Recurrences of Clostridium difficile infections lead to hospital readmissions and high costs, in addition to the suffering and frustration for the patients. Fidaxomicin has recently been introduced as a new antibiotic that has been shown to significantly reduce the recurrence of this infection. Despite this superiority, its high cost has led to very restrictive policies in its use, as such that many institutions only use it in patients with multiple recurrences. While waiting for new predictive clinical tools, we propose the development of scoring systems that allow the more high-risk patients to be treated earlier.
Las recurrencias de la infección por Clostridium difficile ocasionan reingresos hospitalarios y costes elevados, además de sufrimiento y frustración para los pacientes que las padecen. Recientemente se ha incorporado fidaxomicina, un nuevo antibiótico con cuya utilización se ha demostrado una reducción significativa de las recurrencias de esta infección. A pesar de esta superioridad, su elevado coste ha ocasionado políticas muy restrictivas en su utilización hasta el punto de que en muchas instituciones solo se emplea para pacientes con múltiples episodios. En espera de herramientas clínicas predictivas, proponemos el desarrollo de sistemas de puntuación que permitan tratar más precozmente a pacientes de alto riesgo.
Recurrences are a major challenge of Clostridium difficile (C. difficile) infection (CDI). They lead to increased morbidity, hospital readmissions, high costs and reduced quality of life in patients and are also new opportunities for disease transmission.1
Despite adequate treatment with vancomycin or metronidazole, up to 25% of patients experience recurrence of infection, generally within the first 30 days of treatment.2 Pivotal clinical trials comparing fidaxomicin and vancomycin have demonstrated a similar efficacy (88% vs 86%) for curing the initial episode. However, recurrence rates were significantly lower (26% vs 14%) in those patients treated with fidaxomicin.3
Use of fidaxomicin has, however, been restricted due to its high cost and, in practice, is limited to patients with multiple recurrent infections. Nevertheless, experience published to date is limited to patients with a first recurrence of infection and there are very limited data on patients with multiple recurrence of CDI.4 The fact that use in initial episodes is limited means that patients with a high risk of recurrence do not benefit and they have to wait until traditional treatments fail. However, systematic administration of fidaxomicin to all patients is probably not cost-efficient.5,6 The best option would therefore be to identify those patients with a high risk of recurrence in order to be able to use the drug selectively. A scoring system based on data extracted from published literature has been established at our hospital that, although not validated, allows organised prescription of fidaxomicin in a way that is not so restrictive.
The objective of this study is to analyse our centre's experience with this screening system and to provide information on the use of fidaxomicin in Spain, outside the context of a clinical trial.
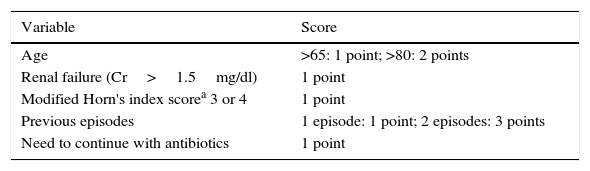
MethodsDuring the study period (May 2013 to March 2015), patients with microbiologically confirmed symptomatic CDI were routinely evaluated by a member of the Infectious Diseases Department. When patients scored 4 or more on the scoring system described in Table 1, treatment with fidaxomicin was considered.
Criteria for fidaxomicin use.
| Variable | Score |
|---|---|
| Age | >65: 1 point; >80: 2 points |
| Renal failure (Cr>1.5mg/dl) | 1 point |
| Modified Horn's index scorea 3 or 4 | 1 point |
| Previous episodes | 1 episode: 1 point; 2 episodes: 3 points |
| Need to continue with antibiotics | 1 point |
By clinical assessment: 1: mild (single mild illness), 2 moderate (more severe illness but uncomplicated recovery expected), 3: severe (complications or multiple conditions requiring treatment), 4: fulminant (catastrophic).
Horn's index, according to Hu et al.8 (patient's severity based on clinical assessment): 1, mild (mild illness); 2, moderate (more severe illness but uncomplicated recovery expected); 3, severe (complications or multiple conditions requiring treatment); 4, fulminant (catastrophic life-threatening illness).
