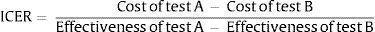The need to reduce the time it takes to establish a microbiological diagnosis and the emergence of new molecular microbiology and proteomic technologies has fuelled the development of rapid and point-of-care techniques, as well as the so-called point-of-care laboratories. These laboratories are responsible for conducting both techniques partially to response to the outsourcing of the conventional hospital laboratories. Their introduction has not always been accompanied with economic studies that address their cost-effectiveness, cost-benefit and cost-utility, but rather tend to be limited to the unit price of the test. The latter, influenced by the purchase procedure, does not usually have a regulated reference value in the same way that medicines do. The cost-effectiveness studies that have recently been conducted on mass spectrometry in the diagnosis of bacteraemia and the use of antimicrobials have had the greatest clinical impact and may act as a model for future economic studies on rapid and point-of-care tests.
La necesidad de reducir el tiempo de diagnóstico microbiológico y la irrupción de nuevas tecnologías relacionadas con la microbiología molecular y la proteómica han favorecido el desarrollo de técnicas rápidas y de realización en el lugar de asistencia al paciente (point-of-care), así como de los denominados laboratorios point-of-care, espacios que concentran la realización de ambas técnicas como respuesta, en parte, a la externalización de los laboratorios convencionales de los hospitales. Su introducción no siempre se ha acompañado de evaluaciones económicas (estudios de coste-efectividad, coste-beneficio y coste-utilidad) y suelen limitarse al precio unitario de la prueba. Este último, influido por el procedimiento de compra, no suele tener un valor de referencia regulado, como en el caso de los medicamentos. Los análisis de coste-efectividad que mayor repercusión han tenido han sido los realizados recientemente con la espectrometría de masas en el diagnóstico de la bacteriemia y el uso de antimicrobianos y pueden servir como modelo de futuros estudios económicos de las pruebas rápidas y point-of-care.
As is the case for all of the activities involved in clinical patient management decisions, the diagnostic processes performed in Clinical Microbiology Departments and laboratories are continually evaluated in terms of their diagnostic efficacy and cost-effectiveness.1 Rapid microbiological diagnostic tests are one of the areas that has been studied the most in this respect, not only because of the benefits of reducing technique duration in the diagnostic process and response time, but also in how they affect laboratory work organisation as well as patient management and clinical impact. These technique evaluations therefore usually include those performed where patients are treated (point-of-care tests). Although these tests were developed to be used outside the laboratory, they are often performed within a laboratory or in what are now termed point-of-care laboratories.2,3 Self-diagnostic tests are usually excluded from this category, as their entry into the market has not been free of controversy. Although these tests are similar to point-of-care procedures, they are designed so that patients can perform them themselves. They may also be used by those interested in the diagnosis of an infectious agent, and they are available in pharmacies or even through web pages, although regulation of the latter is deficient.4,5
Rapid Microbiological diagnosis has been evaluated relatively often, above all in areas of knowledge where new technologies have become available. Its use has given rise to a high level of clinical impact.6,7 Among other techniques, the use of mass spectrometry in the diagnosis of bacteraemia stands out, as do molecular techniques that target specific multiresistant pathogens such as methicillin-resistant Staphylococcus aureus or enterobacteriaceae that produce carbapenemases. There are also diagnostic panels that include different microorganisms associated with a single clinical entity, such as gastroenteritis, pneumonia, neurological infection or sepsis.7–11 The majority of evaluations cover diagnostic performance in terms of sensitivity, specificity, positive and negative predictive power or pre-test probability Some evaluations cover cost analysis or budgetary impact and basically cover economic parameters (test price per unit multiplied by the volume of tests used). Complete economic evaluations of cost-effectiveness, cost-benefit or cost-utility are less common. Other rapid tests which are increasingly used in microbiology are lateral-flow immunochromatographic assays. These too have been analysed more often in terms of their diagnostic values, while the economic impact of their clinical use has been analysed less frequently.3
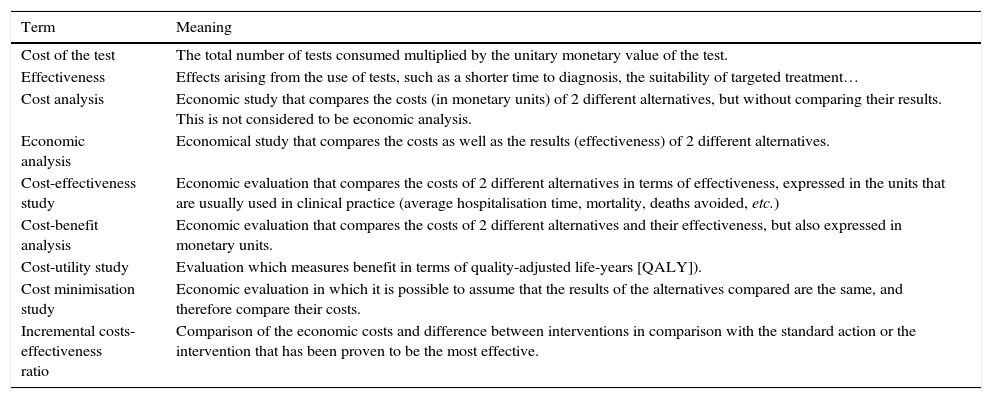
In this work we review the general concepts that are used in evaluating the economic impact of rapid diagnostic techniques in Clinical Microbiology, apart from the exclusive price of the test itself, which goes beyond the overall clinical impact and the benefit for the patient. Self-diagnostic systems are not included, given that this would require different considerations from rapid and point-of-care tests. Table 1 includes a glossary of terms to facilitate the reading of this paper.
