Tuberculosis (TB) is one of the most significant infections in immunosuppressed patients due to its high frequency and high morbidity and mortality. TB is the leading cause of death among HIV-infected patients. The diagnosis and early treatment of latent tuberculosis infection is vital to preventing it progression to disease. Similarly, the early diagnosis of TB is key to improving the prognosis of patients and preventing its transmission. The clinical expression of TB in immunosuppressed patients is conditioned by the patient's degree of immunosuppression. It is important to keep this peculiarity in mind so as not to delay the diagnosis of suspected TB. TB treatment is basically the same in immunosuppressed patients as in the general population and any differences mainly derive from pharmacological interactions. We examined the diagnosis and treatment of TB and latent tuberculosis infection in immunosuppressed patients.
La tuberculosis (TB) es una de las infecciones más importantes en pacientes inmunodeprimidos debido a su elevada frecuencia y alta morbimortalidad. La TB es la principal causa de muerte entre pacientes infectados por VIH. El diagnóstico y tratamiento precoz de la infección tuberculosa latente es clave para evitar su progresión a enfermedad. Del mismo modo, el diagnóstico precoz de la TB es clave para mejorar el pronóstico de los pacientes y evitar su transmisión. La expresión clínica de la TB en pacientes inmunodeprimidos está condicionada por el grado de inmunodepresión de los pacientes. Es importante tener presente esta peculiaridad para no retrasar el diagnóstico de sospecha de TB. Las bases del tratamiento de la TB en inmunodeprimidos son las mismas que en la población general. Sus peculiaridades derivan principalmente de las interacciones farmacológicas. Examinamos las bases de diagnóstico y tratamiento de la TB y la infección tuberculosa latente en pacientes inmunodeprimidos.
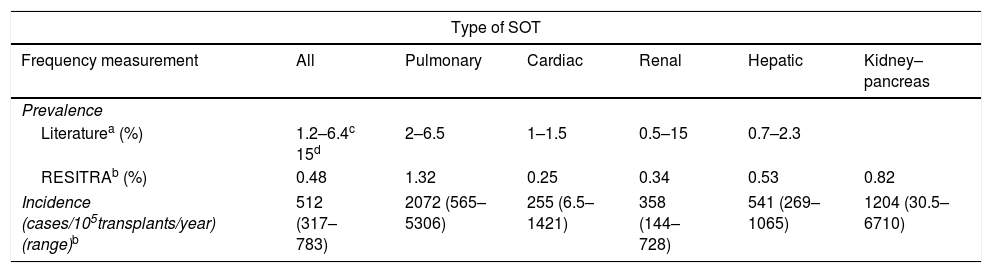
Tuberculosis (TB) is one of the most significant opportunistic infections which may affect solid-organ transplant (SOT) recipients due to its high morbidity and mortality. The prevalence of TB in SOT recipients ranges between 1.2% and 15%,1 i.e. between 20 and 74 times higher than in the general population. Table 1 shows the prevalence and incidence of TB in SOT recipients in most of the series in the literature1,2 compared to the information available in the Red Española de Infección en Trasplante [Spanish Network of Infection in Transplantation] (RESITRA).2
Prevalence and incidence of tuberculosis in SOT recipients (8).
| Type of SOT | ||||||
|---|---|---|---|---|---|---|
| Frequency measurement | All | Pulmonary | Cardiac | Renal | Hepatic | Kidney–pancreas |
| Prevalence | ||||||
| Literaturea (%) | 1.2–6.4c 15d | 2–6.5 | 1–1.5 | 0.5–15 | 0.7–2.3 | |
| RESITRAb (%) | 0.48 | 1.32 | 0.25 | 0.34 | 0.53 | 0.82 |
| Incidence (cases/105transplants/year) (range)b | 512 (317–783) | 2072 (565–5306) | 255 (6.5–1421) | 358 (144–728) | 541 (269–1065) | 1204 (30.5–6710) |
Although all SOT recipients should be considered a high-risk group for TB, this risk varies among the different types of transplant. The incidence of TB is particularly high in lung transplant recipients. Lung transplant recipients have a 5.6 times greater risk of developing TB than recipients of other types of transplant, and have a 73.3 times greater risk than non-immunosuppressed patients.2
Chronology and clinical characteristicsMost cases of TB in SOT recipients are caused by reactivation of a latent infection in the recipient when starting immunosuppressive treatment. It tends to occur in the first year after transplantation, with a median time of nine months. However, up to one third of patients may develop TB later than this. It has been observed that kidney transplant recipients develop symptoms later than other types of transplant recipients. This may be partly due to the lower intensity of the immunosuppression received compared to, for example, lung or heart transplant recipients.2 Furthermore, patients with a prior history of TB are more at risk of reactivation during the first months after transplantation, regardless of the type of immunosuppression received.
Pulmonary TB is the most common type of disease in the context of transplants. However, the number of patients who develop extrapulmonary or disseminated forms is higher than in the general population. These extrapulmonary or disseminated forms are more common in the first six months post-transplant, which coincides with the period of maximum immunosuppression.
Unlike that which occurs in the general population, TB in SOT recipients may be asymptomatic and the diagnosis in these cases is established using surveillance cultures. It is not uncommon for the diagnosis to be made during the post-mortem. Moreover, up to one third of patients may have a normal chest X-ray.
ProphylaxisProphylaxis should ideally be started before transplantation. If it is not possible to complete it before transplantation, it should be completed afterwards. The drug of choice is isoniazid (INH) (300mg/day) supplemented with vitamin B6 for nine months.1,3 The possibility of hepatotoxicity due to INH should be borne in mind; monitoring liver enzymes and discontinuing prophylaxis is recommended if these show a three-fold increase and the patient is symptomatic, or if these show a five-fold increase in asymptomatic patients.
