Little is known about the characteristics of high-risk papillomavirus (HR-HPV) infection in men. The aims of this cross-sectional study were: (a) to investigate HR-HPV prevalence and genotype distribution in men, sexual partners of women presenting with high-grade cervical intraepithelial neoplasia (HG-CIN), according to epidemiological characteristics, and (b) to assess type-specific concordance between partners.
MethodsA total of 125 men were recruited within the first 6 months after HG-CIN diagnosis of their partner. Samples from the coronal sulcus, glans penis shaft, and scrotum were tested with linear array HPV genotyping assay (Roche Diagnostics, Mannheim, Germany). Type-specific concordance within 120 couples was studied. Epidemiological factors were evaluated by multivariate logistic regression analysis. SPSS 19 (IBM, Chicago, USA).
ResultsThe prevalence of HR-HPV infection in males was 50.4% (63/125). HPV16/53/52/51/66/31 were the most frequent genotypes (24/10.4/9.6/8.8/8/7.2%, respectively). Current smoking was associated with an increased risk for HR-HPV infection in men (38.2% (21/55) vs 60% (42/70), OR 2.4, p=0.025). Among 60 infected couples, 62% shared at least one genotype: 41.7% couples were concordantly HPV16 positive and 18.3% were HPV16 negative (kappa value: 0.21). The proportion of women with the same genotype as their male partner was higher than the proportion of men sharing the same genotype as their female partner: 58.7% (37/63) vs 30.8% (37/120), p<0.0001.
ConclusionsSexual partners of women with HG-CIN are a significant reservoir and vector of HPV infection, a fact that could contribute to making viral clearance more difficult to achieve in their partners after treatment of their HG-CIN lesions.
Las características de la infección por papilomavirus de alto riesgo (VPH-AR) en el varón apenas se conocen. Los objetivos de este estudio transversal fueron: (a) investigar la prevalencia de VPH-AR y la distribución de genotipos en varón pareja sexual de mujer con neoplasia intraepitelial cervical de alto grado (CIN-AG) y su epidemiología, y (b) evaluar la concordancia tipo-específica entre parejas.
MétodosSe seleccionaron hombres (n=125) en los primeros 6 meses tras el diagnóstico de CIN-AG de su pareja. Se genotiparon muestras del surco coronario, base del glande y escroto (Linear Array, Roche Diagnostics, Mannheim, Alemania). Se estudió la concordancia tipo-específica entre parejas (n=120). Los factores epidemiológicos se evaluaron mediante regresión logística multivariante, SPSS 19 (IBM, Chicago, USA).
ResultadosLa prevalencia de VPH-AR en el hombre fue del 50,4% (63/125). VPH16/53/52/51/66/31 fueron los genotipos más frecuentes (24/10,4/9,6/8,8/8/7,2%, respectivamente). El tabaquismo se asoció con un mayor riesgo de infección por VPH-AR en el hombre (38,2% [21/55] vs 60% [42/70], OR 2,4, p=0,025). Entre 60 parejas infectadas, el 62% compartieron al menos un genotipo: el 41,7% fueron concordantes VPH16 positivas y el 18,3% VPH16 negativas (valor kappa: 0,21). La proporción de mujeres con el mismo genotipo que su pareja fue mayor que la de hombres con el mismo genotipo que su pareja: 58,7% (37/63) vs 30,8% (37/120), p<0,0001.
ConclusionesLas parejas sexuales de mujeres con CIN-AG constituyen un importante reservorio y vector de infección por VPH; esto podría contribuir a que el aclaramiento viral de sus parejas tras el tratamiento de CIN-AG fuese más difícil de alcanzar.
