Plasma HIV p24 is considered a significant predictor of CD4+ T cell decline and progression to AIDS in HIV-infected patients. We evaluated the p24 levels in patients on triple therapy and after switching to ritonavir-boosted protease inhibitor monotherapy (mtPI/rtv), as well as the relationships with virological and immunological evolution.
Materials and methodsPlasma samples from patients participating in two studies of simplification to mtPI/rtv were analysed for presence of p24, using a boosted enzyme-linked immunosorbent assay specific for mature p24. Only patients with available samples at baseline (on triple therapy) and during a follow-up of at least 12 months after switching to mtPI/rtv were included.
ResultsA total of 233 samples from 51 patients were analysed. After switching to mtPI/rtv and a median follow-up of 24 months, 14 patients maintained continuous undetectable viraemia, and 37 patients experienced a total of 49 transient viraemic episodes. Unexpectedly, the evolutionary p24 patterns were uniform for most patients, both before and after switching to mtPI/rtv, independently of the virological behaviour, fitting into one of three categories: persistent undetectable p24 levels, positive p24, matching only with the viraemic episodes, and persistent detectable p24 levels. The last group showed lower CD4+ T cell counts and percentages, as well as lower CD4+/CD8+ T cell ratios after 12 and 24 months of follow up.
ConclusionTreatment simplification to mtPI/rtv does not influence the behaviour of p24 in plasma. Patients with continuous positive p24, despite undetectable viraemia, showed worse immunological evolution.
El antígeno p24 se considera un buen predictor de caída de los recuentos de linfocitos T CD4+ y de progresión a sida en pacientes infectados por el VIH. En este estudio hemos evaluado la presencia de p24 en plasma y su relación con la evolución virológica e inmunológica durante la monoterapia con inhibidores de la proteasa potenciados (mtPI/rtv).
Material y métodosSe analizaron muestras de pacientes que participaron en 2 estudios de simplificación con mtPI/rtv. Las concentraciones de p24 se midieron mediante un ELISA potenciado específico para p24 madura. Solo se incluyeron los pacientes con muestras disponibles basalmente (en triple terapia) y durante≥12 meses de seguimiento con mtPI/rtv.
ResultadosSe analizaron un total de 233 muestras de 51 pacientes. Tras la simplificación y una mediana de seguimiento de 24 meses, 14 pacientes mantuvieron una viremia indetectable de forma continuada, mientras que se observaron 49 episodios de viremia transitoria en los 37 restantes. Las determinaciones de p24 fueron estables en la mayoría de los pacientes, tanto antes como después del cambio a mtIP/rtv, independientemente del comportamiento virológico, incluyéndose en una de las siguientes categorías: p24 indetectable persistentemente, p24 positiva coincidiendo solo con los episodios de viremia transitoria, y p24 detectable en todas las determinaciones. Este último grupo mostró recuentos y porcentajes de linfocitos T CD4+ más bajos, así como cocientes CD4+/CD8+ inferiores tras 12 y 24 meses de seguimiento.
ConclusiónLa simplificación a mtPI/r no modifica el comportamiento de la p24 en plasma. Los pacientes con detección persistente de p24 en plasma a pesar de una viremia indetectable muestran una peor evolución inmunológica.
Several clinical trials have shown that monotherapy based on specific ritonavir-boosted protease inhibitors (mtPI/rtv) may be effective alternatives for the simplification of antiretroviral therapy (ART) in patients with long-standing virological suppression.1,2 On the other hand, the dynamics of HIV-1 RNA under protease inhibitor-based triple therapy (TT) and mtPI/rtv are well known, but there are no data about the behaviour of viral proteins, which play a significant role in the pathogenesis of HIV infection, during the simplification to mtPI/rtv. Among HIV proteins, p24 can be measured easily in plasma. Beside used as an inexpensive diagnostic assay and alternative to HIV-RNA testing for monitoring treatment, several studies have shown that the presence of p24 in plasma are related to CD4+ T cell decline, progression to acquired immune deficiency syndrome and survival in patients with no ART or on mono- or dual-nucleoside reverse-transcriptase inhibitor therapy.3–12 However, its significance in the scenario of combined antiretroviral therapy is unclear and unknown during the monotherapy. In this study, we have evaluated the dynamics of p24 levels throughout mtPI/rtv, particularly during transient viraemic episodes (tVEs), and the relationships with virological and immunological evolution.