Other alternatives to prophylaxis include INH (up to 900mg) twice a week for nine months in directly observed therapy, rifampicin (RF) (up to 600mg/day) for four months (with or without INH) and rifapentine (RP) (750mg if <50kg; 900mg if >50kg) with weekly INH for three months by directly observed therapy.4 The combination of RF with pyrazinamide (Z) for two months has been linked to cases of severe hepatotoxicity and is generally not recommended.4,5 For regimens including RF or RP, prophylaxis is only advised before transplantation, due to the interactions between these drugs and the immunosuppressive treatment.
It is advisable to delay the administration of INH until after transplantation in liver transplant candidates.4,5 Levofloxacin prior to transplantation could be an alternative to INH, although adverse effects, mainly tenosynovitis, have been reported.6
TreatmentThe recommendations for the treatment of TB in transplant patients are similar to those indicated in the general population4,5,7,8; there are only two differential aspects: (a) the interaction of rifamycins (RF, rifabutin [RB] or RP) with immunosuppressive drugs from the family of calcineurin inhibitors (ciclosporin, tacrolimus), with rapamycin and with steroids and (b) the duration of treatment.
Use of rifamycins in transplant patientsThe inclusion of rifamycins, particularly RF, in treatment regimens for TB in transplant patients is the most controversial aspect of treatment for these patients. RF reduces the serum concentrations of ciclosporin, tacrolimus, rapamycin (sirolimus), everolimus and corticosteroids. This has been linked to an increased risk of rejection. Therefore, increasing the dose of calcineurin inhibitors by approximately three to five times when RF is used simultaneously in these patients, and closely monitoring the concentrations of the immunosuppressant drugs is recommended.5
RB could be an alternative, given that it is a weaker inducer of cytochrome P450 than RF, but the data on its use are limited.
Use of other anti-tuberculosis drugs in transplant patientsINH and Z have been used extensively in transplant patients with TB. Given the risk of hepatotoxicity, liver enzymes must be closely monitored, particularly in liver transplant recipients.
Administration of aminoglycosides should be weighed up with caution, given the risk of strengthening the nephrotoxicity of these drugs with calcineurin inhibitors.
Fluoroquinolones may be an alternative, especially in patients who develop hepatotoxicity or who have liver dysfunction. However, the indiscriminate use of these drugs in the general population has been associated with an increased resistance of Mycobacterium tuberculosis (M. tuberculosis) to fluoroquinolones in recent years.
An increased incidence of adverse effects linked to prolonged treatment with fluoroquinolones, particularly arthralgia and tendinopathy, has been observed.6 The joint and prolonged use of levofloxacin with Z has been associated with poor tolerance, especially digestive tolerance, in SOT recipient patients.
In special cases of resistance or toxicity, linezolid has been effective in patients with TB.9 However, prolonged use of this drug is linked to the common development of thrombocytopaenia and anaemia and, in some cases, of polyneuropathy, particularly in patients with other comorbidities, such as diabetes or kidney disease.
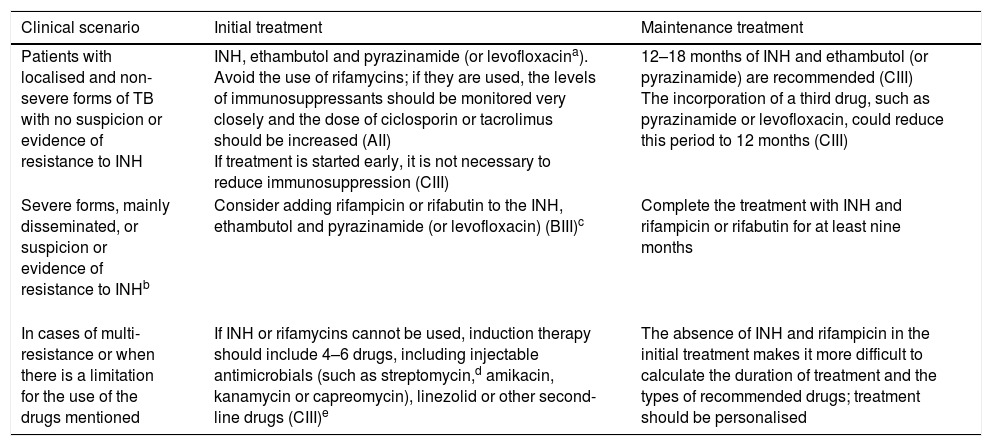
Table 2 lists the recommendations for the treatment of TB in transplant patients based on the consensus document of the Grupo de Estudio de la Infección en el Trasplante [Transplantation Infection Study Group] (GESITRA) of the Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica [Spanish Society of Infectious Diseases and Clinical Microbiology].7
TB treatment in SOT recipients in accordance with the GESITRA recommendations from the Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica [Spanish Society of Infectious Diseases and Clinical Microbiology].
| Clinical scenario | Initial treatment | Maintenance treatment |
|---|---|---|
| Patients with localised and non-severe forms of TB with no suspicion or evidence of resistance to INH | INH, ethambutol and pyrazinamide (or levofloxacina). Avoid the use of rifamycins; if they are used, the levels of immunosuppressants should be monitored very closely and the dose of ciclosporin or tacrolimus should be increased (AII) If treatment is started early, it is not necessary to reduce immunosuppression (CIII) | 12–18 months of INH and ethambutol (or pyrazinamide) are recommended (CIII) The incorporation of a third drug, such as pyrazinamide or levofloxacin, could reduce this period to 12 months (CIII) |
| Severe forms, mainly disseminated, or suspicion or evidence of resistance to INHb | Consider adding rifampicin or rifabutin to the INH, ethambutol and pyrazinamide (or levofloxacin) (BIII)c | Complete the treatment with INH and rifampicin or rifabutin for at least nine months |
| In cases of multi-resistance or when there is a limitation for the use of the drugs mentioned | If INH or rifamycins cannot be used, induction therapy should include 4–6 drugs, including injectable antimicrobials (such as streptomycin,d amikacin, kanamycin or capreomycin), linezolid or other second-line drugs (CIII)e | The absence of INH and rifampicin in the initial treatment makes it more difficult to calculate the duration of treatment and the types of recommended drugs; treatment should be personalised |
Prolonged use of fluoroquinolones may be associated with arthralgia; the combination of pyrazinamide and levofloxacin is not well tolerated due to gastrointestinal side effects.