Glossary of terms habitually used in economic studies evaluating diagnostic tests.
| Term | Meaning |
|---|---|
| Cost of the test | The total number of tests consumed multiplied by the unitary monetary value of the test. |
| Effectiveness | Effects arising from the use of tests, such as a shorter time to diagnosis, the suitability of targeted treatment… |
| Cost analysis | Economic study that compares the costs (in monetary units) of 2 different alternatives, but without comparing their results. This is not considered to be economic analysis. |
| Economic analysis | Economical study that compares the costs as well as the results (effectiveness) of 2 different alternatives. |
| Cost-effectiveness study | Economic evaluation that compares the costs of 2 different alternatives in terms of effectiveness, expressed in the units that are usually used in clinical practice (average hospitalisation time, mortality, deaths avoided, etc.) |
| Cost-benefit analysis | Economic evaluation that compares the costs of 2 different alternatives and their effectiveness, but also expressed in monetary units. |
| Cost-utility study | Evaluation which measures benefit in terms of quality-adjusted life-years [QALY]). |
| Cost minimisation study | Economic evaluation in which it is possible to assume that the results of the alternatives compared are the same, and therefore compare their costs. |
| Incremental costs-effectiveness ratio | Comparison of the economic costs and difference between interventions in comparison with the standard action or the intervention that has been proven to be the most effective. |
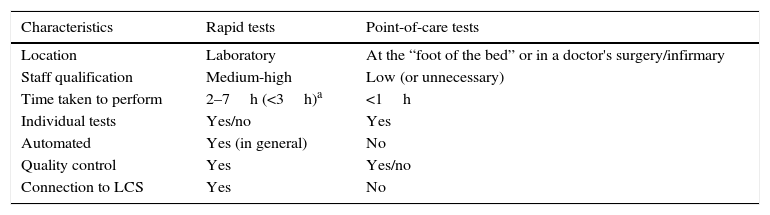
Table 2 shows the characteristics and general differences between rapid tests themselves undertaken in Clinical Microbiology laboratories and point-of-care tests performed where patients receive care, including doctors’ or nurses offices and hospital units.
General characteristics of rapid tests and those which are performed at the point-of-care for microbiological diagnosis.
| Characteristics | Rapid tests | Point-of-care tests |
|---|---|---|
| Location | Laboratory | At the “foot of the bed” or in a doctor's surgery/infirmary |
| Staff qualification | Medium-high | Low (or unnecessary) |
| Time taken to perform | 2–7h (<3h)a | <1h |
| Individual tests | Yes/no | Yes |
| Automated | Yes (in general) | No |
| Quality control | Yes | Yes/no |
| Connection to LCS | Yes | No |
LCS: laboratory computer system.
Rapid diagnostic tests in Microbiology usually take place in the laboratory itself and usually take less time before the result is obtained than the duration of a typical working day lasting 7–8h. Nevertheless, the majority of test results are available in 3–5h. This difference of 2–3h is essential so that the results reach the requester in the same working day and are immediately effective. In the case of discontinuous care a longer time would give rise to the risk of the requester not being efficiently informed of the results so that clinical decisions would be put off until the next day.
