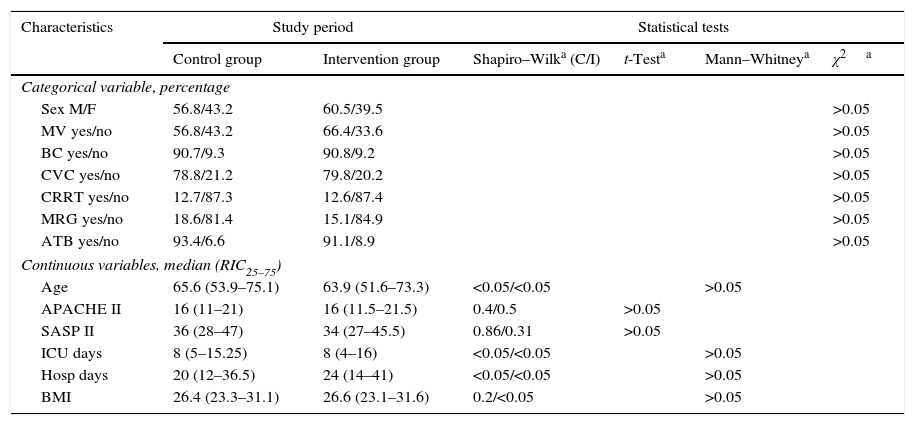
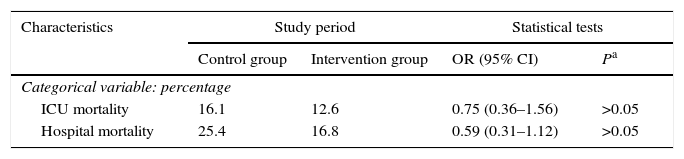
Patient characteristics were similar in both groups in terms of age, sex, degree of severity in the first 24h (APACHE II, SAPS II), BMI, central venous catheter, bladder catheter, the need for assisted respiration or renal replacement therapy, the presence of multiresistant germs (colonisation or infection) and antibiotic treatment, days of admission in the ICU and hospitalisation (Table 1), as well as intra-ICU and hospital mortality (Table 2). Respecting the type of disease a higher proportion of medical patients were included in the control group, and there were more surgical patients in the intervention group. Nevertheless, the differences were not statistically significant (Table 3). No skin alterations were detected that were considered to be side effects of the chlorhexidine. We found no differences in the SCORAD score between the two groups studied.
Comparison of patient characteristics in the study groups.
| Characteristics | Study period | Statistical tests | ||||
|---|---|---|---|---|---|---|
| Control group | Intervention group | Shapiro–Wilka (C/I) | t-Testa | Mann–Whitneya | χ2a | |
| Categorical variable, percentage | ||||||
| Sex M/F | 56.8/43.2 | 60.5/39.5 | >0.05 | |||
| MV yes/no | 56.8/43.2 | 66.4/33.6 | >0.05 | |||
| BC yes/no | 90.7/9.3 | 90.8/9.2 | >0.05 | |||
| CVC yes/no | 78.8/21.2 | 79.8/20.2 | >0.05 | |||
| CRRT yes/no | 12.7/87.3 | 12.6/87.4 | >0.05 | |||
| MRG yes/no | 18.6/81.4 | 15.1/84.9 | >0.05 | |||
| ATB yes/no | 93.4/6.6 | 91.1/8.9 | >0.05 | |||
| Continuous variables, median (RIC25–75) | ||||||
| Age | 65.6 (53.9–75.1) | 63.9 (51.6–73.3) | <0.05/<0.05 | >0.05 | ||
| APACHE II | 16 (11–21) | 16 (11.5–21.5) | 0.4/0.5 | >0.05 | ||
| SASP II | 36 (28–47) | 34 (27–45.5) | 0.86/0.31 | >0.05 | ||
| ICU days | 8 (5–15.25) | 8 (4–16) | <0.05/<0.05 | >0.05 | ||
| Hosp days | 20 (12–36.5) | 24 (14–41) | <0.05/<0.05 | >0.05 | ||
| BMI | 26.4 (23.3–31.1) | 26.6 (23.1–31.6) | 0.2/<0.05 | >0.05 | ||
APACHE II: Acute Physiology And Chronic Health Evaluation II; C: control; Hosp days: days of admission to hospital; ICU days: days of admission in the intensive care unit; MRG: multiresistant germs; I: intervention; BMI: body mass index; SAPS II: Simplified Acute Physiology Score II; BC: bladder catheter; CRRT: continuous renal replacement therapy; CVC: central venous catheter; MV: mechanical ventilation.