If INH cannot be used, initial and maintenance treatment which includes four drugs for at least 18 months is recommended (CIII).
The use of rifampicin or rifabutin requires increasing the doses of ciclosporin or tacrolimus and close monitoring of the levels of these drugs (AII). Resistance to rifampicin is associated with cross-resistance to rifabutin and rifapentine; therefore, they are not alternatives to consider (DII).
In cases of streptomycin resistance, there is no cross-resistance with other injectable drugs (amikacin, kanamycin or capreomycin); however, cross-resistance between amikacin and kanamycin is universal. The combination of injectable drugs is not recommended due to its intolerance and side effects (DII).
There are two reasons for debate in these patients: the duration of treatment and the type of drugs which should be used after the first two months, especially if RF is not used in the first two months or if this should be discontinued due to its adverse effects. Some authors recommend a daily treatment regimen for six months in transplant patients,4 although treatment for less than nine months has been linked to increased mortality.1 The duration of treatment should be extended in patients with: osteoarticular disease (from six to nine months); central nervous system involvement (from nine to twelve months); severe disseminated disease (from six to nine months); cavitating pulmonary TB with positive sputum cultures after two months of treatment (nine months); and in patients with second-line drugs or RF-free regimens, given that rifamycins have a potent bactericidal activity against M. tuberculosis.4 In these cases, initial treatment should last two months, but the maintenance therapy should be longer.
Tuberculosis in haematopoietic stem cell transplant recipientsAlthough TB is approximately 10 times less common in recipients of haematopoietic stem cell transplants (HSCT) than SOT recipients, the frequency is 10–40 times greater in HSCT recipients than in the general population.10 The incidence of TB in autologous HSCTs ranges from 0.05% to 0.26%, which is comparable to the general population. However, the incidence in allogeneic HSCTs is greater: between 0.1% and 5.5%.11
It is estimated that one quarter of cases are due to the reactivation of latent M. tuberculosis infection. The median time for the onset of TB is 257 days post-HSCT.
Clinical presentationTB in HSCT recipients typically presents with an indolent course. Pulmonary TB is the most common manifestation and the symptoms are similar to those of the general population. However, the classic pulmonary findings, such as infiltrate or apical cavitation, tend to be absent. Radiological findings may be non-specific, lobar and segmental diffuse infiltration, interstitial pneumonitis, acute respiratory distress syndrome, peripheral pulmonary nodules or, even, diffuse alveolar haemorrhage. Clinical and radiological manifestations may resemble an invasive fungal infection.
More than 15% of patients may have extrapulmonary TB which affects the liver, spleen, kidney, bone, bone marrow, central nervous system and joints.
ProphylaxisThe indications and recommendations for the treatment of latent tuberculosis infection (LTBI) are the same as those for SOTs. If possible, treatment should be started before the conditioning therapy, or when this is completed, when the risk of disease is high.12
TreatmentThe management of active TB in HSCTs is the same as in SOTs.
Tuberculosis in patients on biological therapiesBiological agents, including tumour necrosis factor (TNF) antagonists, are used to manage some rheumatic and autoimmune diseases. The use of biological therapies generates concern, due to the long-term effects of persistent blocking or inhibiting of cytokines or their receptors (TNF, IL-1, IL-6R) or of cells (lymphocytes B and T) that play an essential role in the immune system defence mechanisms against infection or against cancer. To date, five anti-TNFα drugs are used in clinical practice: etanercept, adalimumab, infliximab, golimumab and certolizumab pegol. In order to determine the long-term safety of biological therapies, different national registries have been created. In 2000, the Sociedad Española de Reumatología [Spanish Society of Rheumatology] created BIOBADASER, the Spanish registry for adverse events related to biological therapies in rheumatic diseases.13 The main problem associated with the use of biological therapies is a moderate increase in the risk of serious infections compared to that observed with conventional therapies. The infection in which the contribution of BIOBADASER has been fundamental is TB. In 2000, a high incidence of TB compared to the general population was detected in Spanish patients treated with anti-TNFα. The relative risk (RR) of TB in patients with rheumatoid arthritis treated with infliximab compared to patients not exposed to this therapy was 19.9 (95% CI: 16.2–24.8) in 2000 and 11.7 (95% CI: 9.5–14.6) in 2001,14 including extrapulmonary and disseminated forms in 65% of cases. The alert resulted in the issuance in March 2002 of the official recommendations on TB prevention in patients with LTBI who started treatment with anti-TNFα. This led to an 80% reduction in new cases.15 The validity of these recommendations was subsequently ratified, when it was proven that there is a seven-fold greater risk of TB if they were not met.16
ProphylaxisIn patients with LTBI, treatment with INH 5mg/kg/day (up to 300mg/day) for nine months is recommended. In the event of allergy or intolerance to INH, RF (10mg/kg/day; up to 600mg) for four months could be used. In any case, it is recommended that biological therapy is not started until one month after the start of anti-TB prophylaxis.17
TreatmentThe management of active TB in patients treated with biological therapies is the same as that in the general population.
Tuberculosis in patients undergoing chronic corticotherapyAn increase in the incidence of TB in patients undergoing chronic treatment with corticosteroids has been reported.18 In a retrospective case-control study, the odds ratio for TB was 2.8 (95% CI: 1.0–7.9) for doses lower than 15mg/day of prednisone and 7.7 (95% CI: 2.8–21.4) for doses greater than 15mg/day.19 Moreover, the use of high doses of inhaled corticosteroids (greater than that equivalent to a 500μg/day dose of fluticasone), a dose ≥10mg/day of prednisolone and previous pulmonary TB have been associated with an increased risk of pulmonary TB in patients with chronic obstructive pulmonary disease.20
Therefore, given the risk of developing TB in patients undergoing chronic treatment with corticosteroids, it is recommended that LTBI is ruled out before starting this treatment.