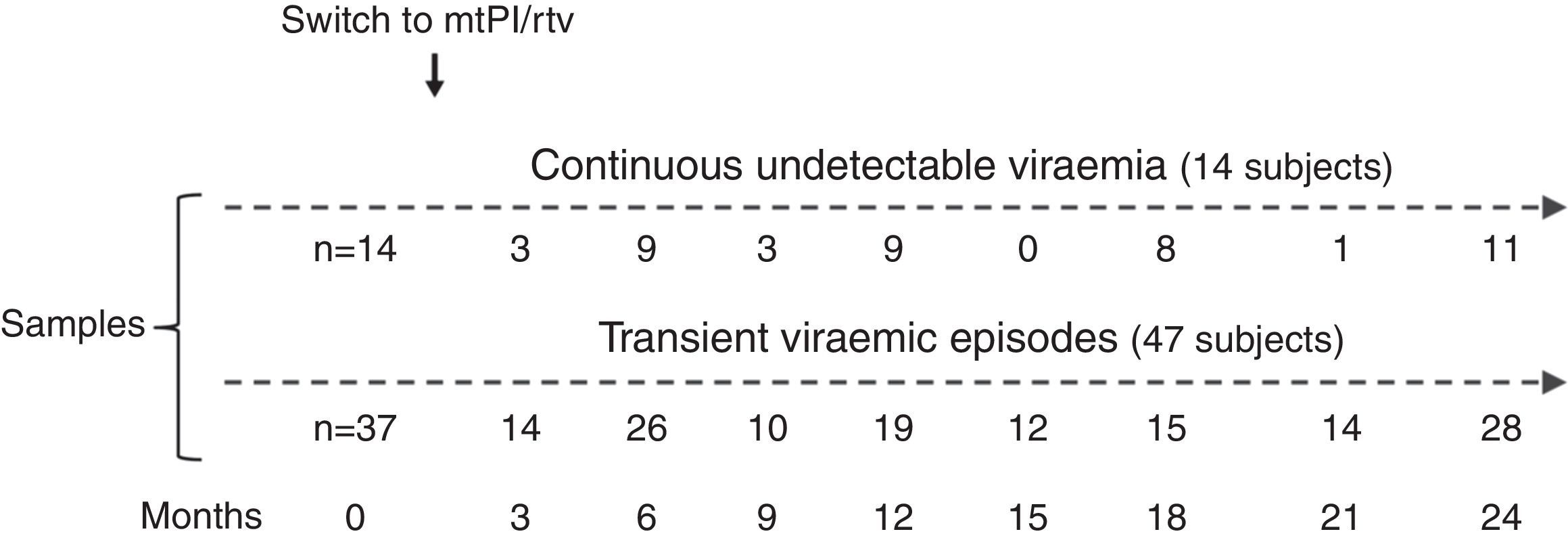
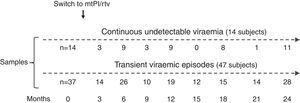
Material and methodsThe patients analysed here participated in two studies of simplification to mtPI/rtv after at least 6 months with an undetectable viral load on TT and a lack of major resistance mutations to the PI used [lopinavir/ritonavir (LPV/rtv) 400/100mg twice a day or darunavir/ritonavir (DRV/rtv) 800/100mg once daily], with a total of 277 patients enrolled (LPV/rtv, 127; DRV/rtv, 150). Both studies were approved by the Spanish Agency for Medicines and Healthcare Products and the Coordinating Committee on Ethics in Biomedical Research of Andalucía, and all patients provided informed consent. The details and clinical results of the studies are published elsewhe.13–15 The patients’ assessments and blood sampling were performed at baseline (the day before switching to mtPI/rtv) and for up to 2 years. Due to the exhaustion of samples in the course of other studies, in this sub-study only a subset of 51 subjects who had enough stored plasma samples at baseline (on TT) and during a follow-up of at least 12 months for further analysis were included. There were no differences in the patients’ characteristics at baseline between the 277 enrolled patients in both studies of mtPI/r and the 51 patients selected for this sub-study. Patients were classified according to their virological behaviour during the follow-up as continuous undetectable viraemia (cUV), and B) tVEs, defined as events of HIV-RNA >20copies/mL preceded and followed by undetectable viral loads, irrespective of the viral load value.
