Cumulative susceptibility reports are a valuable tool for the empirical treatment of urinary tract infections, especially in the current context of increasing resistance rates. Our objective was to analyze the antimicrobial susceptibility of bacterial isolates in urine cultures of pediatric patients during a five-year period.
MethodsRetrospective study of urine cultures from 2011 to 2015. Identification and antimicrobial susceptibility tests were performed using the Vitek-2 system (BioMérieux®) and categorized according to EUCAST criteria. Antimicrobial susceptibility data were analyzed by gender and age groups (neonates, 1 month to 5 years, 5–15 years) before being compared with data obtained from patients over the age of 15 years.
ResultsDuring the study period, 17164 urine cultures were processed from 7924 patients under 16 years of age. Antimicrobial susceptibility rates in these patients were: ampicillin 36.3%, amoxicillin/clavulanic acid 75.3%, cefuroxime 83.2%, co-trimoxazole 68.9%, ciprofloxacin 85.3%, fosfomycin 85.5%, nitrofurantoin 84.4% and 3rd generation cephalosporins 89–91%. Aminoglycosides (>92%) and carbapenems (95%) maintained the highest susceptibility rates. The prevalence of ESBL-producing isolates was significantly lower in children under the age of 16 years (1.5% vs. 4.1%). In patients under the age of 16 years, Escherichia coli isolates in girls were significantly more sensitive (p<0.0001) to ampicillin (41% vs. 30%) and amoxicillin/clavulanic acid (82% vs. 72%) than in boys.
ConclusionsThe compilation of cumulative susceptibility reports disaggregated by age or gender reveals significant differences. In our setting, cefuroxime may be considered the first-line empirical treatment in pediatric patients.
Los informes de sensibilidad acumulada son una herramienta valiosa para guiar el tratamiento empírico de infecciones urinarias, sobre todo en el contexto actual de crecientes tasas de resistencia. Nuestro objetivo es analizar la sensibilidad antimicrobiana de bacterias aisladas de urocultivos de pacientes pediátricos durante un período de 5 años.
MétodosEstudio retrospectivo de los urocultivos del período 2011-2015. La identificación y estudios de sensibilidad se realizaron con el sistema Vitek-2 (BioMérieux®) y se interpretaron según los criterios de EUCAST. Se analizaron los datos de sensibilidad antimicrobiana según sexo y tramos de edad (neonatos, 1 mes-5 años, 5-15 años) y se compararon con los datos de mayores de 15 años.
ResultadosEn el período analizado se procesaron 17.164 urocultivos de 7.924 pacientes menores de 16 años. Los porcentajes de sensibilidad en estos pacientes fueron: ampicilina 36,3%; amoxicilina/clavulánico 75,3%; cefuroxima 83,2%; cotrimoxazol 68,9%; ciprofloxacino 85,3%; fosfomicina 85,5%; nitrofurantoína 84,4%, y cefalosporinas de tercera generación 89-91%. Aminoglucósidos (>92%) y carbapenemas (95%) mantienen las mayores tasas de sensibilidad. La prevalencia de aislamientos productores de BLEE fue significativamente menor en niños menores de 16 años (1,5% vs. 4,1%). En menores de 16 años, los aislamientos de Escherichia coli procedentes de mujeres fueron significativamente (p<0,0001) más sensibles a ampicilina (41% vs. 30%) y amoxicilina-clavulánico (82% vs. 72%) que en varones.
ConclusionesLa elaboración de informes de sensibilidad acumulados desglosados por edad o sexo permite detectar importantes diferencias. En nuestra área, cefuroxima puede considerarse como primera opción de tratamiento empírico en pacientes pediátricos.
Urinary tract infection (UTI) is the second most common infection in pediatric patients, with Escherichia coli being its first cause. Because of the possible complications that may lead to long-term hospital stay and, especially in children, to renal scarring, hypertension or chronic kidney disease,1,2 often require physicians to prescribe empirical antimicrobial therapy. This leads to growing consumption of antibiotics increasing selective pressure and favoring antimicrobial resistance. Current guidelines establish that uncomplicated UTIs in children should be treated empirically with short-course oral antimicrobials.2,3 But, in recent years, increasing resistance to antimicrobial agents has been reported worldwide. In this context, cumulative reports on antimicrobial susceptibility data provided by microbiological laboratories are important for selecting empirical treatments. This is of particular relevance in case of UTI.4 However, there is a paucity of such data related to pediatrics patients.
The aim of this study is to retrospectively analyze antimicrobial susceptibility data of microorganism isolated from pediatrics patients in our healthcare area during the last years and to compare them with those obtained from adult patients.