Prophylaxis in patients with LTBI and treatment of active TB in patients undergoing chronic corticotherapy are the same as that for the general population.
Tuberculosis in patients with human immunodeficiency virus infectionEpidemiologyIn 2015, the estimated number of new cases of TB worldwide in individuals with the human immunodeficiency virus (HIV) infection was 1.2 million, which accounted for 11% of TB cases globally. In the same year, 0.4 million HIV patients died from TB worldwide (22% of deaths from TB: 1.8 million people).21 This means that TB currently remains the main cause of death in HIV-infected patients.21 The annual TB reactivation risk among individuals with HIV without antiretroviral therapy (ART) is 3–16% per year. This implies approximately the same risk (∼5%) of developing TB which individuals not infected with LTBI have throughout their lifetime.22 Unlike other opportunistic infections, and although its incidence is greater as immunosuppression increases, TB may occur in any HIV-infected patient, regardless of his/her CD4 lymphocyte count.22 Furthermore, although ART reduces the incidence of TB in HIV patients, the risk of TB among these patients continues to be greater than in the general population. The development of TB is also an independent factor of the increased risk of disease progression and death in HIV-infected patients. Therefore, despite being a preventable and curable disease, TB is still one of the main health problems in HIV-infected patients.
Anti-tuberculosis prophylaxis in patients with human immunodeficiency virus infectionIn individuals with HIV, LTBI has traditionally been defined by the presence of a positive (≥5mm of induration after 48–72h) tuberculin skin test (TST) in the absence of evidence of tuberculosis.23–25 Screening for LTBI should be performed in all individuals with HIV when they are diagnosed. In individuals with a negative TST and CD4+ count <200cells/ml, the TST should be repeated after starting the ART and reaching a T4 count of >200cells/ml. In individuals with a high risk of exposure to active TB, repeating the TST on an annual basis is recommended.23 Experience with the TST in HIV-infected patients is very high, and numerous clinical trials have demonstrated the benefit of treating individuals with a positive TST. However, the diagnosis of LTBI using TST has at least three limitations26: (1) two visits are required for its determination, (2) its specificity is reduced in patients who have received the Bacillus Calmette-Guérin (BCG) vaccine, as it results in false positives, and (3) its sensitivity is low in patients with advanced immunosuppression, which causes false negatives. These limitations have inspired the development of the interferon-γ release assay (IGRA) for the diagnosis of LTBI. Compared to the TST, the IGRA has greater specificity and lower cross-reactivity with BCG, although its sensitivity is also reduced in situations of severe immunosuppression.24,25,27 In contrast, we do not have sufficient tests demonstrating that treatment of LTBI with a diagnosis based on IGRA reduces the risk of TB in HIV-infected patients, and this is a significant limitation for its use. Joint use of both procedures for the screening of LTBI is not recommended in HIV-infected patients, as this is a more expensive and difficult strategy, and its benefit is unknown.23,28
Anti-TB prophylaxis should be indicated in all patients with LTBI.23,25 In addition, prophylaxis should be indicated, regardless of the TST or IGRA result, to all HIV-infected patients in close contact with bacilliferous TB patients.23,25 The benefit of anti-TB prophylaxis in patients with a negative TST or in patients with cutaneous anergy has not been proven.23,24,29 In a study conducted in an area of high TB prevalence (South Africa), it was demonstrated that treatment with isoniazid reduced the risk of TB, regardless of the TST or IGRA result.30 However, this finding cannot be extrapolated to areas with a lower prevalence of TB.
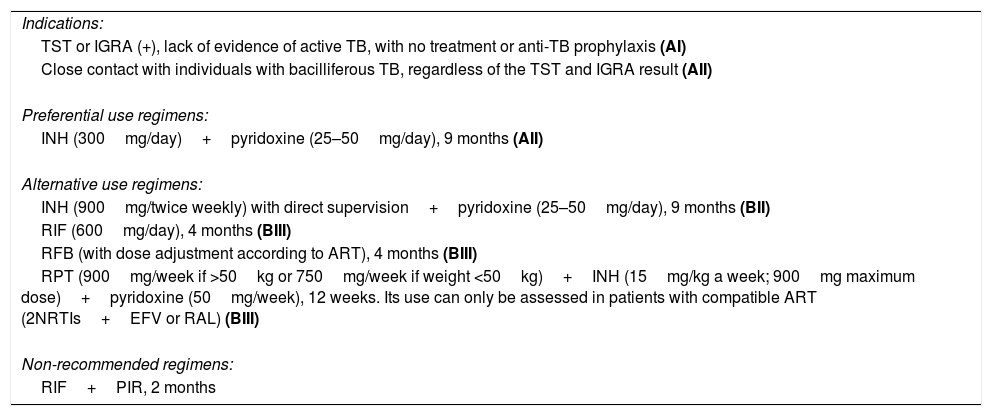
Isoniazid for nine months is the regimen of choice in anti-TB prophylaxis in HIV-infected patients, due to the high level of evidence available on its efficacy and due to its safety profile.23,25 Concomitant administration of pyridoxine at a dose of 25–50mg/day to prevent the development of peripheral neuropathy is recommended.23,25 The main limitation of this regimen is its duration. Shorter regimens have been developed, but there is less experience and a lower level of evidence with these regimens. Table 3 shows the regimens of preferential and alternative use in anti-TB prophylaxis in HIV-infected patients.