Laboratory proceduresHIV-RNA levels were measured in fresh plasma samples via polymerase chain reaction (COBAS AmpliPrep/COBAS TaqMan HIV-1 Test, v 2.0, Roche Diagnostic System, Branchburg, NJ, USA; limit of quantification, 20copies/mL). For p24 determination, the plasma samples were immediately frozen at −80°C after being processed and stored until tested. After the disruption of immune complexes following the method of Schüpbach et al.,16 we measured p24 levels using a signal amplification-boosted enzyme-linked immunosorbent assay (ELISA) specific for mature p24 with no cross-reactivity to other HIV proteins, specifically the polyprotein precursor p55 (HIV-1 p24 ELISA kit with the ELAST Amplification System, PerkinElmer Life and Analytical Sciences, Waltham, MA, USA). We generated a standard curve with seven serial dilutions ranging from 0.01 to 100pg/mL of p24 diluted in plasma from healthy volunteers, with a linear regression coefficient of r2=0.995 and a sensitivity limit of 0.5pg/mL. The intra- and inter-assay variabilities were below 20% (y=319.76x−24.67). For each run, all standard dilutions and patient samples were run in duplicate.
Statistical analysisThe results are expressed as medians (M) and ranges. We used the Mann–Whitney U and Kruskal–Wallis H tests to compare different groups and the Wilcoxon signed-rank test for paired samples. The relationship between quantitative variables was assessed using Spearman's rank correlation coefficients (ρ test). The differences were considered to be statistically significant for p-values less than 0.05. The statistical analyses were performed using SPSS software (v.19.0, Chicago, IL, USA).
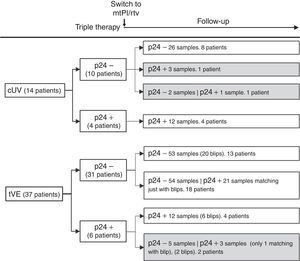
ResultsA total of 233 samples from 51 patients who switched to mtPI/rtv ((LPV/rtv, 14; DRV/rtv, 37 patients) were analysed, with a median of 4 samples per subject (range, 2–6); the number of samples and the time-points when they were taken are shown in Fig. 1. At baseline, all patients were on TT (53 and 8 patients on PI- and non-nucleoside reverse-transcriptase inhibitor-based regimens, respectively) and had an undetectable viral load for a median of 46 months (range, 6–180). The median time of follow-up was 24 months (range, 12–24).