Unlike point-of-care tests, samples for rapid diagnosis have to be taken to a laboratory and generally prepared before processing. Specific training may be required for staff, and the technology tends to be more complex than the equipment used in point-of-care techniques. As rapid diagnostic tests are performed in a laboratory, they are included in quality management systems and their results are included in laboratory computer systems. The current tendency is for rapid diagnostic tests to be automated and used for specific clinical entities (such as a community-acquired respiratory infection, gastroenteritis or meningitis) rather than for a diagnostic problem associated with a single pathogen. Nevertheless, some examples cover a single problem, such as the detection of Clostridium difficile, methillicin-resistant Staphylococcus aureus or cabapenemase-producing enterobacteriaceae.12
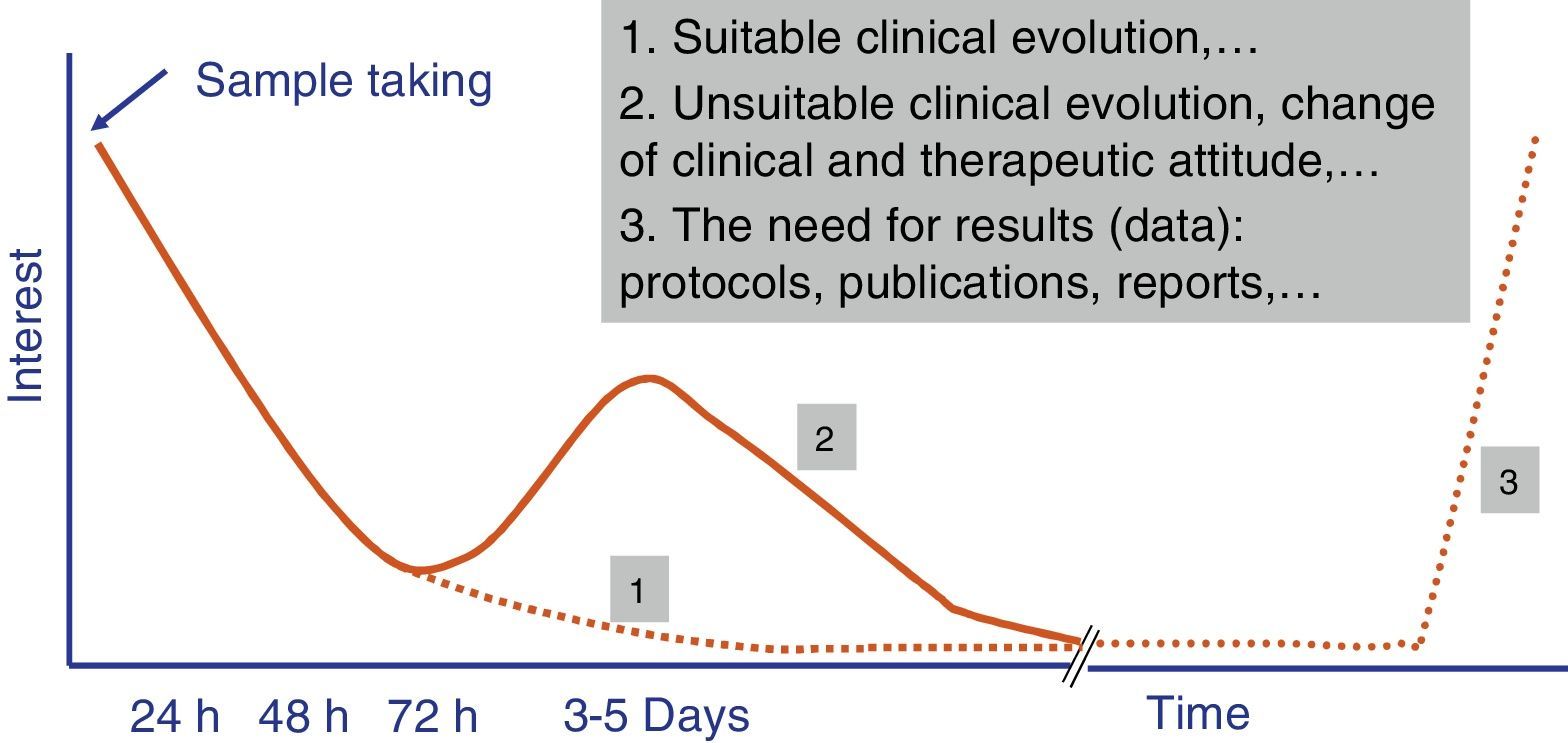
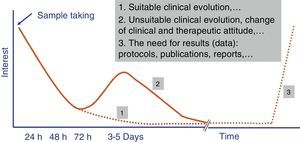
Independently of their economic cost or the accuracy of the diagnostic results of fast tests, how long they take to perform is one of the parameters that leads to their success or failure in Microbiology laboratory diagnostic processes. A time longer than 4–5h does not ensure that rapid tests fit in properly with laboratory workflows, and it also restricts the requesting doctor's interest in the result (Fig. 1). The doctor always has to be as close as possible to the point where the sample is taken to make a therapeutic decision (e.g., to start antimicrobial treatment or to modify one before a second dose), preventive treatment (isolation or not of the patient), clinical decision (admission to a ward or an intensive care unit [ICU]) or even diagnostic decisions (taking additional samples for other microbiological tests).
The evaluation of rapid tests has to take into account not only the specific time taken to perform them, but also the times associated with the pre-analytic process and subsequent processing of the result. Due to this the current tendency is for these tests to be performed in less than 3–5h, and this fulfils the objectives described in the previous paragraph. Likewise, the possibility that these tests can replace conventional ones is another key reason for their success.13 In this case the fact that tests are not duplicated and that one replaces another is highly appreciated. Another point that is appreciated is that more human resources are not needed for the new tests, as this is what usually most increases economic costs.