Indications and regimens in HIV-infected patients.
| Indications: |
| TST or IGRA (+), lack of evidence of active TB, with no treatment or anti-TB prophylaxis (AI) |
| Close contact with individuals with bacilliferous TB, regardless of the TST and IGRA result (AII) |
| Preferential use regimens: |
| INH (300mg/day)+pyridoxine (25–50mg/day), 9 months (AII) |
| Alternative use regimens: |
| INH (900mg/twice weekly) with direct supervision+pyridoxine (25–50mg/day), 9 months (BII) |
| RIF (600mg/day), 4 months (BIII) |
| RFB (with dose adjustment according to ART), 4 months (BIII) |
| RPT (900mg/week if >50kg or 750mg/week if weight <50kg)+INH (15mg/kg a week; 900mg maximum dose)+pyridoxine (50mg/week), 12 weeks. Its use can only be assessed in patients with compatible ART (2NRTIs+EFV or RAL) (BIII) |
| Non-recommended regimens: |
| RIF+PIR, 2 months |
The letters in bold refer to the evidence of the recommendations based on the Infectious Diseases Society of America's (IDSA) criteria, 2001.
STRENGTH OF RECOMMENDATION:
A: good evidence to support a recommendation for use.
B: moderate evidence to support a recommendation for use.
C: poor evidence to support a recommendation.
D: moderate evidence to support a recommendation against use.
E: good evidence to support a recommendation against use.
QUALITY OF EVIDENCE:
I: evidence from ≥1 properly randomised, controlled trial.
II: evidence from ≥1 well-designed clinical trial, without randomisation; from cohort or case-controlled analytic studies (preferably from >1 centre); from multiple time series; or from dramatic results from uncontrolled experiments.
III: evidence from opinions of respected authorities, based on clinical experience, descriptive studies or reports of expert committees.
Treatment of LTBI reduces the risk of developing TB by 62%, and the risk of death by 26% in HIV-infected patients.31 Both ART and treatment with isoniazid independently reduce the risk of TB, mortality and the development of AIDS events.32 It has been demonstrated that, among patients who receive ART, treatment with isoniazid reduces the risk of TB versus placebo by 37%.30 Therefore, screening for LTBI and its appropriate treatment are an essential strategy for the improvement of expectations and quality of life of HIV-infected patients.
Clinical characteristics of tuberculosis in patients with human immunodeficiency virus infectionThe clinical expression of TB in HIV-infected patients is determined by the patients’ degree of immunosuppression. Thus, patients with severe immunosuppression (<200 and, in particular, <50 CD4+ lymphocytes/ml) tend to present with extrapulmonary or disseminated forms of TB, while those patients with a good immunological situation (>200 and, in particular, >500 CD4+ lymphocytes/ml) more often present with localised pulmonary forms of the disease, similar to those of patients without HIV.33 In the case of pulmonary TB, radiological expression is closely correlated with the patients’ degree of immunosuppression, meaning that the presence of pulmonary infiltrate or cavitation are less common as the degree of immunosuppression increases.34 This radiological anergy, for which the greatest expression is the normal chest X-ray in the presence of sputum smear microscopy or positive sputum cultures, is not uncommon among patients with severe immunosuppression. In HIV-infected patients, TB may adopt a sub-clinical or oligosymptomatic course. However, in severely immunosuppressed patients, TB may present in the form of symptoms of sepsis with a severe and rapidly progressive course. Therefore, in HIV-infected patients, the awareness of symptoms or classic radiological signs suggestive of TB (cough, fever, cavitary pulmonary infiltrate in the upper lobes, night sweats and weight loss) is lower and reduces as immunosuppression increases. It is very important to bear in mind these clinical characteristics of TB in HIV patients in order to properly establish, and not delay, the diagnosis of suspected TB and allow for early treatment. The clinical manifestations of extrapulmonary TB are not substantially different from those of the general population.35 In general, and as a rule, the diagnosis of suspected TB in HIV-infected patients should be taken into account in any disease, regardless of the organ or system affected.24,35
Diagnosis of tuberculosisThe initial assessment of an HIV-infected patient with suspected TB should always include a chest X-ray, even in the absence of respiratory symptoms.24,33,34 Similarly, specific staining and a sputum culture should be performed in all patients with suspected TB, even in cases in which the X-ray is normal.24,34 The sensitivity of sputum staining in HIV-infected patients is lower than in the general population, and may prove to be falsely negative, particularly in patients with severe immunosuppression. In contrast, the sensitivity of the sputum culture is not affected by HIV infection or by the degree of immunosuppression.36 In HIV-infected patients, performing three stains and sputum cultures in different samples increases the diagnostic sensitivity.24,37
Furthermore, as occurs with clinical-radiological expression, histopathological findings of TB are determined by the patients’ degree of immunosuppression.33 It is therefore important to bear in mind that in HIV-infected patients with severe immunosuppression, the typical granulomatous reaction observed in patients without HIV may be absent. For this reason, screening using stains and appropriate techniques for all histological samples, regardless of the presence or absence of granulomas, should be performed in HIV-infected patients with suspected TB.
In extrapulmonary TB, the efficiency of staining and culturing is lower than for sputum. In these cases, the combined efficiency of staining, culturing and histopathological analysis of the material obtained by fine-needle aspiration or biopsy is high. The efficiency of the blood culture and urine culture from mycobacteria depends on the experience of each laboratory, and may be relatively high in patients with severe immunosuppression and disseminated TB. Given the ease of obtaining these samples, both procedures should be performed routinely in these patients.
The use of nucleic acid amplification tests (NAATs) in the diagnosis of TB is high due to the fact that they are faster than mycobacterial cultures, have greater sensitivity than stains and allow for the rapid detection of resistances to anti-TB drugs. In samples with negative stain/positive culture, the positivity of just one NAAT is 50–80%, and that of three NAATs increases up to 90%.24 Furthermore, they enable M. tuberculosis to be quickly differentiated from other mycobacteria (2h) and directly in sputum. This is very useful in HIV-infected patients with severe immunosuppression, in whom non-tuberculous mycobacterial infections are common. Lastly, they enable mutations associated with resistance to RF to be detected in sputum with a sensitivity and specificity in HIV-infected patients of 80% and 89%, respectively, somewhat lower than that observed in non-HIV-infected patients (88% and 98%, respectively).