Human papillomavirus infection (HPV) is estimated to be the most common sexual transmitted infection.1 Although these infections are typically transient and asymptomatic, some of them will result in anogenital warts, dysplastic and/or neoplastic lesions, which cause a substantial disease burden in both sexes and generate a considerable economic distress within societies.2 Most infections are asymptomatic or subclinical and become undetectable over time.3
There has been immense progress in understanding the natural history of HPV infection in women disease. Recently there has been an interest in understanding the relationship between HPV infection and disease in men.4
Epidemiological studies show that high-risk (HR) HPV infection is necessarily the sexual transmitted cause of invasive cervical cancer and its precursor lesion, cervical intraepithelial neoplasia (CIN).5 There are no consistent data on the natural history of HPV in the male population even though these viruses are prevalent in males.6 Bosch et al.7 assessed the contribution of the males’ genital HPV DNA status to the risk of development cervical neoplasia in their sexual partners, confirming their hypothesis that men could be vectors of HPV types typically found in cervical cancer. Presence of HR-HPV in the husbands’ penis conveys a 5-fold risk of cervical cancer to their wives.8 Some studies have demonstrated a higher risk of cervical cancer among second wives of men whose previous wife died of cervical cancer.9 It has been recognized widely that the risk of infection is associated with sexual behavior.10 Nevertheless, the biology and dynamics of HPV transmission among sexual partners (SP) is still a cause for debate and have not already been completely established. The gap may be due to the limited number of studies on HPV male genital infections. A better understanding of HPV infection in men is an essential component of prevention programs aimed to reduce cervical cancer and other HPV related diseases.11
Earlier studies of HPV infection in men used a variety of clinical and histological techniques to establish a diagnosis, but polymerase chain reaction (PCR) has emerged as the most sensitive available method for the detection of latent HPV infection.12 The infectious diseases literature supports the lack of the Food and Drug Administration (FDA) approval of HPV tests for HPV detection in men and the absence of adequate therapy for established HPV infection in this population.13 Although routine HPV testing is not necessary for men in general population, findings from emerging research in high-risk population suggest that HPV infection is pervasive and persistent in these groups, warranting the adoption of additional screening measures.3
The aims of the present study were: (a) to investigate HR-HPV prevalence and genotype distribution in men, sexual partners of women presenting high-grade cervical intraepithelial neoplasia (HG-CIN) according to several epidemiological characteristics and (b) to assess type-specific concordance between partners.
Materials and methodsPopulationA cross-sectional study was conducted by the Urology Department of the University Hospital of Vigo, Spain from January 2013 to June 2015. We recruited 125 asymptomatic men, more than 18 years old, whose SP (regular sexual intercourse for more than 1 year) had presented high grade squamous cervical lesions (cervical intraepithelial neoplasia (CIN) grade 2 (n=55) or CIN grade 3-carcinoma in situ (n=70)) in the previous 6 months. A stable relationship was defined as a duration of longer than 6 months, regardless of sexual intercourse with other partners. Women were diagnosed by cytology, colposcopy and histological examination in this time and HPV detection was performed when possible (n=120). Men were invited to fill in a questionnaire on life-style habits, including sexual behavior (SB). The delay between treatment (conization) of high grade cervical lesions and sampling of men for virological studies was less than 3 months. Penile scraping was obtained and submitted to the PCR assay in order to identify HPV carriers although there are no licensed tests for HPV detection in men and there are no recommendations for male screening. The study protocol (cod. 2013/470) received approval from the ethics committee of clinical investigation of Galicia (Santiago de Compostela, Spain). Information concerning the research project was provided to all participants. Because the significance of a positive HPV test in men is unknown, study personnel spent a considerable amount of time educating men about HPV. We explained that a positive test for the virus do not necessarily put them at risk for disease. Written informed consent was obtained from all patients (male and female partner). Participants did not receive incentive for study involvement. Women and men were not vaccinated against HPV previously to the inclusion in this study.
Specimen collectionThree dry cytobrush for each male were used to scrape the genitalia in order to collect exfoliated cells from different penile areas: the dorsal and ventral area of the penile, external and internal surface of prepuce, coronal sulcus, glans and distal urethra. The three samples were combined and collected into one single vial containing TE buffer pH 8.0 Molecular Biology grade (AppliChem GmbH, Darmstadt, Germany). Samples were maintained at 2–8°C and processed within 24–72h after collection.
Epidemiological surveyThe following variables were obtained by questionnaire in male: age, age at first sexual intercourse (FSI), number of SP in the year preceding the study, number of SP up to date of the study and tobacco use.
HPV DNA detection and genotypingDNA was isolated using QIAamp MinElute Media Kit (Qiagen, Hilden, Germany). The extracted nucleic acids were stored at −20°C. An aliquot of the original sample was also stored at −20°C. Amplification and detection were carried out using the Linear Array HPV Genotyping Test (Linear Array. Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions. We described the distribution of 21 HR-HPV genotypes classified as HR (HR-HPV, IARC Group 1 carcinogens) or probable/possible HR (pHR-HPV, IARC Group 2A/B carcinogens) by the International Agency for Research on Cancer Monograph Working Group14 (HPV16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 67, 68, 69, 70, 73, 82 – including IS39 subtype). This test also detects human beta-globin in order to control the sample adequacy and quality as well as success in DNA extraction and PCR (internal control). Linear array does not have individual probe for HPV52 but uses a probe that simultaneously detects HPV52, 33, 35 and 58. Additional specific PCR was performed in case of HPV33, 35 and/or 58 infection in order to properly detect confection of these three genotypes with HPV52.15
Data analysisEpidemiological men characteristics were described overall, stratified by HPV infection and histological diagnosis of their partner. For all calculations of means, standard deviation was calculated. The association between age at first sexual intercourse and HPV infection was evaluated using t-Student test. Qualitative variables were compared using the chi-square test. Multivariate logistic regression analysis was used to evaluate the factors independently associated with the detection of HR-HPV infection in male by use Step-Wise Logistic regression models. Only variables with p≤0.1 in the bivariate analysis were included in the multivariate analysis. Odds ratio (OR) and their 95% confidence interval (CI) were calculated. Type specific concordance analysis among the couples in which both partners were HPV positive was performed and kappa value was calculated. Data were analyzed using SPSS version 19.0 for Windows IBM, Chicago, USA. A p value <0.05 were considered statistically significant.