A clearly successful example was the introduction in Microbiology departments and laboratories of mass spectrometry to identify bacteria and fungi. This is technique is rapid and has been shown to be cost-effective. It has meant that identification tests based on biochemical reactions are now secondary and even token.14,15 On the other hand, even when techniques which take longer than 5–7h have clear clinical benefits, they have not become as widespread as the companies that developed them or the doctors who demand microbiological studies expected. One example of this is the technique that combines genetic sequence amplification with the subsequent detection of the amplified product by mass spectrometry (electrospray ionisation time of flight mass spectrometry).16 In spite of the clear interest in detecting microorganisms directly in blood samples without the need for previous culture, as is the case with MALDI TOF protocols, it has not been implemented in the way that was expected. The high cost of these determinations is also a barrier again their use in normal clinical practice. Moreover, and unlike the MALDI TOF system, it does not cover all possible pathogens as it is only aimed at the most usual ones, so that the conventional process has to be maintained, thereby increasing the cost of diagnosis even further.1
A systematic review was undertaken of recently published literature about different molecular systems, one of them in combination with proteomics, used to diagnose sepsis in direct blood samples. It concluded that although these methods have a high level of sensitivity and specificity, they had not been correctly evaluated in randomised clinical trials to determine their efficacy in terms of clinical parameters (mortality, duration of admission in the ICU, etc.). Due to this, and in spite of avoiding the need to inoculate blood cultures and wait for growth to occur, it cannot be concluded that they are advantageous in terms of efficacy in comparison with the current standard. Nevertheless, and although this is based on estimates that are not evidence-based, they may be favourable in cost-benefit terms.17
Point-of-care techniquesPoint-of-care techniques in Microbiology usually take less than one hour. They have been developed to establish a microbiological diagnosis at the place where a patient is being treated, either during admission or when seen in a doctor's office or nursing department.18 In general sample preparation is either minimal or non-existent and the staff who perform the test (a doctor or nurse) need no very specific training, and the technology used is very simple. They usually answer a single diagnostic question and usually cover a single pathogen. They are not usually automated. A negative aspect is that they are not usually associated with quality control programs and are only subject to internal controls, and their results are not usually recorded in computer systems (electronic clinical histories or laboratory computer systems) (Table 2).19,20
Diagnostic demands must be similar for rapid tests and point-of-care ones, and they should be evaluated clinically and economically in the same way.7,13 Nevertheless, in economic evaluation point-of-care techniques usually have more advantages than rapid techniques. This is because the unit cost of these tests tends to be low, skilled personnel are not required to perform them and their associated apparatus, if any is required, is not complex. This is why they are usually cost-effective in comparison with conventional methods.3 Likewise, the launch onto the market of tests that use the same concept as the initial one are usually at a lower price and have a better cost-effectiveness ratio.7
Point-of care laboratoriesIn recent years a major debate has arisen about where point-of-care tests should take place: in the laboratory or at the place where the patient is being treated.20 Some studies evaluate the advantages and disadvantages of performing such tests in the Microbiology laboratory itself, above all with a view to the outsourcing and centralisation of its services.21,22 The American Society for Microbiology clearly recommends the use of these rapid techniques even outside the geographical area of the laboratory, on condition that tests are selected, introduced and evaluated by the microbiologists themselves.23
To resolve this debate specific laboratory areas known as point-of-care laboratories are now being designed. These are exclusively for rapid and point-of-care tests,24 and they also have the purpose of overcoming the negative effects of the said outsourcing and centralisation, as they make it impossible to perform rapid tests in the centres involved other than simple point-of-care ones. Nevertheless, this concept has been broadened to include consolidated Microbiology departments and laboratories within hospitals as a means of offering a better service and responding to current needs and tendencies in microbiological diagnosis.3 These laboratories work under a 24hours 7 days a week or continuous care concept, in very close association with those who request diagnostic tests. These individuals wait for results before taking decisions and usually share patient clinical data with the doctors in charge of these laboratories to ensure the best choice of diagnostic strategy.1
Due to all of the above considerations, “point-of-care” laboratories perform rapid techniques as well as point-of-care ones in hospitals that usually lack a standard laboratory and which send samples to a central laboratory, while continuing to perform the techniques sufficient for a first decision. They have also been implemented in consolidated hospitals to ensure continuous care.2,3 This fact has led to an increased interest in diagnostics companies in innovating in the field of rapid tests and entering this area of work. In spite of this interest, there are still no studies which offer an overall evaluation of point-of-care laboratories from an economic point of view, apart from their diagnostic efficiency.
The economic cost of rapid and point-of-care testsIn the economic study that is usually performed for all laboratory tests, including rapid ones, one of the first challenges for the evaluator is to discover the real cost of test. This may be per unit or adjusted to diagnostic equipment or kits that also include the necessary controls for their development and interpretation. This data is not usually accessible in the catalogues or web pages of diagnostic companies, and the price will depend on the purchase procedure used by each institution. This may take the form of direct purchase, centralised purchase (which is usually negotiated), or by means of competition with bidding for offers and options for improvement in connection with diagnostic activity (renewal and maintenance of computer equipment, making available external quality controls apart from those of the equipment used, certification and accreditation processes, the preparation of spaces or renting the equipment that is necessary for diagnosis, etc.). On the majority of occasions competitions with options for improvements oblige suppliers to raise the cost.
In other cases the price is increased when suppliers are paid over long periods of time, so that each test is affected by the financial costs necessary for the normal working of the supplier. The lack of a uniform hospital purchasing procedure means that there is no single reference price for each test, as is the case for medicines with prices regulated by the Ministry for Health, Social Services and Equality.25
The cost based on price of purchase is often analysed exclusively, and this may differ from the actual cost once the final number of results issued by the laboratory is taken into account, given that possible repetitions or tests consumed during controls and internal quality exercises are not taken into account, and this is also the case for inter-laboratory exercises in accreditation and certification systems.