Materials and methodsA retrospective observational study of urine cultures analyzed in the period of January 2011to December 2015 at the Microbiology Service of the University Hospital Marqués de Valdecilla (Santander, Northern Spain) was conducted. Our institution covers a healthcare area of 330000 inhabitants (44800 children under the age of 15 years).
All urine samples were inoculated on Cysteine lactose electrolyte deficient agar plates (CLED, Oxoid™, Wesel, Germany) with a 0.001mL calibrated loop. In case of immunosuppressed patients or urine samples obtained by suprapubic bladder aspiration or catheterism, sheep blood agar plates were also inoculated. Colony counts >104 (or 102 in case of suprapubic bladder aspiration and 103 in case of catheterism) were considered positive. Isolation of more than 2 microorganisms was considered an indication of a contaminated urine sample.
Identification and susceptibility testing has been performed with GP-ID, GN-ID, AST-626, AST-589 and AST-244 cards (Vitek-2, bioMérieux, L’Étoile, France). Ampicillin, amoxicillin plus clavulanate, cefuroxime, cefoxitin, cefotaxime, ceftazidime, cefepime, imipenem, ertapenem, amikacin, gentamicin, tobramycin, nalidixic, ciprofloxacin, trimethoprim-sulfamethoxazole, fosfomycin and nitrofurantoin were studied. Categorical interpretation of susceptibility data was defined following EUCAST breakpoints,5 except for amoxicillin-clavulanic acid (ratio 2:1 in the Vitek cards we used, which was interpreted according to CLSI breakpoints.6 Ciprofloxacin-susceptible enterobacteria with a MIC of nalidixic acid >16mg/L (indicating low-level quinolone resistance) was reported as ciprofloxacin intermediate in our laboratory.
Antimicrobial susceptibility data (first isolate of each microorganism per patient and year) was stratified by gender and age of the patient, and classified into three pediatric groups (neonates, 1 month to 5 years and 5–15 years). These data was compared with that obtained from patients older than 15 years. We analyzed susceptibility data of all microorganisms grouped together and for each microorganismseparately. Not all antimicrobial agents analyzed were tested for all microorganisms, due to the different designs of Vitek-2 cards we used. To avoid bias in the overall analysis of all microorganisms grouped; we deducted the susceptibility of antimicrobials not tested considering microorganism identification and other antimicrobial susceptibility results. For example, amoxicillin–clavulanate, ertapenem and imipenem were interpreted as susceptible in case of ampicillin-susceptible enterococci; cephalosporins and aminoglycosides were considered as resistant in enterococci; ampicillin, amoxicillin plus clavulanate, cefuroxime, cefoxitin and cefotaxime were interpreted as resistant in Pseudomonas spp., etc.7,8
The presence of ESBL producing microorganisms was analyzed and compared between the different pediatric age groups. ESBL-producing strains were characterized by PCR targeting TEM, SHV and CTX-M gene families and by sequencing of the obtained amplicons.9–11
Comparative analysis of antimicrobial susceptibility results was determined using χ2, and when it was not suitable, a Fisher's exact test was used.4 A p value of ≤0.05 was considered statistically significant.
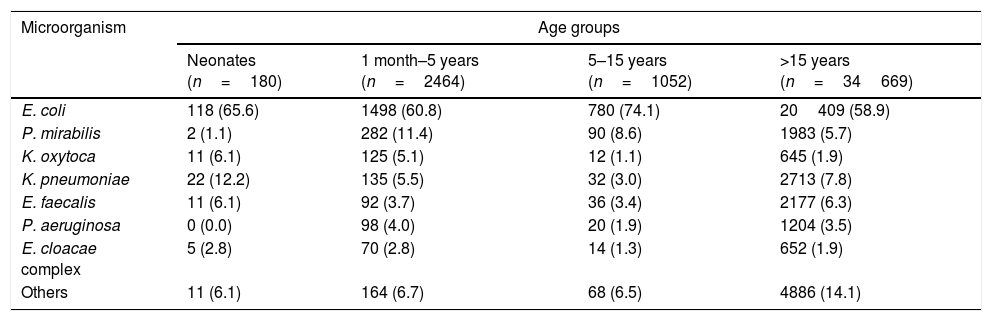
ResultsDuringthe study period 17164 urine samples from 7924 patients younger than 16 years were processed, they came from primary care (55.1%), hospitalized patients (6.5%), outpatient visits (21.8%), and emergency departments (16.6%). Urine culture was defined as positive in 3751 samples (21.8%) and E. coli was the most frequently isolated uropathogen (2370 patients, 63.2%), followed by Proteus mirabilis (374 patients, 10%), Klebsiella pneumoniae (189 patients, 5%), Klebsiella oxytoca (148 patients, 3.9%), Enterococcus faecalis (138 patients, 3.7%), Pseudomonas aeruginosa (118 patients, 3.1%) and Enterobacter cloacae complex (81 patients, 2.2%) (Table 1). All these species together represent 91.3% of the isolates recovered from urine cultures. Only 7.4% of the isolates were recovered from hospitalized patients.