Lipoarabinomannan (LAM) is a polysaccharide of the M. tuberculosis cell wall which may be detected in the urine of patients with TB. In HIV-infected patients with CD4 <100cells/ml, it has a sensitivity of 35–56% and a specificity of 95%.37
A baseline study of resistance to anti-TB drugs should be performed in all HIV-infected patients with TB.
Tuberculosis treatment in patients with human immunodeficiency virus infectionIn Spain, it is recommended to start anti-TB treatment in HIV-infected patients with a combination of four drugs,23–25,38 including H, RF, Z and E. The use of RB has been suggested as an alternative to RF in order to minimise possible interactions with some antiretroviral drugs (mainly protease inhibitors). However, the evidence of its efficacy in HIV-infected patients is low, its use increases the complexity of anti-TB treatment by stopping the use of co-formulated combinations and, lastly, it would require an adjustment of the RB dose which, in the absence of controlling the plasma levels of the drug, could facilitate the selection of resistance to rifamycins.38 Therefore, the use of RB instead of RF should be considered for cases in which the use of protease inhibitors is absolutely essential.38 The use of RP instead of RF has the same disadvantages commented for RB and is also not recommended.38
After two months of treatment have been completed, and once clinical improvement, correct adherence to treatment and the baseline resistance profile have been confirmed, the administration of Z and E will be discontinued. Treatment with H and RF (maintenance phase) will continue for a further 4–7 months. Furthermore, E may be withdrawn once the susceptibility of the strain to RF and H has been confirmed. It must be kept in mind that, when H is used, it is necessary to associate pyridoxine (vitamin B6) at a dose of 10–50mg/day in order to avoid the development of peripheral neuropathy.38
Anti-TB treatment should be administered daily (seven days a week) whenever possible.38 Intermittent regimens during the treatment induction period have been associated with a greater risk of relapses and failure. Therefore, they should only be used once the induction phase has been completed, when there are problems for daily administration and when it can be guaranteed that the administration of the medication will be supervised.38
The optimal duration of treatment for drug-susceptible TB in HIV-infected patients is disputed. A randomised clinical trial conducted in the USA showed excellent and comparable efficacy of regimens lasting six and nine months.39 However, the study design lacked sufficient power to draw firm conclusions. Moreover, in two clinical trials conducted in areas with a high incidence of TB, the risk of recurrence among patients treated for six months was greater than in those treated for nine or twelve months.40,41 However, in these studies the patients did not receive ART, and no distinction was made between relapses and re-infections. In one of them, intermittent regimens were used from the start of treatment. Therefore, it is not possible to extrapolate these results to areas with different health and epidemiological situations. In this situation, in Spain and other western countries, it is recommended that most HIV-infected patients with TB are treated for six months, and that this is extended to nine months in the event of persistency in the positivity of the sputum after two months,24,38 a CD4+ lymphocyte count below 100cells/μl and in patients with doubtful adherence to treatment.38 In cases of TB which affect the central nervous system (meningitis, tuberculoma), prolonging the treatment until at least 9–12 months have been completed is recommended.38 The clinical trials do not provide any information that makes it possible to further specify the duration of treatment in this scenario. In cases of TB with involvement of the pericardium or the central nervous system, the use of corticosteroids should be considered.24,38
The treatment of drug-resistant TB is specified in another chapter of this monograph.
When should antiretroviral treatment in human immunodeficiency virus-infected patients with tuberculosis be started?Establishing the optimal time to start ART in HIV-infected patients with TB has been a subject of debate for a long time. Currently, the information from clinical trials with a suitable design has shed light on this issue.
The SAPIT42 study included 642 patients with T4 levels <500cells/ml, who were distributed randomly to start ART during TB treatment (integrated, early or delayed ART) or after treatment completion (sequential ART). Higher mortality was observed in the sequential ART group than in the integrated ART group (5.4 versus 12.1 deaths/100 patients per year; p=0.003), regardless of the CD4+ lymphocyte count. Subsequently,43 the branches of early integrated ART (in the first four weeks of treatment) and delayed ART (after completing the intensive phase) were compared, and a 68% reduction in mortality (8.5 versus 26.3 deaths/100 patients per year; p=0.06) was observed in the early ART group in the sub-group of patients with baseline CD4+ lymphocyte counts below 50cells/μl.
The CAMELIA44 study included 661 patients with CD4+ lymphocyte counts below 200cells/μl, who were distributed randomly to start ART two weeks after having started TB treatment (early ART) or after completing the intensive phase of anti-TB treatment (delayed ART). The median CD4+ lymphocyte count was 25cells/μl. Starting ART early reduced the risk of death by 34% compared to starting ART late (8.28 versus 13.77 deaths/100 patients per year; p=0.002).