Cost analysis only includes the economic cost of a test, i.e., its unit value multiplied by the number of tests performed in the time period set for analysis. However, complete economic evaluation such as cost minimisation analysis (Table 1) allows us to compare 2 different diagnostic options and assume that the results in terms of health (effectiveness) are identical for both, so that the final result refers to the costs which include direct costs associated with the test (technical and medical personnel) as well as indirect costs such as electricity and air conditioning, etc.
The evaluation of rapid tests: cost-effectiveness, cost-benefit and cost-utilityThe economic evaluation of laboratory diagnostic tests in the current context of healthcare cost increases has become a management need, to select the ones with the most efficient results. Cost-effectiveness analysis is the most widespread tool, as its results in health may be expressed in terms of results, deaths avoided, reductions in the duration of hospitalisation or the adjustment of antibiotic treatments. It makes it possible to determine which intervention should be the priority to maximise the benefit produced using the available economic resources.
Other evaluation strategies use cost-benefit analysis (Table 1). Its main characteristic is that it translates health benefits into monetary units, so that the term used for comparison is always similar. This situation also makes it possible to analyse net monetary gain or the cost-benefit ratio.26
Cost-effectiveness analysis shows the numerical relationship between the costs (measured in monetary units) of a specific intervention (e.g., the intrinsic cost of a rapid or point-of-care test) and its consequences (measured in the same units used in clinical practice). The relative value of the intervention is normally expressed as a coefficient in which the denominator is a gain in a health or management indicator and the numerator is the cost associated with the increase in health. This coefficient is denominated the average cost-effectiveness. In the case of rapid diagnostic or point-of-care tests the denominator may be evaluated from 2 different viewpoints. The first would be a laboratory-based approach in which the reduction in the time to obtaining results, increased working capacity or the availability of time for the technician could be indicators. The second would be based more on the positive consequences of the intervention for the patient as a result of the rapid laboratory or point-of-care test. This would include, for example, reduced hospitalisation time or time spent without effective treatment, or the time taken before starting a suitable treatment, switching from an empirical to a targeted treatment and a reduction in mortality, etc.
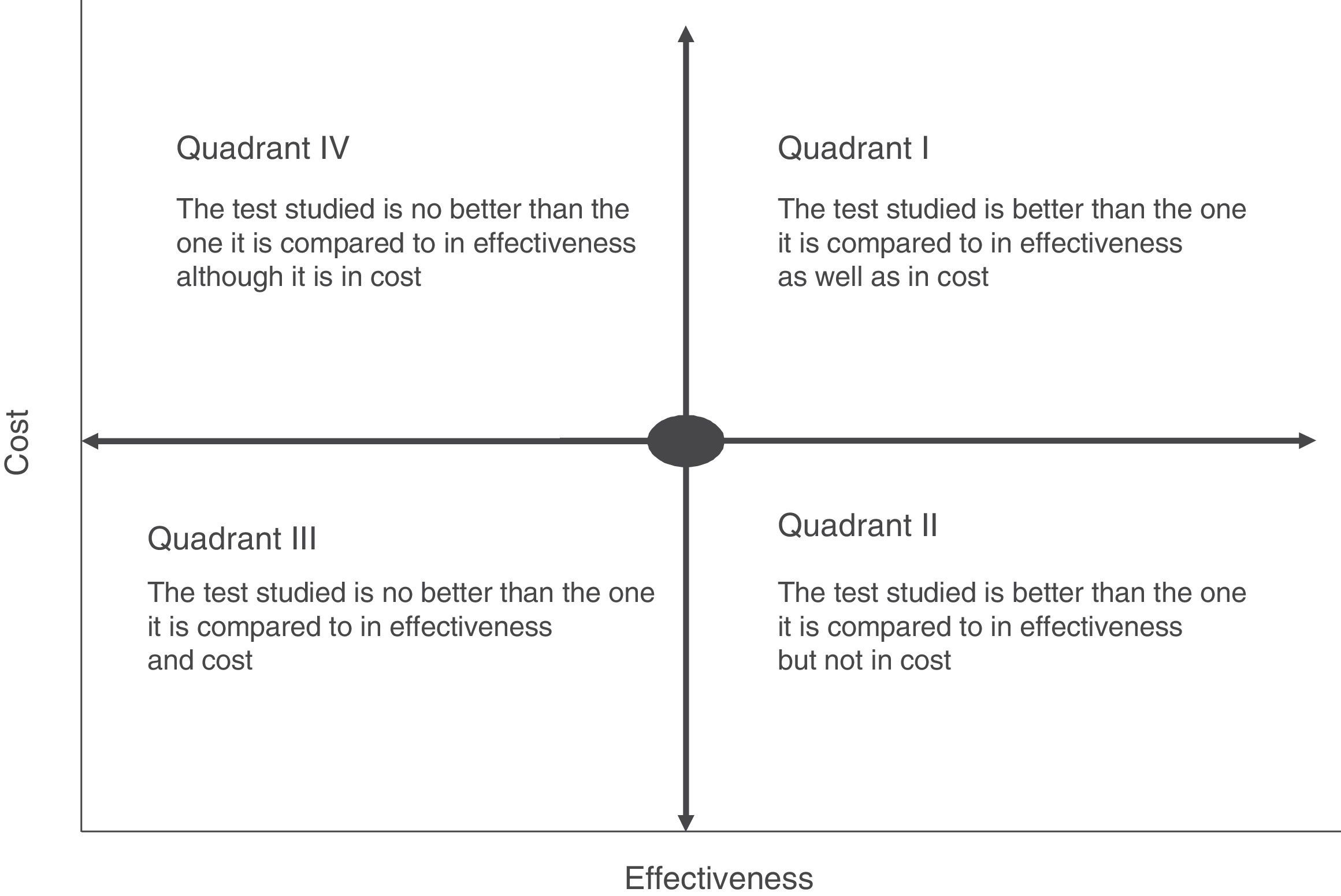
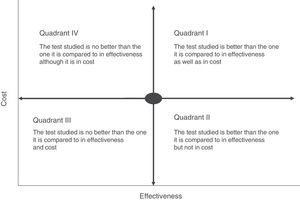
Cost-effectiveness analysis is usually shown in the form of a matrix known as the cost-effectiveness plane. This simultaneously shows effectiveness and the economic cost of the tests (Fig. 2). For example, if a rapid or point-of-care test is measured in terms of costs and outcome–such as the average hospitalisation of patients–we may find different scenarios in which the most advantageous situation would correspond to the most effective and least costly interventions (low cost test with a high impact on hospitalisation duration) (quadrant II, Fig. 2). On the contrary, the least favourable would be a situation in which the rapid tests have a high cost and little affect on stay (quadrant IV, Fig. 2). In cost-effectiveness tests the outcome is usually shown in quadrants corresponding to the