Distribution of isolates by microorganism and age group.
| Microorganism | Age groups | |||
|---|---|---|---|---|
| Neonates (n=180) | 1 month–5 years (n=2464) | 5–15 years (n=1052) | >15 years (n=34669) | |
| E. coli | 118 (65.6) | 1498 (60.8) | 780 (74.1) | 20409 (58.9) |
| P. mirabilis | 2 (1.1) | 282 (11.4) | 90 (8.6) | 1983 (5.7) |
| K. oxytoca | 11 (6.1) | 125 (5.1) | 12 (1.1) | 645 (1.9) |
| K. pneumoniae | 22 (12.2) | 135 (5.5) | 32 (3.0) | 2713 (7.8) |
| E. faecalis | 11 (6.1) | 92 (3.7) | 36 (3.4) | 2177 (6.3) |
| P. aeruginosa | 0 (0.0) | 98 (4.0) | 20 (1.9) | 1204 (3.5) |
| E. cloacae complex | 5 (2.8) | 70 (2.8) | 14 (1.3) | 652 (1.9) |
| Others | 11 (6.1) | 164 (6.7) | 68 (6.5) | 4886 (14.1) |
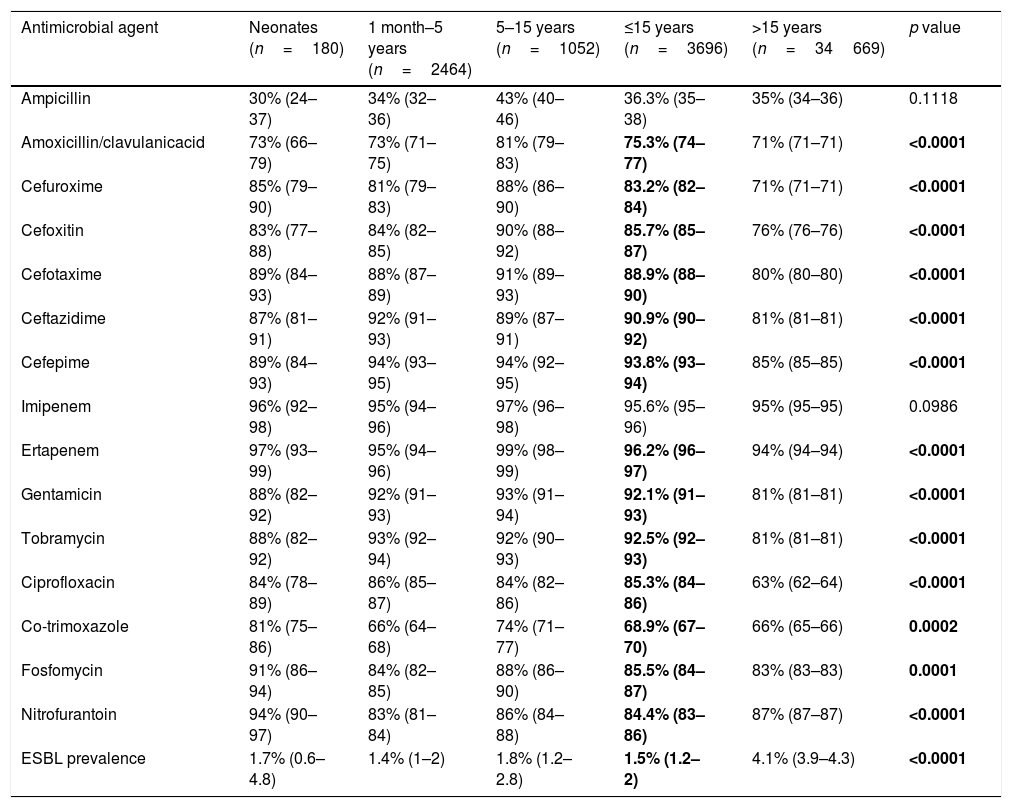
Global antimicrobial susceptibility results (with 95% confidence intervals) and ESBL prevalence distributed in different age groups are shown in Table 2. Non-susceptibility rates higher than 20% were observed for oral antimicrobials such ampicillin, amoxicillin–clavulanate and co-trimoxazole in the pediatric population. Cefuroxime and ciprofloxacin presented non-susceptibility rates between 15 and 20%. On the other hand, high rates of susceptibility, near 90%, were observed for aminoglycosides, third- and fourth-generation cephalosporins. The highest susceptibility rate was observed to carbapenems (>95%). Comparing the 3 pediatrics groups with patients older than 15 years, we found statistically significant differences in the rates of susceptibility for cefuroxime, cefoxitin, cefotaxime, tobramycin and ciprofloxacin, which were all more susceptible in the pediatric population.