Data were collected on the demographic and clinical characteristics of the patients. Until May 2014, CDI was diagnosed by direct detection of toxins in stool samples or toxigenic C. difficile culture. After that date, a three-step algorithm was used for direct toxin and GDH detection in stool samples, also performing PCR in conflicting cases. The presence of suggestive symptoms (diarrhoea with more than 3 unformed stools a day) within 60 days of completion of fidaxomicin treatment, with microbiological confirmation of toxigenic C. difficile in stools, was defined as CDI recurrence.
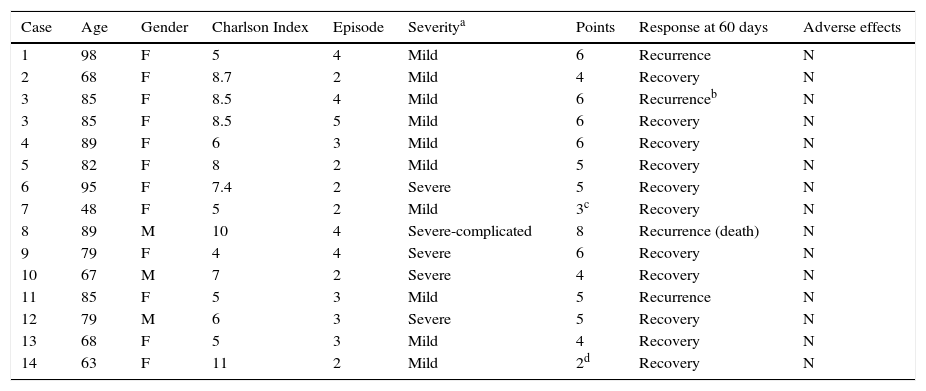
ResultsFifteen patients (16 episodes) received fidaxomicin during the study period. These accounted for 4.9% of all patients diagnosed with CDI during this period. Two of the patients were not tested because they died within 48hours of starting fidaxomicin. Also, two patients with scores below 4 according to the agreed scoring system received fidaxomicin due to special circumstances not contemplated by the scoring system. One of these patients had had a recent kidney transplant and the other had cancer requiring chemotherapy.
The median age of the 13 patients analysed was 80.5 years and the majority (84.6%) were women. Six patients (43%) were treated for their second episode and the others were treated for their third episode or later. Five of the episodes were severe and 9 were mild. Fidaxomicin was prescribed for 10 days in all cases and one patient received two treatment cycles. During treatment with fidaxomicin, no adverse effects attributable to the drug were described. Only one of the patients died during follow-up due to complications directly related to CDI. The characteristics of the patients are described in Table 2.
Patient characteristics and results.
| Case | Age | Gender | Charlson Index | Episode | Severitya | Points | Response at 60 days | Adverse effects |
|---|---|---|---|---|---|---|---|---|
| 1 | 98 | F | 5 | 4 | Mild | 6 | Recurrence | N |
| 2 | 68 | F | 8.7 | 2 | Mild | 4 | Recovery | N |
| 3 | 85 | F | 8.5 | 4 | Mild | 6 | Recurrenceb | N |
| 3 | 85 | F | 8.5 | 5 | Mild | 6 | Recovery | N |
| 4 | 89 | F | 6 | 3 | Mild | 6 | Recovery | N |
| 5 | 82 | F | 8 | 2 | Mild | 5 | Recovery | N |
| 6 | 95 | F | 7.4 | 2 | Severe | 5 | Recovery | N |
| 7 | 48 | F | 5 | 2 | Mild | 3c | Recovery | N |
| 8 | 89 | M | 10 | 4 | Severe-complicated | 8 | Recurrence (death) | N |
| 9 | 79 | F | 4 | 4 | Severe | 6 | Recovery | N |
| 10 | 67 | M | 7 | 2 | Severe | 4 | Recovery | N |
| 11 | 85 | F | 5 | 3 | Mild | 5 | Recurrence | N |
| 12 | 79 | M | 6 | 3 | Severe | 5 | Recovery | N |
| 13 | 68 | F | 5 | 3 | Mild | 4 | Recovery | N |
| 14 | 63 | F | 11 | 2 | Mild | 2d | Recovery | N |
Source: Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 Update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010; 31: 431–455.
Three patients suffered recurrences after being treated with fidaxomicin for their fourth episode and one patient who suffered a recurrence during her third episode treated with fidaxomicin was cured by a faecal microbiota transplant. Therefore, 71.4% of episodes did not result in recurrences after using fidaxomicin during the study period. The six patients who were treated for the second episode were CDI free after 60 days of follow-up, while four of the nine episodes treated for the third recurrence or later resulted in a new recurrence.