highest cost and high efficacy (quadrant I, Fig. 2). In this case the choice of a test will depend on the degree to which increased costs are accepted and the calculation of its effectiveness within the overall context of patient management. The majority of occasions this effectiveness should not be calculated by evaluating a single indicator, as this may lead to rejection of a test because of its high cost and its a priori lack of effectiveness. An example of this would be interventions that reduce the duration of hospital stay which would have a major affect on the quality perceived by the patient or the possible reduction in the adverse effects associated with hospitalisation. Thus the effect of the introduction of mass spectrometry in the identification of microorganisms in grown haemoculture flasks has clearly been proven to be effective in terms of patient stay time. However, it requires a large initial investment due to the cost of the MALDI TOF system, and this has prevented it from being introduced in some institutions.15 However, if we add that shorter hospitalisation also reduces the possibility of acquiring a nosocomial infection the intervention would be shown to be more effective, above all in countries with a high incidence of infection by multi-resistant bacteria. The challenge in this case would be to be able to calculate how many infections could be prevented by reducing hospitalisation time, given that the unit cost of infections of this type and their impact on the healthcare system have been measured clearly in several studies.27–29
To make it possible to compare several interventions, they are usually ranked according to what is known as their incremental cost-effectiveness value. I.e., the calculated value is referred to and compared with the interventions that are measured in the same way, using the denominated action standard or simply with the action (in this case, a rapid or point-of-care test) that has to date been shown to be the most effective. Also, and following the current movement which asks “what not to do?”, an intervention may sometimes be compared with non-intervention (http://www.choosingwisely.org/). Incremental cost-effectiveness therefore refers to the additional cost per unit of benefit, which is also additional. If we take 2 interventions (A and B), this value would be calculated using the following formula:
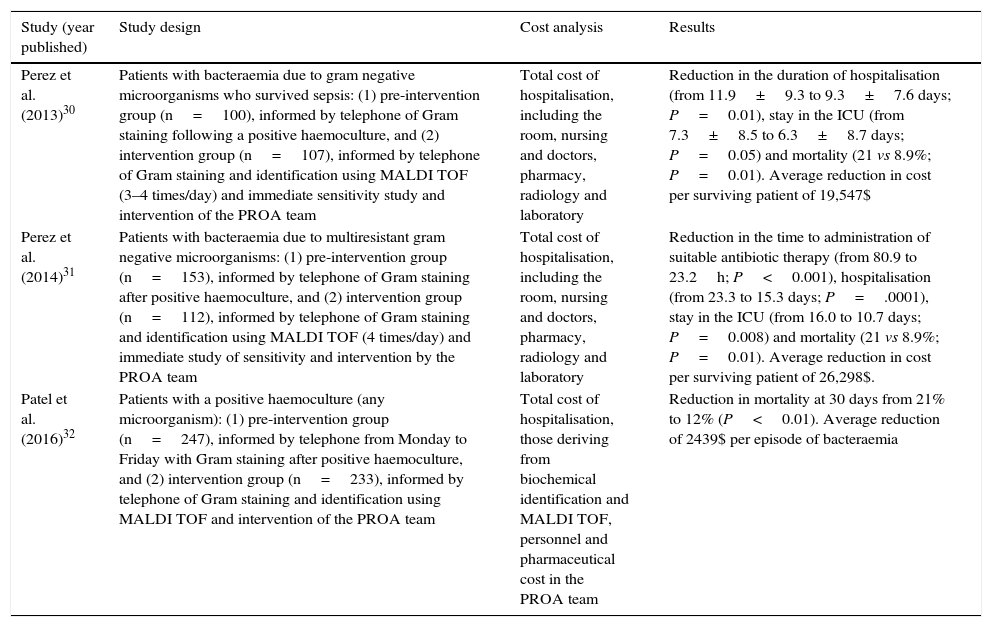
ICER: Incremental cost-effectiveness ratio.Different studies have recently been published that analyse the economic impact arising from the use of MALDI TOF as a rapid technique for the identification of microorganisms using grown haemoculture flasks.30–32 It is interesting because the analysis leads to improved use of the antimicrobial agents associated with the denominated “Programs for the optimisation of the use of antimicrobial agents” (PROA) due to faster supply of data when the MALDI TOF system is used in comparison with conventional methods based on traditional biochemical tests. Mortality and the duration of hospitalisation are measured as outcomes.33,34Table 3 shows a summary of some of these studies, their design, the observed outcomes and economic quantification. Although each study uses a different method to calculate costs, above all the indirect costs, all of them conclude that there is an economic advantage in the intervention following the rapid supply of data using the MALDI TOF system.30–32
Clinical and economical impact analysis in connection with the use of rapid mass spectrometry identification techniques (MALDI TOF) combined with actions to improve the use of antimicrobial agents in the diagnosis of bacteraemia.