Percentages of susceptible (95% confidence intervals) organisms and ESBL prevalence among four age groups of patients.
| Antimicrobial agent | Neonates (n=180) | 1 month–5 years (n=2464) | 5–15 years (n=1052) | ≤15 years (n=3696) | >15 years (n=34669) | p value |
|---|---|---|---|---|---|---|
| Ampicillin | 30% (24–37) | 34% (32–36) | 43% (40–46) | 36.3% (35–38) | 35% (34–36) | 0.1118 |
| Amoxicillin/clavulanicacid | 73% (66–79) | 73% (71–75) | 81% (79–83) | 75.3% (74–77) | 71% (71–71) | <0.0001 |
| Cefuroxime | 85% (79–90) | 81% (79–83) | 88% (86–90) | 83.2% (82–84) | 71% (71–71) | <0.0001 |
| Cefoxitin | 83% (77–88) | 84% (82–85) | 90% (88–92) | 85.7% (85–87) | 76% (76–76) | <0.0001 |
| Cefotaxime | 89% (84–93) | 88% (87–89) | 91% (89–93) | 88.9% (88–90) | 80% (80–80) | <0.0001 |
| Ceftazidime | 87% (81–91) | 92% (91–93) | 89% (87–91) | 90.9% (90–92) | 81% (81–81) | <0.0001 |
| Cefepime | 89% (84–93) | 94% (93–95) | 94% (92–95) | 93.8% (93–94) | 85% (85–85) | <0.0001 |
| Imipenem | 96% (92–98) | 95% (94–96) | 97% (96–98) | 95.6% (95–96) | 95% (95–95) | 0.0986 |
| Ertapenem | 97% (93–99) | 95% (94–96) | 99% (98–99) | 96.2% (96–97) | 94% (94–94) | <0.0001 |
| Gentamicin | 88% (82–92) | 92% (91–93) | 93% (91–94) | 92.1% (91–93) | 81% (81–81) | <0.0001 |
| Tobramycin | 88% (82–92) | 93% (92–94) | 92% (90–93) | 92.5% (92–93) | 81% (81–81) | <0.0001 |
| Ciprofloxacin | 84% (78–89) | 86% (85–87) | 84% (82–86) | 85.3% (84–86) | 63% (62–64) | <0.0001 |
| Co-trimoxazole | 81% (75–86) | 66% (64–68) | 74% (71–77) | 68.9% (67–70) | 66% (65–66) | 0.0002 |
| Fosfomycin | 91% (86–94) | 84% (82–85) | 88% (86–90) | 85.5% (84–87) | 83% (83–83) | 0.0001 |
| Nitrofurantoin | 94% (90–97) | 83% (81–84) | 86% (84–88) | 84.4% (83–86) | 87% (87–87) | <0.0001 |
| ESBL prevalence | 1.7% (0.6–4.8) | 1.4% (1–2) | 1.8% (1.2–2.8) | 1.5% (1.2–2) | 4.1% (3.9–4.3) | <0.0001 |
Bold values correspond to results in wich the difference between ≤ 15 years groups was statistically significant (p >0,05).
We found no significant difference in the activity of any of the antibiotics over the 5 years study period among pediatric patients.
