Tropheryma whipplei is the causative agent of Whipple disease. T. whipplei has also been detected in asymptomatic carriers with a very different prevalence. To date, in Spain, there are no data regarding the prevalence of T. whipplei in a healthy population or in HIV-positive patients, or in chronic fatigue syndrome (CFS). Therefore, the aim of this work was to assess the prevalence of T. whipplei in stools in those populations.
MethodsStools from 21 HIV-negative subjects, 65 HIV-infected, and 12 CFS patients were analysed using real time-PCR. HIV-negative and positive subjects were divided into two groups, depending on the presence/absence of metabolic syndrome (MS). Positive samples were sequenced.
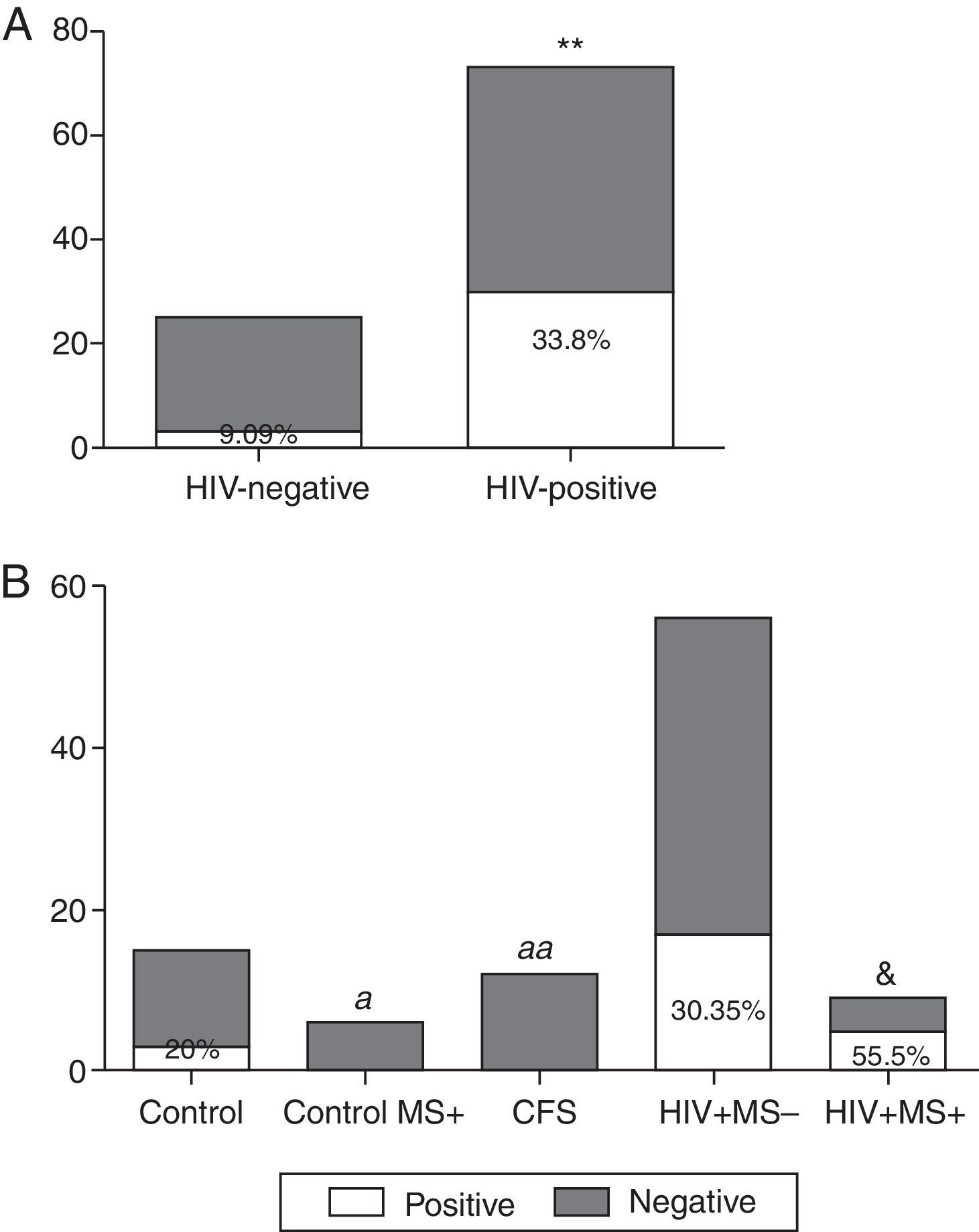
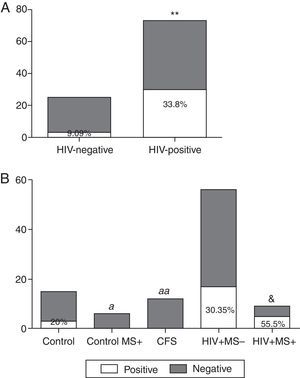
ResultsThe prevalence of T. whipplei was 25.51% in 98 stool samples analysed. Prevalence in HIV-positive patients was significantly higher than in HIV-negative (33.8% vs. 9.09%, p=0.008). Prevalence in the control group with no associated diseases was 20%, whereas no positive samples were observed in HIV-negative patients with MS, or in those diagnosed with CFS. The prevalence observed in HIV-positive patients without MS was 30.35%, and with MS it was 55.5%. The number of positive samples varies depending on the primers used, although no statistically significant differences were observed.