DiscussionThe risk of recurrence increases considerably after the second episode of CDI.6 Recurrence is also more common in patients over the age of 64, in those with decompensated underlying diseases (modified Horn's index of 3 or 4 points), those suffering from chronic kidney disease and those requiring continuation of antibiotics after treating CDI.7–9 Other risk factors are associated with immune responses to C. difficile toxins and infection with the hypervirulent 027 strain.10,11 Awareness of such risk factors for recurrence may be useful when screening patients for treatment with drugs that reduce the risk of recurrence but this does not help properly quantify each patient's actual probability of recurrence. The need for predictive models or clinical decision tools is therefore proposed.
Using the cohort of patients included in the pivotal fidaxomicin trials, D’Agostino et al.12 published a predictive model for recurrence in which the probability of recurrence was determined by age (greater or less than 75 years), number of unformed bowel movements during the previous 24hours (greater or less than 10), creatinine level (greater or less than 1.2mg/dl) and prior episodes of CDI. Based on whether the patient had received fidaxomicin or vancomycin and these four risk factors, the predicted risk of recurrence, according to the resulting model, was between 10% and 18% and between 37% and 54% for those treated with fidaxomicin and vancomycin, respectively. The drawback of this model is the fact that it is based on patients included in clinical trials, with the biases that this may entail, and it has not been validated externally afterwards. At an earlier date, Hu et al.8 had described a model based on multivariate analysis of a cohort. The variables considered were age, Horn's index and the need to continue antibiotics. Unfortunately, the number of patients considered was too low.
Another approach for deciding which patients to administer fidaxomicin to are pharmacoeconomic studies. A recent study in Spain found that treatment with fidaxomicin is dominant versus vancomycin in patients with cancer, renal impairment or those requiring continued antibiotic therapy.13
In a cost-effectiveness study conducted in Canada, the authors concluded that faecal transplantation by colonoscopy was the most cost-effective strategy for recurrent C. difficile infection, while fidaxomicin would be the dominant option where faecal transplant is not available.14 Nevertheless, other aspects, such as convenience and access, should be considered when making therapeutic decisions.
While waiting for more robust prediction tools, one pragmatic approach involves implementing risk scoring systems that, although not validated, allow institutions to organise and draft protocols for prescribed methods. We make decisions at our institution based on this strategy. Our scoring system has been determined to be too restrictive since we have only administered fidaxomicin to 4.9% of the patients diagnosed with CDI. The success rate is high, taking into account the number of prior episodes before receiving fidaxomicin. It is important to note that none of the patients treated after their first relapse experienced any recurrence during follow-up, while four of the nine patients who had experienced two or more recurrences failed with fidaxomicin. In our opinion, these data suggest that fidaxomicin should not be used too late when managing the disease.
Using a similar but less restrictive approach, Edward H. Eiland et al.15 treated 60 patients with fidaxomicin, obtaining a high rate of clinical success, a low rate of recurrence and a low readmission rate. His less restrictive scoring system screened 60 patients (15% of all patients diagnosed with CDI), of whom 34 (56.7%) received fidaxomicin for their first episode. More recently, a multicentre study has demonstrated that the introduction of fidaxomicin is associated with a better clinical outcome in several hospitals in England.16
Our experience is obviously very limited and we cannot ensure that no patients with 4 points were not eventually treated with fidaxomicin, given that scores were not logged for all patients diagnosed with CDI. However, despite these limitations, we believe that our experience may be of use to other institutions interested in managing prescription of this drug by incorporating similar scoring systems that do more than simply count previous episodes.
In our opinion, it is important to urgently develop validated predictive tools that help screen patients better for treatment with expensive but more effective drugs. Until such a tool is developed, doctors need to establish some type of criterion to allow certain patients to be able to access better treatment without having to wait for the current treatment to fail, which results in suffering, risks and costs for the health system.
Conflicts of interestJavier Cobo has received consulting and speaker fees from Astellas and MSD. All other authors declare that they have no conflicts of interest.
Please cite this article as: Vivancos-Gallego MJ, Jiménez-López MÁ, Gioia F, Ibañez-Segura D, Romero-Vivas J, Cobo J. Uso de un sistema de puntuación para la prescripción de fidaxomicina en la infección por Clostridium difficile. Enferm Infecc Microbiol Clin. 2018;36:34–37.