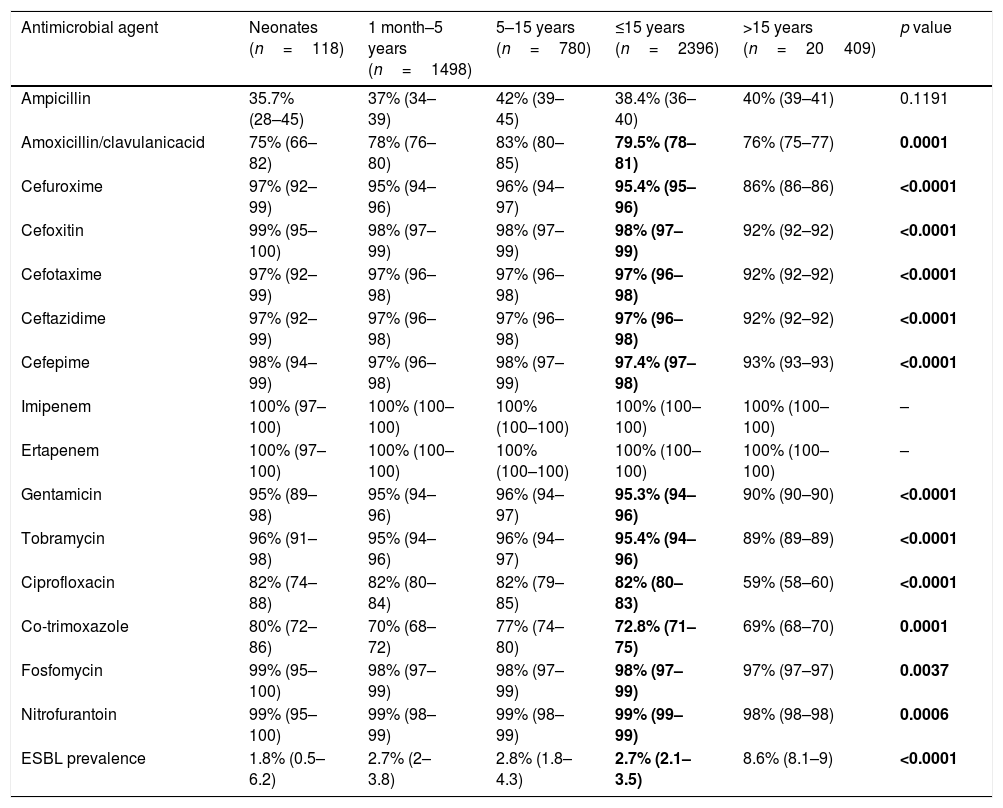
Antibiotic susceptibility of specific bacteriaE. coli showed susceptibility rates higher than 95% to cephalosporins, carbapenems, aminoglycosides, nitrofurantoin and fosfomycin (Table 3). Otherwise, other important antimicrobials for treatment of UTIs (especially community-acquired) such as ampicillin, amoxicillin–clavulanate, ciprofloxacin and co-trimoxazole did not exceed 80% of susceptibility. Ninety-nine percent of E. coli isolates recovered in pediatric patients were from community origin. E. coli isolates recovered from women aged ≤15 were statistically (p<0.0001) more susceptible to ampicillin [41% (39–43) vs. 30% (27–34)] and amoxicillin-clavulanic acid [82% (80–84) vs.72% (68–75)] compared with those cultured from men of the same age.
Susceptibility (95% confidence intervals) and ESBL prevalence of E. coli among four age groups of patients.
| Antimicrobial agent | Neonates (n=118) | 1 month–5 years (n=1498) | 5–15 years (n=780) | ≤15 years (n=2396) | >15 years (n=20409) | p value |
|---|---|---|---|---|---|---|
| Ampicillin | 35.7% (28–45) | 37% (34–39) | 42% (39–45) | 38.4% (36–40) | 40% (39–41) | 0.1191 |
| Amoxicillin/clavulanicacid | 75% (66–82) | 78% (76–80) | 83% (80–85) | 79.5% (78–81) | 76% (75–77) | 0.0001 |
| Cefuroxime | 97% (92–99) | 95% (94–96) | 96% (94–97) | 95.4% (95–96) | 86% (86–86) | <0.0001 |
| Cefoxitin | 99% (95–100) | 98% (97–99) | 98% (97–99) | 98% (97–99) | 92% (92–92) | <0.0001 |
| Cefotaxime | 97% (92–99) | 97% (96–98) | 97% (96–98) | 97% (96–98) | 92% (92–92) | <0.0001 |
| Ceftazidime | 97% (92–99) | 97% (96–98) | 97% (96–98) | 97% (96–98) | 92% (92–92) | <0.0001 |
| Cefepime | 98% (94–99) | 97% (96–98) | 98% (97–99) | 97.4% (97–98) | 93% (93–93) | <0.0001 |
| Imipenem | 100% (97–100) | 100% (100–100) | 100% (100–100) | 100% (100–100) | 100% (100–100) | – |
| Ertapenem | 100% (97–100) | 100% (100–100) | 100% (100–100) | 100% (100–100) | 100% (100–100) | – |
| Gentamicin | 95% (89–98) | 95% (94–96) | 96% (94–97) | 95.3% (94–96) | 90% (90–90) | <0.0001 |
| Tobramycin | 96% (91–98) | 95% (94–96) | 96% (94–97) | 95.4% (94–96) | 89% (89–89) | <0.0001 |
| Ciprofloxacin | 82% (74–88) | 82% (80–84) | 82% (79–85) | 82% (80–83) | 59% (58–60) | <0.0001 |
| Co-trimoxazole | 80% (72–86) | 70% (68–72) | 77% (74–80) | 72.8% (71–75) | 69% (68–70) | 0.0001 |
| Fosfomycin | 99% (95–100) | 98% (97–99) | 98% (97–99) | 98% (97–99) | 97% (97–97) | 0.0037 |
| Nitrofurantoin | 99% (95–100) | 99% (98–99) | 99% (98–99) | 99% (99–99) | 98% (98–98) | 0.0006 |
| ESBL prevalence | 1.8% (0.5–6.2) | 2.7% (2–3.8) | 2.8% (1.8–4.3) | 2.7% (2.1–3.5) | 8.6% (8.1–9) | <0.0001 |
Bold values correspond to results in wich the difference between ≤ 15 years groups was statistically significant (p >0,05).