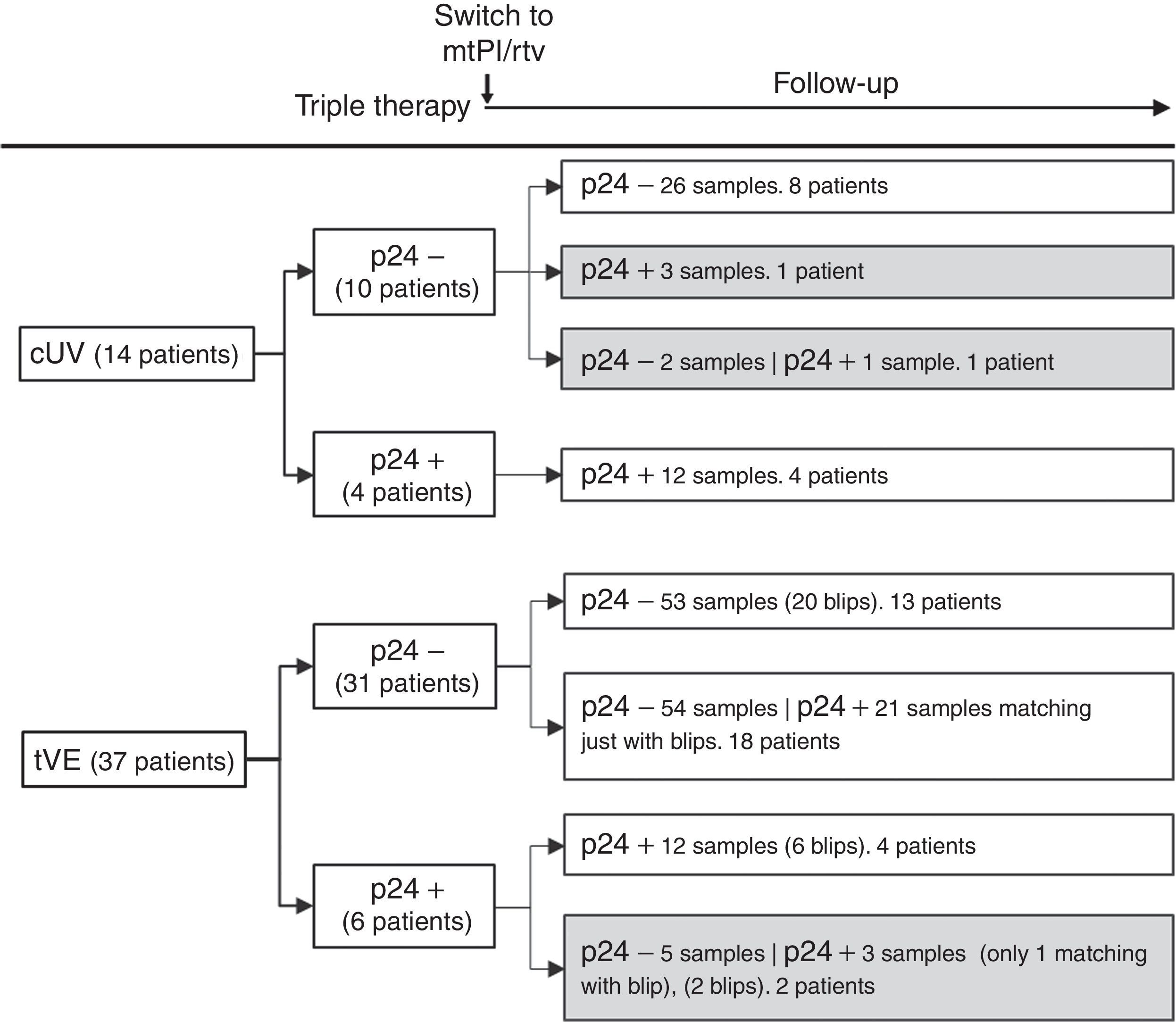
Fourteen patients maintained cUV during the follow-up. Among them, 8 patients showed negative p24 results both at baseline and throughout all the follow-up (n=38 samples). By contrast, 4 patients showed positive p24 results both at baseline [11.7pg/mL (range, 1.23–24.57)] and in all the samples after switching to mtPI/rtv [n=20; 6.42pg/mL (5.06–39.92); p=0.001]. Additionally, 1 patient showed a negative p24 result at baseline (on TT) and 3 positive p24 results (0.91–5.38pg/mL) after switching to mtPI/rtv. The remaining patient showed only 1 out of 4 positive p24 results (1.23pg/mL) while on mtPI/rtv (Fig. 2).
The remaining 37 patients experienced a total of 49 tVEs (27 patients, 1 episode; 8 patients, 2 episodes; 2 patients, 3 episodes), with a median HIV-RNA level of 155copies/mL (IQR, 82–249; range, 28–1990copies/mL). However, only 28 out of these 49 tVEs (57.1%) matched with detectable p24 levels [6.18pg/mL (range, 0.59–25.85)] despite similar viral loads than in those tVEs with negative p24 [143copies/mL (range, 28–1430) vs. 166copies/mL (range, 28–1990); p=0.407]. There was no correlation between the p24 and HIV-RNA values in positive samples for both measurements.
Evolutive p24With the exception of 4 patients, the evolutive p24 patterns were uniform for all other patients both before and after switching to mtPI/rtv independently of the virological behaviour. Thus, 47 out of 51 patients fit into one of the following three categories: (1) 21 patients (100 samples) maintained undetectable p24 levels, both at baseline and after switching to mtPI/rtv, even though 13 patients presented 20 tVEs [135copies/mL (range, 28–1490)]; (2) 18 patients (83 samples) showed a positive p24 result [3.14pg/mL (range, 0.59–11.46)] matching just only with the viraemic episodes [n=21, 225copies/mL (range, 40–1990copies/mL)]; and (3) 8 patients (32 samples) maintained continuous detectable p24 levels, both at baseline and after switching to mtPI/rtv, although only 6 tVEs were observed in 4 patients [(119copies/mL (range, 82–145)]. In the last group, p24 levels increased during mtPI/rtv [baseline, 4.10pg/mL (range, 0.91–24.57) versus last determination [12.73pg/mL (5.70–39.92); p=0.012].