Comparison between the mortality of patients in both study groups.
| Characteristics | Study period | Statistical tests | ||
|---|---|---|---|---|
| Control group | Intervention group | OR (95% CI) | Pa | |
| Categorical variable: percentage | ||||
| ICU mortality | 16.1 | 12.6 | 0.75 (0.36–1.56) | >0.05 |
| Hospital mortality | 25.4 | 16.8 | 0.59 (0.31–1.12) | >0.05 |
ICU: intensive care unit.
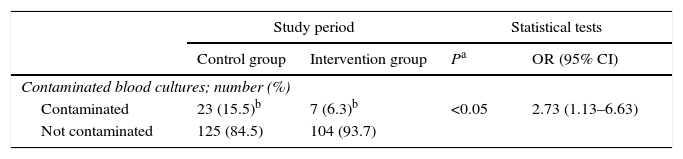
259 blood cultures were extracted, 148 in the control group and 111 in the intervention group, of which 30 were considered to be contaminated (23 and 7, respectively) (Table 4). This showed that there was a higher proportion of contaminated blood cultures during the control group period than was the case during the intervention group period (15.5% and 6.3%, respectively). The difference between these proportions amounted to 9.23% (95% CI: 1.34–16.7%). This is a statistically significant difference, with an odds ratio of 2.73 (95% CI: 1.13–6.63) (Table 5).
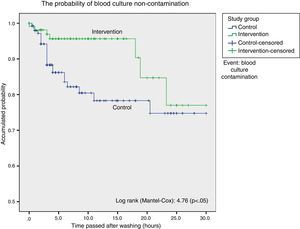
Survival analysis considered blood culture contamination to be an event and showed a statistically significant difference in favour of the intervention group. We found the probability of blood culture contamination was lower up to 18h after bathing in the group bathed using chlorhexidine, after which both curves become closer (Fig. 1).
Cox regression study, taking the presence of multiresistant germs, previous antibiotic treatment, degree of severity and days of admission in the ICU to be confusion variables, showed that bathing without chlorhexidine increased the risk of blood culture contamination: HR 3.05 (95% CI: 1.14–8.12; P<.05).
DiscussionThere is now increasing interest in improving the quality of healthcare while also restricting the cost of the same. In the context of patients admitted to critical care units, actions such as daily bathing with chlorhexidine has the aim of improving both aspects.
Some studies show that daily hygiene using chlorhexidine in ICU patients gives rise to a decontamination effect that prevents infections from developing14–18 and reduces the possibility of crossed transmission.19
Bleasdale et al.12 show a significant reduction in cases of bacteraemia as well as a tendency to reduce the incidence of contaminated blood cultures. Popovich et al.18 found that as well as reducing catheter bacteraemia, bathing with chlorhexidine also reduced the possibility of blood culture contamination in critical medical patients. Nevertheless, in a subsequent publication20 these authors once again show a significant reduction in the incidence of contaminated blood cultures in surgical patients admitted to the UCI who were bathed daily using chlorhexidine, although they did not find a fall in catheter bacteraemia. A recent study by Noto et al.21 does not show a reduction in the incidence of infections associated with healthcare procedures or a fall in contaminated blood cultures in patients bathed every day with chlorhexidine. Among other weak points this work did not monitor adherence to protocols and provides weak evidence of the effect of chlorhexidine on the incidence of nosocomial infections.
Septimus et al.,22 in another recent study that includes a large sample of patients admitted to an ICU, but without ensuring that a skin disinfection protocol was followed or that a uniform blood sample extraction technique was used, conclude that daily bathing using chlorhexidine and the use of nasal mupirocin is effective in reducing bacteraemia and blood culture contamination. This work does not discriminate the individual benefits of mupirocin and chlorhexidine.