ESBL-producing E. coli isolates were significantly (p<0.0001) less frequent in pediatric patients than in adults (neonates 1.8%, 1 month–5 years 2.7%, 5–15 years 2.8%, >15 years 8.6%). They were detected in 51 patients aged ≤15, and CTX-M type were the most frequent enzymes (66.7%, CTX-M group 1 in 12 patients, CTX-M group 9 in 22 patients), followed by SHV-12 (29.4%) and SHV-2 (3.9%).
P. mirabilis was the second most prevalent microorganism and was most frequently isolated in the age group of 1 month to 5-year-old patients. This species showed high susceptibility rates to cefuroxime [97% (95–98)], third and fourth-generation cephalosporins [98% (96–99)], ertapenem [97% (95–98)] and tobramycin [93% (90–95)]. On the other hand, the highest resistance rates were observed for amoxicillin [64% (59–69)] and nitrofurantoin (intrinsic resistance). Isolates from younger patients were statistically more susceptible to ciprofloxacin [90% (87–93) vs. 62% (60–64)], co-trimoxazole [72% (67–76) vs. 60% (58–62)], fosfomycin [80% (76–84) vs. 69% (67–71)] and gentamicin [87% (83–90) vs. 78% (76–80)] compared with isolates recovered from patients older than 15 years old. Only one ESLB-producing isolate was detected (CTX-M group 1).
Susceptibility to fosfomycin was low in case of K. pneumoniae [62% (55–69)] and K. oxytoca [55% (47–63)] isolates, whereas both organisms were highly susceptible (97–100%) to ciprofloxacin, third and fourth-generation cephalosporins and aminoglycosides. Around 90% of Klebsiella spp. isolates were susceptible to oral antimicrobials such as amoxicillin–clavulanate [92% (86–95)] and co-trimoxazole [87–88% (81–91)]. Compared with isolates recovered from patients older than 15 years, Klebsiella spp. was statistically more susceptible to ciprofloxacin in younger patients [98% (96–99) vs. 88% (87–89)]. Prevalence of ESBL-producing isolates was 1.18% (n=4), including 1 K. oxytoca (TEM-52) and 3 K. pneumoniae (two CTX-M group 1 and one TEM-52). Only one GES-type carbapenemase producing K. oxytoca isolate was detected, in an 8-year-old boy.
E. faecalis, the most frequently isolated gram-positive microorganism in urine cultures, remains highly susceptible to ampicillin (100%), vancomycin (100%) and nitrofurantoin (99%). Following EUCAST breakpoints for uncomplicated UTIs, E. faecalis isolates from pediatric patients were significantly more susceptible to ciprofloxacin [94% (81–98) vs. 72% (69–75)] than in adults.
P. aeruginosa and E. cloacae complex were isolated less frequently in pediatric population and adults, but important and significant differences in susceptibility to a wide group of antimicrobials were observed when comparing the two age groups. In P. aeruginosa, the isolates from pediatric patients were more susceptible to piperacillin–tazobactam [98% (93–99) vs. 85% (83–87)], ceftazidime [99% (95–100) vs. 87% (85–89)], imipenem [92% (86–96) vs. 80% (78–82)], ciprofloxacin [94% (88–97) vs. 59% (56–62)], gentamicin [100% (97–100) vs. 69% (66–72)] and tobramycin [100% (97–100) vs. 77% (75–79)]. In E. cloacae complex, pediatric isolates were more susceptible to cefepime [91% (83–96) vs. 78% (74–81)], gentamicin [99% (94–100) vs. 82% (79–85)], tobramycin [98% (92–100) vs. 81% (78–84)] and ciprofloxacin [96% (89–99) vs. 74% (70–77)]. Susceptibility rates to oral agents as co-trimoxazole and fosfomycin were 85% (76–91) and 48% (37–59) respectively, with similar values in adults. Only two ESBL-producing E. cloacae isolates were detected (one CTX-M group 1 and one CTX-M group 9).