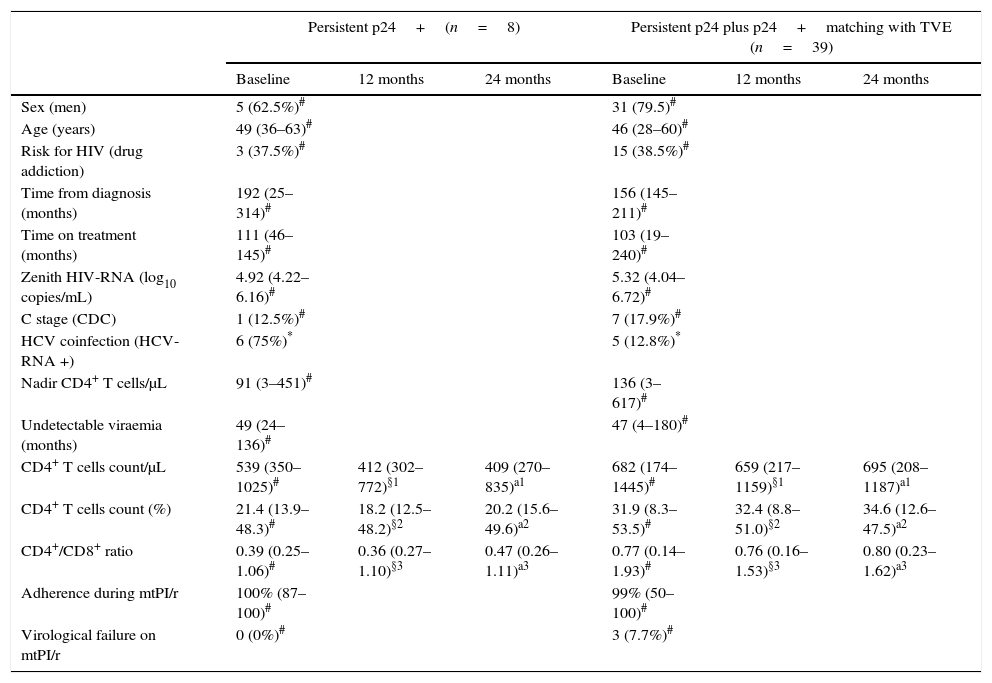
As there were no differences in the characteristics at baseline or during the follow-up between patients with persistent negative p24 levels and those who only showed positive p24 levels matching with the tVEs, we grouped these subjects together and compared them (group A) to patients who always showed positive p24 levels (group B). A higher frequency of active chronic hepatitis C was the only difference between both groups at the time of switching to mtPI/r (Table 1). In group B there were no linear correlations between the absolute values of p24 and CD4+ T cell counts, percentages or CD4+/CD8+ ratios. However, after 1 and 2 years of follow-up both CD4+ T cell counts and percentages of CD4+ T cells were significantly lower in group B (Table 1).
Results are expressed as number (percentage) or median (range).
| Persistent p24+(n=8) | Persistent p24 plus p24+matching with TVE (n=39) | |||||
|---|---|---|---|---|---|---|
| Baseline | 12 months | 24 months | Baseline | 12 months | 24 months | |
| Sex (men) | 5 (62.5%)# | 31 (79.5)# | ||||
| Age (years) | 49 (36–63)# | 46 (28–60)# | ||||
| Risk for HIV (drug addiction) | 3 (37.5%)# | 15 (38.5%)# | ||||
| Time from diagnosis (months) | 192 (25–314)# | 156 (145–211)# | ||||
| Time on treatment (months) | 111 (46–145)# | 103 (19–240)# | ||||
| Zenith HIV-RNA (log10 copies/mL) | 4.92 (4.22–6.16)# | 5.32 (4.04–6.72)# | ||||
| C stage (CDC) | 1 (12.5%)# | 7 (17.9%)# | ||||
| HCV coinfection (HCV-RNA +) | 6 (75%)* | 5 (12.8%)* | ||||
| Nadir CD4+ T cells/μL | 91 (3–451)# | 136 (3–617)# | ||||
| Undetectable viraemia (months) | 49 (24–136)# | 47 (4–180)# | ||||
| CD4+ T cells count/μL | 539 (350–1025)# | 412 (302–772)§1 | 409 (270–835)a1 | 682 (174–1445)# | 659 (217–1159)§1 | 695 (208–1187)a1 |
| CD4+ T cells count (%) | 21.4 (13.9–48.3)# | 18.2 (12.5–48.2)§2 | 20.2 (15.6–49.6)a2 | 31.9 (8.3–53.5)# | 32.4 (8.8–51.0)§2 | 34.6 (12.6–47.5)a2 |
| CD4+/CD8+ ratio | 0.39 (0.25–1.06)# | 0.36 (0.27–1.10)§3 | 0.47 (0.26–1.11)a3 | 0.77 (0.14–1.93)# | 0.76 (0.16–1.53)§3 | 0.80 (0.23–1.62)a3 |
| Adherence during mtPI/r | 100% (87–100)# | 99% (50–100)# | ||||
| Virological failure on mtPI/r | 0 (0%)# | 3 (7.7%)# | ||||
CD4+ T cell counts, percentage of CD4+ T cell and CD4+/CD8+ T cells ratio both at baseline (on triple therapy) and at 1 and 2 years after switching to ritonavir-boosted protease inhibitor monotherapy as function of plasma p24 behaviour.