The published studies which show that daily washing with chlorhexidine leads to a reduction in blood culture contamination use wipes with 2% chlorhexidine. In our work we used sponges with 4% chlorhexidine digluconate, leading to a drastic reduction in the contamination rate, the effect of which persisted for up to 18h after washing. Although our study did not aim to compare the different commercial preparations of chlorhexidine at different concentrations, it may be thought that higher concentrations may have a greater protective effect against blood culture contamination.
The study by Popovich et al.23 showed the importance of chlorhexidine concentration. This study used colorimetric techniques to analyse the concentration of the said antiseptic on specific zones of the skin of patients in a medical ICU and made quantitative microbiological cultures of germs from the same zones of the skin. They thereby established that an inverse relationship exists between microbial density and chlorhexidine concentration, the activity of which persists for up to 24h after it is applied.
The results of our study show a high incidence of blood culture contamination in our ICU in spite of the revision and diffusion of the blood culture extraction protocols. The use of daily washing with chlorhexidine has a good cost-efficacy ratio, given the reduction in costs arising due to blood culture contamination.
Nevertheless, our study has methodological limitations. One of these is the fact that it took place in a single hospital with a small sample and a before and after design which may inappropriately attribute the effect studied to the intervention. We also started with an unacceptably high blood culture contamination rate, even though the extraction protocols had been revised and training programs had been implemented. Although these factors may weaken the results by introducing distortions, we believe that this is the most practical type of study.
No development of resistant microorganisms or allergic reactions to the use of chlorhexidine were detected.19 In our case, nor were any cutaneous reactions observed due to the use of this antiseptic.
There is ongoing debate about the efficacy of different antiseptics in preventing the contamination of blood cultures. Alcohol solutions are more effective decontaminants than water-based ones.24,25 Although alcohol-free 2% chlorhexidine antiseptic solution was used to decontaminate the skin in our blood culture extraction protocol, we believe that the results are due to the use of chlorhexidine soap in daily washing, which reduced blood culture contamination. Studies in the future will be needed to evaluate the effect of alcohol-based antiseptic as skin decontaminants in patients washed every day with chlorhexidine.
ConclusionsThe use of 4% chlorhexidine soap in the daily washing of critical patients reduces the incidence of blood culture contamination, and its effect lasts over time. A low rate of blood culture contamination is a quality of care marker. It gives rise to cost savings due to the use of diagnostic tests and unnecessary treatments, as well as aiding the clinical management of patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed are in accordance with the ethical norms of the responsible human experimentation ethical committee, the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they followed the protocols of their centre of work regarding the publication of patient data.
Right to privacy and informed consentThe authors declare that they obtained the informed consent of the patients or subjects included in the paper. This document is held by the corresponding author.
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to thank the Nursing staff of the Intensive Care Unit for their collaboration in patient hygiene using the materials studied.
2% water-based chlorhexidine
Vein compressor
Gauze
Sterile wipe
70° alcohol
Sterile latex gloves
Surgical mask
IV needle
20ml syringe or needle+holder
Blood culture flasks (anaerobic/aerobic)
Hygienic hand washing. Putting on a surgical mask.
Select extraction site.
Disinfect the selected extraction zone with 2% water-based chlorhexidine and let it dry. Once disinfected the puncture point must not be touched, unless this is done in a sterile way. Put the sterile cover into place and put on sterile gloves.
Remove the plastic caps from the flasks.
The rubber cap is not sterile and must be disinfected.
Disinfect the rubber cap using 70° alcohol.
Let it dry.
Extract 20ml blood and inoculate 10ml in the anaerobic flask and 10ml in the aerobic flask.
Blood is extracted by direct puncture of a vein or artery.
The gauze must not touch the needle when it is withdrawn.
The needle of the syringe does not have to be changed to inoculate the flasks.
Do not touch the flask cap membrane after it has been disinfected with 70° alcohol.
Record patient data on the flasks (request label) without covering the barcode or batch number.
Send the flasks to the laboratory as soon as possible, where they will be incubated in a stove within a blood culture processing automatic system (BacT/ALERT).
Please cite this article as: Garrido-Benedicto P, Cueto-Quintana P, Farré-Termens E, Mariné-Cabré M, Riba-Reig J, Molina-Chueca R. Efecto de la higiene diaria con clorhexidina sobre la incidencia de contaminaciones de hemocultivos en el paciente crítico. Enferm Intensiva. 2017;28:97–104.