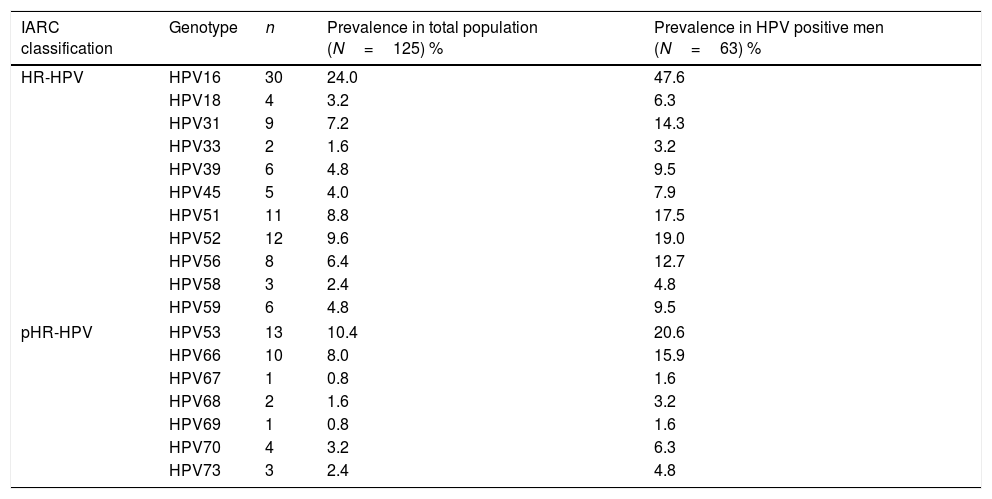
ResultsA total of 125 men were included. Average age of men was 38.2±8.9 years old; 80% were younger than 45 years old. Human beta-globin was detected in all cases. Prevalence of HR-HPV infection in men was 50.4% (63/125). Multiple HR-HPV infections were detected in 30.4% (38/125) of this population. HPV16 was detected in 47.6% (30/63) of infected men. The most frequently detected genotypes were HPV16, HPV53, HPV52, HPV51, HPV66 and HPV31. Their prevalence is shown in Table 1.
Type-specific prevalence in HPV positive men.
| IARC classification | Genotype | n | Prevalence in total population (N=125) % | Prevalence in HPV positive men (N=63) % |
|---|---|---|---|---|
| HR-HPV | HPV16 | 30 | 24.0 | 47.6 |
| HPV18 | 4 | 3.2 | 6.3 | |
| HPV31 | 9 | 7.2 | 14.3 | |
| HPV33 | 2 | 1.6 | 3.2 | |
| HPV39 | 6 | 4.8 | 9.5 | |
| HPV45 | 5 | 4.0 | 7.9 | |
| HPV51 | 11 | 8.8 | 17.5 | |
| HPV52 | 12 | 9.6 | 19.0 | |
| HPV56 | 8 | 6.4 | 12.7 | |
| HPV58 | 3 | 2.4 | 4.8 | |
| HPV59 | 6 | 4.8 | 9.5 | |
| pHR-HPV | HPV53 | 13 | 10.4 | 20.6 |
| HPV66 | 10 | 8.0 | 15.9 | |
| HPV67 | 1 | 0.8 | 1.6 | |
| HPV68 | 2 | 1.6 | 3.2 | |
| HPV69 | 1 | 0.8 | 1.6 | |
| HPV70 | 4 | 3.2 | 6.3 | |
| HPV73 | 3 | 2.4 | 4.8 | |
HR-HPV, high-risk HPV genotypes; pHR, probable/possible high-risk genotypes; IARC, International Agency for Research on Cancer. Crude HPV prevalence calculated in 63 HPV positive patients: 25 single and 38 multiple infections.