The STRIDE (ACTG-5221) study included 806 patients with baseline CD4+ lymphocyte counts below 250cells/μl, who were distributed randomly to start ART after two weeks of anti-TB treatment (early ART), or after completing the intensive phase of treatment (delayed ART). A 68% reduction in mortality (15.5 versus 26.6 deaths/100 patients per year; p=0.02) was observed in the group of patients who received early ART in the sub-group of patients with baseline CD4+ lymphocyte counts below 50cells/μl.45
Lastly, the TB-HAART46 study included 1538 patients with pulmonary TB and with baseline CD4+ lymphocyte counts ≥220cells/μl, who were distributed randomly to start ART after two weeks of anti-TB treatment (early ART), or after completing TB treatment (sequential ART). A 68% reduction in mortality (15.5 versus 26.6 deaths/100 patients per year; p=0.02) was observed in the group of patients who received early ART in the sub-group of patients with baseline CD4+ lymphocyte counts below 50cells/μl. The study's composite outcome variable (TB treatment failure, recurrence or death) was observed in 8.5% of the early ART group and in 9.2% of the sequential ART group (RR 0.91; 95% CI: 0.64–1.30; p=0.9). Due to these results, and unlike the SAPIT, STRIDE and CAMELIA studies, the TB-HAART study concluded that ART, in patients with CD4+ lymphocyte counts >220cells/μl, could be delayed until TB treatment is completed. To assess the implication of these results, it is necessary to bear in mind that the recommendation of starting ART in all HIV-infected patients, regardless of the CD4+ lymphocyte count, aims to not only prevent progression of the disease, but also to reduce transmission of the virus and limit the harmful effect on possible comorbidities.47
From the above, the following conclusions can be drawn: (1) starting ART during TB treatment reduces the risk of death in HIV-infected patients. (2) This benefit is observed irrespective of the CD4+ lymphocyte count. (3) In patients with CD4+ lymphocyte counts below 50cells/μl, starting ART in the first four weeks of TB treatment reduces the risk of death. (4) In patients with CD4+ lymphocyte counts above 50cells/μl, starting ART once the induction phase of TB treatment has been completed reduces the risk of adverse effects and immune reconstitution inflammatory syndrome (IRIS) without compromising survival.48
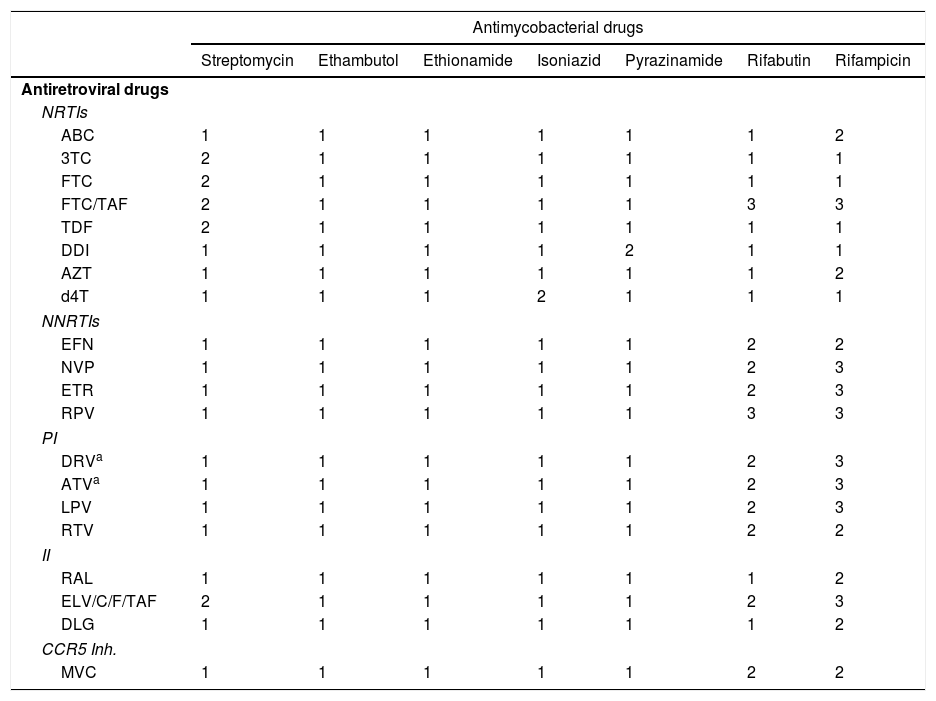
Drug–drug interactions between antiretroviral and anti-tuberculosis drugsThe reason for the most relevant drug interaction between ART and anti-TB treatment is that rifamycins are potent inducers of CYP3A4, a very important enzyme system for the metabolism of most families of antiretroviral drugs38,47 (see Table 4). The interaction between rifamycins and nucleoside-analogue reverse-transcriptase inhibitors (NRTIs) 3 TC, FTC, TDF and abacavir is not clinically significant and, therefore, their concomitant use does not require a dose adjustment.38,47 However, rifamycins may reduce the absorption of TAF and its plasma levels, which could result in the loss of its efficacy and the onset of resistance.47 There are no clinical studies which have evaluated the clinical significance of this potential interaction.47 RF stimulates the metabolism of non-nucleoside-analogue reverse transcriptase inhibitors and elicits a reduction of its plasma concentrations. Of the non-nucleoside-analogue reverse transcriptase inhibitors, the pharmacokinetics of efavirenz and nevirapine are least affected by RF. Of these, efavirenz, at a dose of 600mg/day, has the higher level of evidence42–45 and is therefore considered the drug of choice in the treatment of HIV infection in patients with TB.38 In contrast, RF causes a significant reduction in levels of rilpivirine and etravirine, which prevents its concomitant use. The combined use of ritonavir-boosted protease inhibitor (PI/r) with RF is contraindicated.38
Drug–drug interactions between antiretroviral drugs and antimycobacterial drugs.