DiscussionFor the correct choice of UTI treatment, our National Health System (NHS)12 recommends to rely on the knowledge of the local resistances,12 so reporting updated local susceptibility patterns of the most frequently microorganisms isolated from urine samples allows the physician to choose more appropriate and effective empirical treatments.13,14
NHS12 guidelines for empirical treatment of afebrile UTI in pediatric patients recommend oral treatment with amoxicillin–clavulanate, first or second-generation cephalosporins, fosfomycin, nitrofurantoin or co-trimoxazole. For an oral empirical treatment of UTI with fever, they recommend third-generation cephalosporins and alternatively amoxicillin clavulanate or second-generation cephalosporins. In case of an empirical treatment of febrile UTI by intravenous route, it seems appropriate to use third generation cephalosporins IV (cefotaxime, ceftriaxone) and alternatively an aminoglycoside (gentamicin, tobramycin), amoxicillin clavulanate IV or second-generation cephalosporins IV.
Ceftazidime, amikacin, carbapenems and quinolones should be reserved for special circumstances. In addition, in patients younger than 3 months, given the possibility of enterococcal infection, it is recommended to associate ampicillin.
Our retrospective study describes susceptibility patterns of microorganisms isolated from urine cultures obtained from pediatric patients mostly non-hospitalized. From a clinical perspective, these data are applicable mainly in our area, as changes in bacterial susceptibility can be influenced not only by circulating clones but, especially, by policies of antimicrobial use in different geographical locations. Most of the urine cultures come from primary care centers, where pediatricians monitor children with urinary tract infection according to protocols agreed with our hospital. The urine cultures of the hospitalized patients are always reviewed by the pediatrician and there are usually no discharges without this result.
Our results show that resistance to ampicillin, amoxicillin–clavulanate and co-trimoxazole was high (exceeding 20%) in isolates from both pediatric patients older than 1 month and adults. The data obtained for both ampicillin and co-trimoxazole are consistent with other published national series.13–16 Susceptibility to amoxicillin–clavulanatevaries according to different reported series13–16 but although its use is very common,12,15 our local results suggest it should not be recommended for empirical treatment. In our area, second-generation cephalosporins represent the first therapeutic option for uncomplicated ITU in pediatric population,3 and they continue to maintain acceptable levels of susceptibility (80–90%) for the pediatric population analyzed.
Fosfomycin has several advantages for its use in children. It is easy to dose, reaches high concentrations in the urine, has low toxicity in children, does not affect the anaerobic intestinal flora and still maintains low levels of resistance (9–16%) in all age groups analyzed. For these reasons, fosfomycin may be considered as a good therapeutic option for UTIs without fever.3 It is possible that in the next years the use of fosfomycin will gain importance, although an increased use in the community could modify the current good rates of susceptibility.17
Nitrofurantoin has been considered an excellent treatment option for uncomplicated cystitis in adults in United States and some countries in southern Europe, where E. coli isolated from urine have shown high rates of resistance to fluoroquinolones and co-trimoxazole.18,19 Following the recommendations of the Spanish Society Childcare, nitrofurantoin is one of the most appropriate antibiotics for prophylaxis of urinary tract infection in children.3 Our study supports this indication in our area, however higher toxicity must be considered if administration is prolonged. Recently the Spanish Agency of Medicinal Products and Medical Devices has restricted its use for acute cystitis only for female from 3 months old with a maximum of 7 days of administration.20
In our area susceptibility levels to aminoglycosides were around 85–90%. Because of its toxicity and parenteral administration, its use is restricted to the hospital setting, especially amikacin, which can even be considered as a therapeutic alternative against ESBL-producing strains.13
Fluoroquinolones are a group of antibiotics highly accepted for treating UTI in adults due to its broad spectrum of action, bactericidal activity, good oral bioavailability and marked postantibiotic effect but they are not recommended in the pediatric population.12 The influence of age on quinolone resistance rates has been documented elsewhere14–16 and was also observed in our study, showing ciprofloxacin resistance in adults greater than 35% compared to 14–16% in the pediatric population, although such resistance levels in pediatric patients (that presumably do not receive quinolones) in our area is not totally expected compared with the study of Treviño et al.21
The prevalence of ESBL isolates recovered in urine samples from pediatric patients was significantly lower than in adults [1.5% (1.2–2) vs. 4.1% (3.9–4.3)]. Probably this is due to the presence of more risk factors for infections with these organisms in the older age group of patients (prior antimicrobial therapy, hospitalization, urologic surgeries, underlying diseases, etc.).16,22,23 On the other hand, the prevalence of ESBL isolates from pediatric population was very low in comparison with other Mediterranean countries. Kizilka et al. have documented in Turkey a high prevalence of ESBL-harboring isolates (43%) in pediatrics UTIs.24 These authors point out prior hospitalization and use of cephalosporins as most frequent risk factors. Also, in a recent study from Spain, Hernández Marco et al. reported 3.5% prevalence of ESBL-producing isolates in children under two years, and bladder reflux of any degree was the most frequent risk factor, being twice more frequent in the ESBL patients group, although no statistically significant differences were found.25 However, highest proportion of recurrences in the ESBL group was found to be significant, as previously described by other authors.24,26
Prevalence of carbapenemase producing isolates is very low in our area. Only one isolate was detected in a urine culture of a pediatric patient, and it showed no significant differences compared with those obtained from older patients (0.03% vs. 0.04%).