Average age of woman was 35.3±8.4. The 87.2% were younger than 45 years old. Histological diagnoses of women were: CIN2 (n=55), CIN3-CIS (n=70). Data of HPV genotype were available in 120 women. HPV16 was detected in 67.5% (81/120) women. HR-HPV infection was detected in both partners in 50% (60/120). Among these infected couples, 62% (37/60) harbored at least one genotype in common. The HPV16 specific concordance was: 41.7% (25/60) couples were concordantly HPV16 positive and 18.3% (11/60) were concordantly HPV16 negative (kappa value: 0.21).
The proportion of women with the same genotype as their male partner was 58.7% (37/63). The proportion of men sharing the same genotype as their female partner was 30.8% (37/120), p<0.0001.
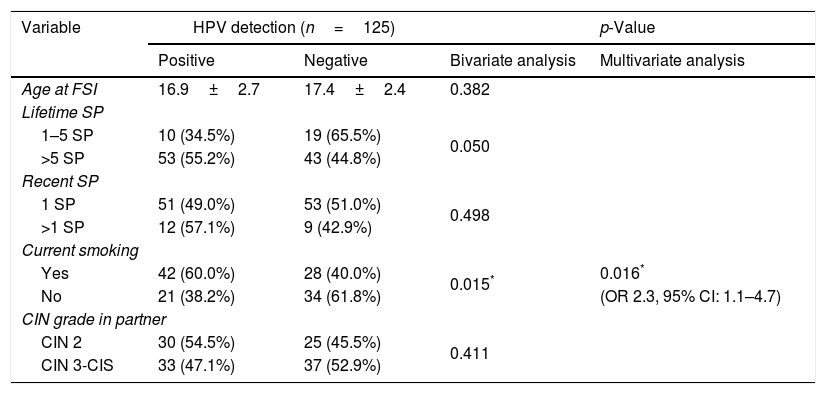
Epidemiological characteristics are shown in Table 2. Current smoking status was associated with an increased risk for HR-HPV infection in men: 38.2% (21/55) vs 60% (42/70), OR 2.3 (95% CI 1.1–4.7), p=0.016.
HPV detection in men according to epidemiological characteristics.
| Variable | HPV detection (n=125) | p-Value | ||
|---|---|---|---|---|
| Positive | Negative | Bivariate analysis | Multivariate analysis | |
| Age at FSI | 16.9±2.7 | 17.4±2.4 | 0.382 | |
| Lifetime SP | ||||
| 1–5 SP | 10 (34.5%) | 19 (65.5%) | 0.050 | |
| >5 SP | 53 (55.2%) | 43 (44.8%) | ||
| Recent SP | ||||
| 1 SP | 51 (49.0%) | 53 (51.0%) | 0.498 | |
| >1 SP | 12 (57.1%) | 9 (42.9%) | ||
| Current smoking | ||||
| Yes | 42 (60.0%) | 28 (40.0%) | 0.015* | 0.016* |
| No | 21 (38.2%) | 34 (61.8%) | (OR 2.3, 95% CI: 1.1–4.7) | |
| CIN grade in partner | ||||
| CIN 2 | 30 (54.5%) | 25 (45.5%) | 0.411 | |
| CIN 3-CIS | 33 (47.1%) | 37 (52.9%) | ||
FSI, first sexual intercourse; SP, sexual partners; CIN: cervical intraepithelial neoplasia; CIS, carcinoma in situ.
Age was expressed as mean±standard deviation.
The hypothesis of this study was that men sexual partners of women presenting HG-CIN may be at high risk for HPV infection; therefore this population could be an important HR-HPV reservoir. HR-HPV infection was prevalent in half male partners in this study. This data falls within the range reported in other studies for sexual partners of women with CIN (30–68%).8,16–19 Bosch et al. have reported 20 years before a low HPV prevalence (17.5%)7 in a similar population, that could be related with different sampling or HPV detection methods.20 Franceschi et al.21 showed the strongest variation by countries, with a higher prevalence of HPV infection among Brazilian SP of woman with CIN compared with those detected in other countries (Colombia, Mexico, Spain). Differences in HPV prevalence by population and country reported in review articles20,22 support that local epidemiological studies are necessary.
As found in previous reports22–24 age did not influence genital HR-HPV prevalence in this population of men younger than 45 years old.
In the present study, HPV16 was the most frequent genotype as found in men in literature.7,19,20,24–26 The most prevalent genotype in women was also HPV16, as reported in women with diagnosis of high-grade squamous intraepithelial cervical lesions, especially in women younger than 45 years old.27,28 Among HR-HPV, HPV52 was the second most frequent genotype in this population of women.