| Study (year published) | Study design | Cost analysis | Results |
|---|---|---|---|
| Perez et al. (2013)30 | Patients with bacteraemia due to gram negative microorganisms who survived sepsis: (1) pre-intervention group (n=100), informed by telephone of Gram staining following a positive haemoculture, and (2) intervention group (n=107), informed by telephone of Gram staining and identification using MALDI TOF (3–4 times/day) and immediate sensitivity study and intervention of the PROA team | Total cost of hospitalisation, including the room, nursing and doctors, pharmacy, radiology and laboratory | Reduction in the duration of hospitalisation (from 11.9±9.3 to 9.3±7.6 days; P=0.01), stay in the ICU (from 7.3±8.5 to 6.3±8.7 days; P=0.05) and mortality (21 vs 8.9%; P=0.01). Average reduction in cost per surviving patient of 19,547$ |
| Perez et al. (2014)31 | Patients with bacteraemia due to multiresistant gram negative microorganisms: (1) pre-intervention group (n=153), informed by telephone of Gram staining after positive haemoculture, and (2) intervention group (n=112), informed by telephone of Gram staining and identification using MALDI TOF (4 times/day) and immediate study of sensitivity and intervention by the PROA team | Total cost of hospitalisation, including the room, nursing and doctors, pharmacy, radiology and laboratory | Reduction in the time to administration of suitable antibiotic therapy (from 80.9 to 23.2h; P<0.001), hospitalisation (from 23.3 to 15.3 days; P=.0001), stay in the ICU (from 16.0 to 10.7 days; P=0.008) and mortality (21 vs 8.9%; P=0.01). Average reduction in cost per surviving patient of 26,298$. |
| Patel et al. (2016)32 | Patients with a positive haemoculture (any microorganism): (1) pre-intervention group (n=247), informed by telephone from Monday to Friday with Gram staining after positive haemoculture, and (2) intervention group (n=233), informed by telephone of Gram staining and identification using MALDI TOF and intervention of the PROA team | Total cost of hospitalisation, those deriving from biochemical identification and MALDI TOF, personnel and pharmaceutical cost in the PROA team | Reduction in mortality at 30 days from 21% to 12% (P<0.01). Average reduction of 2439$ per episode of bacteraemia |
PROA: program of optimisation in the use of antimicrobial agents; ICU: Intensive Care Unit.
In general and in connection with rapid and point-of-care tests, economic analysis has rarely been performed in terms of cost-utility. This evaluation usually measures quality-adjusted life-years (QALY), or years of healthy life gained, in which it is also necessary to establish an economic standard to evaluate the usefulness of the intervention.35 In the majority of countries no threshold has been defined above which healthcare technology is considered to be acceptable in terms of cost-utility. A value of 30,000€/QALY (or 20,000£/QALY) is usually accepted by consensus, this having been defined by the National Institute for Health and Clinical Excellence (NICE) in Great Britain.36 In Spain, Royal Decree-Law 9/2011 established the creation of two committees on the cost-effectiveness of healthcare products and medicines. These eventually became the Spanish Healthcare Technology and National Health Care Evaluation Agencies Network, supported by the Ministry of Health and the regional government agencies created for this purpose.37,38 Its main purpose is to issue technical reports on the evaluation of healthcare technologies by means of systematic review of the scientific literature to examine their efficacy, effectiveness and safety. Likewise, contextualisation is used to evaluate their impact on the healthcare system, taking legal, ethical, economic, social and organisational aspects into account (http://www.redets.msssi.gob.es/home.htm). In the majority of cases these reports have led to the creation of clinical practice guides. It should be underlined that since their creation no action evaluations have been performed in connection with infectious diseases and Clinical Microbiology, so that rapid and point-of-care techniques associated with this area have not been evaluated.