| Antimycobacterial drugs | |||||||
|---|---|---|---|---|---|---|---|
| Streptomycin | Ethambutol | Ethionamide | Isoniazid | Pyrazinamide | Rifabutin | Rifampicin | |
| Antiretroviral drugs | |||||||
| NRTIs | |||||||
| ABC | 1 | 1 | 1 | 1 | 1 | 1 | 2 |
| 3TC | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| FTC | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| FTC/TAF | 2 | 1 | 1 | 1 | 1 | 3 | 3 |
| TDF | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| DDI | 1 | 1 | 1 | 1 | 2 | 1 | 1 |
| AZT | 1 | 1 | 1 | 1 | 1 | 1 | 2 |
| d4T | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| NNRTIs | |||||||
| EFN | 1 | 1 | 1 | 1 | 1 | 2 | 2 |
| NVP | 1 | 1 | 1 | 1 | 1 | 2 | 3 |
| ETR | 1 | 1 | 1 | 1 | 1 | 2 | 3 |
| RPV | 1 | 1 | 1 | 1 | 1 | 3 | 3 |
| PI | |||||||
| DRVa | 1 | 1 | 1 | 1 | 1 | 2 | 3 |
| ATVa | 1 | 1 | 1 | 1 | 1 | 2 | 3 |
| LPV | 1 | 1 | 1 | 1 | 1 | 2 | 3 |
| RTV | 1 | 1 | 1 | 1 | 1 | 2 | 2 |
| II | |||||||
| RAL | 1 | 1 | 1 | 1 | 1 | 1 | 2 |
| ELV/C/F/TAF | 2 | 1 | 1 | 1 | 1 | 2 | 3 |
| DLG | 1 | 1 | 1 | 1 | 1 | 1 | 2 |
| CCR5 Inh. | |||||||
| MVC | 1 | 1 | 1 | 1 | 1 | 2 | 2 |
3TC: lamivudine; ABC: abacavir; ATV: atazanavir; AZT: zidovudine; d4T: stavudine; DDI: didanosine; DLG: dolutegravir; DRV: darunavir; EFV: efavirenz; ETR: etravirine; EVG: elvitegravir; FTC: emtricitabine; FTC/TAF: emtricitabine/tenofovir alafenamide; II: integrase inhibitors; LPV: lopinavir; MVC: maraviroc; NNRTIs: non-nucleoside-analogue reverse-transcriptase inhibitors; NRTIs: nucleoside-analogue reverse-transcriptase inhibitors, we include tenofovir in this group; NVP: nevirapine; PI: protease inhibitors; RAL: raltegravir; RPV: rilpivirine; RTV: ritonavir; TDF: tenofovir.
1. With no clinically significant interaction.
2. Potentially clinically significant interaction. Perhaps additional monitoring, an alteration of the drug dose or of the time of administration is required.
3. Co-administration is contraindicated.
RF has an inductive effect on the enzyme responsible for the degradation of raltegravir, UDP-glucuronosyltransferase, which reduces the AUC of raltegravir by 40%. A phase II clinical trial48 included 155 HIV-infected patients without prior ART diagnosed with TB and treated with a regimen which included RF. The patients were randomised to receive tenofovir/emtricitabine along with efavirenz (600mg/day), raltegravir (400mg every 12h) or raltegravir (800mg every 12h). Twenty-four weeks from the start of ART, 67%, 78% and 76% of patients reached undetectable HIV RNA, respectively. Therefore, raltegravir may be an alternative to EFV in HIV patients with TB.38,47 The results of a phase I clinical trial indicate that dolutegravir, at a dose of 50mg every 12h, may be co-administered with RF.49 The combined use of RF with elvitegravir/cobicistat is not recommended.38,47
The use of RB instead of RF to enable the use of PI/r is a strategy which is not problem-free.38 First, the concomitant use of RB and PI/r increases the levels of RB. Therefore, dose adjustments are required. The optimal dose of RB in combination with PI/r has not been clearly established: 150mg every two days or three times a week is the dose which is most commonly recommended. Treatment failures with the onset of resistance to rifamycins have been reported in patients with advanced immunosuppression treated with PI/r and RB. This raises serious doubts about the efficacy of the combination. It also has to kept in mind that RB levels are highly “sensitive” to the omission of any PI/r dose by the patient. Lastly, the use of RB increases the complexity of anti-TB treatment by preventing the use of co-formulations. For all of these reasons, it is recommended to avoid the simultaneous use of RB and PI/r and, if possible, to assess other therapeutic alternatives.38
Tuberculosis and immune reconstitution inflammatory syndromeIRIS is the result of restoration of the ability of the immune system to activate inflammatory mechanisms after starting ART, and is commonly manifested in two scenarios. The first of these is the so-called unmasked IRIS, a situation in which the improvement in immunity clinically reveals sub-clinical TB which had not yet been identified. The second of these is the so-called paradoxical IRIS. In this situation, clinical worsening of the TB occurs in an HIV-infected patient diagnosed and treated appropriately for TB, after starting ART. Several predictive factors for IRIS in patients with TB have been identified, including: CD4 lymphocyte count <50cells/μl, increased CD4+ lymphocyte count after starting ART, high HIV baseline viral load, severity of TB and starting ART early.38 Most cases of IRIS are self-limited and occur within the first three months after starting anti-TB treatment. In the presence of IRIS, anti-TB treatment or ART should not be discontinued. Mild forms of IRIS should be managed with non-steroidal anti-inflammatory drugs. In a randomised placebo-controlled clinical trial conducted in patients with moderate-severe paradoxical IRIS, treatment with prednisone (1.5mg/kg per day for two weeks followed by 0.75mg/kg per day for another two weeks) reduced the patients’ hospitalisation time and the need for invasive procedures.50 The use of steroids in patients with Kaposi's sarcoma should be avoided.
Conflicts of interestThe authors declare that they have no conflicts of interest.
This study was supported by the Red de Investigación en Sida de España [Spanish AIDS Research Network] ISCIII-RETIC (RD 16/0025/0034), by the Plan Estatal de Investigación Científica y Técnica y de Innovación [State Plan for Scientific and Technical Research and Innovation] 2013–2016 and by the Instituto de Salud Carlos III [Carlos III Health Institute], Subdirección General de Redes y Centros de Investigación Cooperativa [General Sub-directorate of Networks and Centres for Cooperative Research], Ministerio de Economía, Industria y Competitividad [Ministry of Economy, Industry and Competitiveness], Red Española de Investigación en Patología Infecciosa [Spanish Network for Research in Infectious Diseases] (REIPI RD 16/0016/0008), co-funded by the European Regional Development Fund “A way to make Europe”, Smart Growth Operational Programme 2014–2020.
Please cite this article as: Machuca I, Vidal E, de la Torre-Cisneros J, Rivero-Román A. Tuberculosis en pacientes inmunodeprimidos. Enferm Infecc Microbiol Clin. 2018;36:366–374.