In terms of HR-HPV detection, the percentage of couples (50%) harboring HR-HPV is similar to that reported by others (32–65%).19,25,29,30 In couples where both members were HPV-positive, more than 60% were infected with one or more of the same HPV types. This level of concordance was observed before, independently of HPV prevalence and is consistent with the high transmissibility of HPV.23,25,29–31 Although HPV16 was detected in a high proportion of infected couples (more than 40%), it is remarkable that HPV16 specific concordance was weak, mainly due to other HPV genotypes detected in partners of women infected by HPV16. The proportion of women with the same genotype as their male partner was higher (almost double) than the proportion of men sharing the same genotype as their female partner. These findings suggest that the epithelial cells of the penile skin are more resistant to HPV infection than the cervical epithelium and the duration of HPV infection is shorter in men than in women.23,29
Limited data exist on the association between HPV infection and smoking in men. In this study current smoking could increase 2.3-fold the risk of HPV prevalent infection in males. These results are similar to the HIM study.1 At present, it is unclear how smoking may influence HPV infection in men, but many possible mechanisms exist. Smoking could potentially increase viral load by weakening the cellular immune response.32
Sexual behavior has been strongly associated with HPV infection and seropositivity in men.13 Features previously associated with HR-HPV were: young age at first sexual intercourse (FSI), a higher number of lifetime sexual partners (LSP) and a higher number of recent SP. In the National Questionnaire of Sexual Health, published by Spanish Government in 2009, it was found that mean age of FSI was 17–18 years old (29.3%) for Spanish men. In this study, younger age at FSI was not a risk factor for HPV infection as other authors have previously reported.18 There are contradictory data that could be attributable not only to the range of birth year of men but also to geographical characteristics.33
Having one or more SP in the preceding year was not related to HPV infection. This variable has been poorly evaluated. The risk of HPV reinfection between a monogamous couple is still a matter of debate.34 In contrast, Rombaldi et al.18 found a high association between both variables. Our data indicate that in this group a risk factor for HPV infection was not lifetime number of SP. Contradictory results about this association were reported.11,21,31
When lesions are not visible, sampling at multiple penile sites could increase the sensitivity of the HPV.11,30 Our sampling method, which involved the collection of cells from de skin of the glans penis, coronal sulcus and distal urethra was adequate for HPV identification and yielded a high proportion of beta-globin detection (100%).
Research has demonstrated that nonavalent, quadrivalent and bivalent HPV vaccines stimulate immunogenicity in males and females.35 On October 16, 2009, the US Food and Drug Administration (FDA) approved the use of quadrivalent vaccine in males 9–26 years old for the prevention of genital warts. Subsequently, the Advisory Committee on immunization Practices (ACID) declined to recommend the quadrivalent vaccine for routine immunization in men.36 Until 2009 HPV-vaccine programs have not been incorporated for girls and young women in Spain. No vaccinated women were included in this study. We assume that the faster way to achieve greatest protection for cervical cancer and its precursors is to vaccinate males as well as female because both genders contribute to the transmission of HPV-infection. Regardless of vaccination strategies adopted, efforts should be made to educate males about HPV and its health implications.
A limitation of the present study is the sample size. Unfortunately, no epidemiological data were provided for the female partners. Thus, we did not know life style habits of women (including sexual behavior) and the history of other sexually transmitted diseases which may influence the risk of infecting their male partners. At the moment, we have not results of the follow-up of the patients and their partners for their HPV status.
Further prospective and controlled studies are needed to know how to control viral transmission and to provide adequate counseling to HPV infected SP.
ConclusionsThe results of the present study suggest that, within healthy male population, sexual partners of women with high grade cervical lesions have a high prevalence of HR-HPV. Therefore this subpopulation could be maintaining the risk of viral transmission and consequently the risk of recurrence of cervical lesions in their couples. Additional studies focused on the relationship between HPV infected male and HPV reinfection and CIN relapse in their partners are necessary to support the introduction of preventive strategies as men follow up or men vaccination.
Authors’ contributionsELD carried out the collection in male, design of study data and drafted the manuscript. SPC performed HPV detection and participated in the study design. AIF carried out the collection in female. All authors read and approved the final manuscript.
Conflict of interestThe authors declare no conflict of interest.
We thank Vigo, Spain Research Unit for the statistical analysis and review of the manuscript. We also thank nursery team, especially Carmen Garcia and Carmen Lago, from the Urology Department of the University Hospital of Vigo, for their excellent help in this research, collecting patients. We thank M Consuelo Reboredo from the Gynecology Department and the laboratory technicians of the Microbiology Department of the University Hospital of Vigo, for their support of the study.