The NICE recently simultaneously published a systematic review and a meta-analysis to estimate costs and analyse the cost-utility (measured in QALY) of direct diagnosis (in blood) of bacteraemia and fungemia using 3 commercial systems (2 of which are based on real-time molecular PCR amplification techniques, while the third is also a PCR molecular technique coupled with subsequent mass spectrometry of the amplified product). They were compared to conventional diagnosis which included identification by biochemical tests as well as the MALDI TOF system using grown haemoculture flasks.17 Using 2 different analytical models none of the systems demonstrated favourable clinical utility or cost-effectiveness based on the published evidence. Nevertheless, of all the systems the one that combines molecular amplification with mass spectrometry displayed the greatest net incremental benefit in terms of quality-adjusted life years, at a cost below 20,000£/QALY).
From the evaluation of diagnostic values to clinical and economic evaluation: towards a new scenario?On 7 March and 5 April 2017 the European Council and Parliament passed the new European regulation 2017/74639 that governs the norms under which healthcare products for human in vitro diagnosis and their accessories may be commercialised in the European Union. This regulation, which revises and supplants previous norms, was published in the Official Bulletin of the European Union and came into force on 26 May 2017 throughout the territory, without the need for transposition into national legislation by the member states. This prevented the disparity of criteria involved in transposing it into each member state of the Union and brings about similar application in all of them. Nevertheless, a 5-year transitory period was left for its gradual implementation, until it comes fully into force on 26 May 2022. This regulation covers aspects prior to commercialisation and application of the CE mark as well as post-commercialisation. It affects all in vitro diagnostic products including those for rapid diagnosis, point-of-care and self-diagnosis, stipulating that the process will be much more technical to ensure exact and reliable results that lead to a clinical benefit arising from its use (screening, monitoring or diagnosis).
According to Regulation 2017/746, for healthcare products to be commercialised in the European Union it is necessary to document, using different studies or trials: (a) their scientific validity, (b) their analytic working and (c) their clinical working. The first of these, scientific validity, demands that the basis of the technique be established through a proven relationship between the parameter (or analyte studied) and the physiological or clinical state which is studied. Analytic working covers the capacity of the product to correctly detect or measure the specific parameter (or analyte) that is quantified, expressing this in terms of analytical sensitivity and specificity, veracity (bias), precision (repeatability and reproducibility), exactitude (the result of veracity and precision), detection and quantification limits, range of measurement, linearity, cut-off point, including the determination of appropriate criteria for the collection and handling of samples and the control of relevant known endogenic and exogenic interferences and crossed reactions. Lastly, clinical working measures the capacity of a product to produce results that are correlated with a clinical situation or a specific physiological or pathological state or process, depending on the population in question and foreseen users. Clinical working must be measured in terms of diagnostic sensitivity and specificity, positive and negative predictive value, the verisimilitude coefficient and foreseen values in healthy and sick populations.
This regulation stipulates the need to perform studies (trials) with a defined plan that includes justification, objectives, design, methodology, supervision, statistical considerations, organisation and development, with the aim of proving or confirming scientific validity, analytic working, and, when applicable, the clinical working of the in vitro diagnostic product. These trials must take place under the responsibility of a promoter, who may be the manufacturer or another physical person. They must also be authorised by the respective clinical committees (or ethics committees) and obey the norms of good clinical practice regarding data confidentiality. Studies that use remnant or surplus samples will be excepted from the need for authorisation by clinical committees. The manufacturer must also ensure that products are updated as knowledge and medical practice progress. The regulation also covers the information which must be offered together with a commercialised product.
In spite of the detailed regulatory process that is defined by Regulation 2017/746 for in vitro diagnostic healthcare products, it does not stipulate the need to carry out economic or cost-effectiveness studies deriving from their use, so that it will have to be the European Union member states that set the criteria for these. In Spain the Ministry of Health will have to take on this responsibility, through the Spanish Healthcare Technology and National Health Care Evaluation Agencies Network (http://www.redets.msssi.gob.es/home.htm) or the Spanish Medicine and Healthcare Products Agency (AEMPS) (https://www.aemps.gob.es/productosSanitarios/inVitro/home.htm). Nevertheless, regulating clinical working trials will aid obtaining data that can be used in economic evaluation studies.
ConclusionsRapid diagnostic tests carried out in a microbiology laboratory or at the point of patient care, as well as those in the so-called point-of-care laboratories, have undergone extensive development in recent years. However, their introduction has not always achieved the expected scope, given that often only the price of the test is evaluated and, if applicable, that of the diagnostic equipment needed for their development, rather than their overall clinical impact. The most usual studies in economic evaluations that also take clinical impact into account include cost-effectiveness analysis, cost-benefit and cost-utility. The new European regulation governing the authorisation of commercialisation of healthcare products for in vitro diagnosis, including rapid tests and those for human use at the point of patient care may help to obtain data which facilitate their economic evaluation. Recently published studies of mass spectrometry in the diagnosis of bacteraemia and their clinical and economic impact may serve as examples for such studies.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Cantón R, Gómez G. de la Pedrosa E. Impacto económico de los métodos de diagnóstico rápido en Microbiología Clínica: precio de la prueba o impacto clínico global. Enferm Infecc Microbiol Clin. 2017;35:659–666.