This is the first study to examine the dynamics of HIV-1 p24 levels during mtPI/rtv. Using a highly improved p24 ELISA, we have observed that ART simplification to mtPI/rtv does not influence the behaviour of p24. Additionally, two unexpected findings for which we do not have a clear explanation were observed. First, whereas it makes sense that TVEs were associated with detectable p24 in plasma matching just with the viraemic episodes, almost the other half of these episodes coursed with undetectable p24 levels, with no apparent differences between them.
Second, a clustering of p24 patterns in most patients. While one group of patients maintained undetectable p24 levels even during TVEs, at the other extreme, there were subjects with positive p24 levels in all samples despite persistent undetectable viral loads. It is improbable that the detected p24 would derive from residual immune complexes given the long-term viral suppression observed in these patients, although we cannot ensure the absence of intermittent viraemia provided that HIV-RNA determinations were performed quarterly. This detection is not likely attributed to the residual viraemia frequently detected by more sensitive tests in patients with plasma HIV-RNA <20copies/mL, as the median p24 levels in these samples (6.5pg/mL) exceeded the maximum level of particle-associated p24 present in the plasma of subjects with HIV-RNA <50copies/mL by 3 orders of magnitude.19,20
About 15% of the samples with undetectable viraemia on both TT and mtPI/rtv showed a positive plasma p24 result. This has been also reported in 20–40% of patients on ART with HIV-RNA <50copies/mL for extended periods.9,10 The source of the p24 antigen detectable in these patients is unclear. One plausible explanation for this observation may be the persistence of on-going tissular HIV-RNA transcription not reflected by the HIV-RNA levels in plasma, as has earlier been demonstrated by the measurement of cell-associated HIV-RNA.17–19
Positive plasma p24 has been inversely correlated with changes in CD4+ T cells in patients with stable suppressed viral loads under combination ART,16,20,21 similar to our finding. On the other hand, chronic HCV infection has been associated with higher levels of immune activation in both CD8+ and CD4+ T cells in HCV/HIV-coinfected patients compared to HIV-monoinfected patients.22,23 Furthermore, higher T cell activation levels have been associated with decreased CD4+ T cell gains during therapy.24,25 In our patients, the only different basal feature was a higher incidence of HCV coinfection in those patients with persistent positive p24. It could be hypothesised that the presence of p24 in plasma only is a surrogate marker associated with a higher immune activation and on-going tissular HIV-RNA transcription. This and the role of plasma p24 as a marker of worse immunological evolution despite successful ART must be clarified in future studies.
The lack of a control group on triple therapy with the same follow-up can be considered the main limitation of this study, although the patients served as their own controls, before and after switching to monotherapy. Likewise, it would have been desirable to have samples from a larger number of patients and in all different time-points throughout the follow-up.
In conclusion, ART simplification to mtPI/rtv does not influence the behaviour of p24. Some patients with undetectable viraemia showed a positive plasma p24 continuously, both on TT and after switching to mtPIr, which may be a surrogate marker of worse immunological evolution.
Financial supportThis work was supported a grant from Consejería de Salud y Bienestar Social, Junta de Andalucía, Spain (exp. P0077-2012).
Conflicts of interestL.F. Lopez-Cortes and P. Viciana have received unrestricted research funding, consultancy fees, and lecture fees from and have served on the advisory boards of Abbott, Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme, and ViiV Healthcare. The other authors have no conflicts of interest to disclose.
The authors are indebted to the patients for their involvement in this study and to M. Rodríguez, I. Hidalgo and R. Martin for their help with specimen processing.