The usual presentation of accumulated susceptibility data includes separate data for microorganism, and in this way, E. coli data could be taken as a reference to guide empirical treatment.12,14 This practice underestimates resistance rates because it excludes P. aeruginosa, E. cloacae and other bacteria with intrinsic resistance to various antimicrobials. However, this attitude may be acceptable to guide treatment in cases of uncomplicated UTIs or non-hospitalized patients, where these resistant microorganisms are rarely recovered. E. coli susceptibility data observed in our study correlate well with those obtained by Trevino et al. for two pediatric groups (0–4 years and 5–14 years), in which amoxicillin–clavulanate resistance of 22.8% and 17.2% were obtained respectively, while 5% gentamicin and 20% co-trimoxazole resistance was described in both age groups.
The data reported in our study were designed to guide empirical treatment, for this reason the duplicate isolates were excluded as recommended.4 In this way, 1088 isolates from 602 patients were excluded for the statistical analysis. With these data, we estimate that up to 22.5% of pediatric patients are at risk for possible treatment failure, reinfection or recurrence, an observation that needs further analysis. Analyzing duplicate E. coli isolates (n=752), no significant differences were observed in antimicrobial susceptibility data. Susceptibility rates of amoxicillin/clavulanate, cefuroxime, cefotaxime, ceftazidime, fosfomycin and ciprofloxacin from duplicate isolates were only 1–2% lower than from first isolates, and the corresponding figure was 4% in case of amoxicillin and co-trimoxazole.
Most clinical guidelines recommend preserve culture and susceptibility tests for cases of complicated UTI.1,12 Although UTIs in male are less frequent and usually more severe,5,27,28 it will be expected that 10 to 20% of women will have a UTI during their lifetime, and in consequence, a higher volume of samples will be sent to laboratory.26–28 Although UTI and vesicoureteral reflux are very common in women, in our area we have observed that E. coli resistance rates are higher in males than females, but we have not been able to find a reason to explain it.
It is possible that antimicrobial susceptibility studies overestimate rates of resistance, especially in microorganisms isolated from urine samples of community patients, since they usually correspond to cases with initial treatment failure or complicated UTIs.28 In a similar way, differences in resistance rates on both age groups (pediatric and adults patients) might be due to different diagnostic and therapeutic attitudes, including that samples from first episodes in young women are not sent for urine culture, and microbiological studies are only considered in complicated cases, when probably patients have already received antimicrobial treatment.
In our opinion, it is important to report accumulated susceptibility profiles disaggregated by sex and age because important differences can be detected, which will allow a better fit of empirical treatment. In our area and based on our results, and more specifically E. coli data, cefuroxime can be considered the first option for oral empirical treatment for febrile and afebrile UTIs in patients older than 6 months, while fosfomycin and nitrofurantoin are the choice for prophylaxis. For patients between 3 and 6 months, intravenous cefuroxime is the treatment prescribed. However, amoxicillin–clavulanate should not be considered as the first choice for ITU therapy.
Given the paucity of data in the pediatric population, it would be advisable to perform additional local studies in order to optimize empirical antibiotic therapy, and even carrying out a study that covers a bigger cohort of hospitalized patients to obtain more relevant conclusions in this group of patients.
Conflict of interestThe authors declare no conflicts of interest.