ConclusionsThere is a high prevalence of asymptomatic carriers of T. whipplei among healthy and in HIV-infected people from Spain. The role of T. whipplei in HIV patients with MS is unclear, but the prevalence is higher than in other populations.
Tropheryma whipplei es el agente etiológico de la enfermedad de Whipple. También se ha detectado, con diferentes prevalencias, en portadores asintomáticos. Hasta la fecha, en España, no hay datos sobre su prevalencia en población sana, en pacientes VIH o con síndrome de fatiga crónica (SFC). Por ello, el objetivo de este trabajo fue evaluar la prevalencia de T. whipplei en heces en dichas poblaciones.
MétodosSe analizaron heces de 21 sujetos VIH-negativos, de 65 pacientes VIH-positivos y de 12 con SFC mediante PCR a tiempo real. Los sujetos VIH-negativos y VIH-positivos se dividieron en 2 grupos dependiendo de la presencia/ausencia de síndrome metabólico (SM). Las muestras positivas se secuenciaron.
ResultadosDe 98 muestras analizadas, la prevalencia de T. whipplei fue del 25,51%. La prevalencia en pacientes VIH-positivos fue significativamente mayor que en los negativos (33,8% vs. 9,09%, p=0,008). Dentro del grupo control (no VIH) la prevalencia fue del 20% en el grupo sin patologías asociadas, mientras que no se observó ningún positivo en los que presentaban SM ni en los pacientes con SFC. La prevalencia en pacientes VIH-positivos sin SM fue del 30,35%, y del 55,5% en pacientes con SM. El número de muestras positivas varió dependiendo de las dianas utilizadas, aunque no se observaron diferencias significativas.
ConclusionesExiste una alta prevalencia de portadores asintomáticos de T. whipplei en individuos sanos y también en pacientes VIH. El papel de T. whipplei en pacientes VIH con SM no está claro, pero la prevalencia es más alta que en otras poblaciones.
Tropheryma whipplei, the etiologic agent of Whipple disease (WD), has been detected in asymptomatic carriers based, mainly, on stools and saliva analysis with very different prevalence1–11 (Supplemental Table 1). The carriage of T. whipplei varies considerably across studies and subjects. Many factors are involved in those differences; one of them is the geographical region of the studied subjects. The prevalence of asymptomatic carriers of T. whipplei in Africa is higher than in Europe7,11 and it is also higher in children than in adults.6 People expose to sewage,3 in contact with patients with WD8,12 or those with poor hygiene conditions,9 also present higher prevalences. Differences between the targets used for the PCR and the samples used are also observed and could explain these differences reported. Therefore, despite WD is rare, the high number of healthy carriers, the ubiquitous presence of the bacteria in the environment3,10,12,13 and the possibility of interhuman transmission6,8–10,12,14,15 make T. whipplei a common bacterium in humans.
There is a controversial issue between WD and AIDS.16 Some authors have described an impairment of the immune function (decreased T-helper/T-suppressor (CD4/CD8) cell ratio) during active disease.17–20 Nevertheless, this disease has not been often reported in HIV-positive subjects.16,19,21 Lozupone et al.,22 in an attempt to determine the relationship between immunodeficiency associated with HIV-infection and alterations of the lung microbiota, discovered a widespread lung colonization of T. whipplei in asymptomatic HIV-positive patients. They also described a decrease in the abundance of this bacterium after antiretroviral therapy.22 However, the potential pathogenic role of T. whipplei in HIV-infection is still unknown.
Chronic fatigue syndrome (CFS) is a debilitating and complex disorder of unknown aetiology. It is characterized by intense physical and mental fatigue, in addition to a set of nonspecific symptoms whose occurrence requires a reduction in the daily activities.23,24 The cause of CFS remains unknown, although since its original description, most of the study groups postulate an infectious origin.23,24 Although classical WD can be sometimes confused with other chronic inflammatory diseases,25 the potential involvement of T. whipplei in this syndrome (CSF) has not been studied yet.
George H. Whipple related Whipple's disease with a fat metabolism problem.26 Metabolic syndrome is a very frequent disorder in “healthy” but also in HIV people.27 Changes in gut microbiota have been associated with the onset of several disorders such as metabolic syndrome and obesity. However, and up to date, it is unknown if T. whipplei could be associated with the presence and development of this syndrome.
To investigate the prevalence of T. whipplei in stools in certain populations is of great interest since in some suggested algorithms for diagnosing WD, T. whipplei screening in stools is the starting point.28 In addition, it is possible that T. whipplei could be involved in several diseases of unknown origin (i.e. CFS) or to be part of the disorders associated with other conditions (i.e. HIV infection related disorders).
To the best of our knowledge, there is not any study carried out in Spain concerning the prevalence of T. whipplei in faeces. Thus, the aim of this study was to determine the prevalence of T. whipplei in stool samples from different WD asymptomatic populations in the North of Spain.
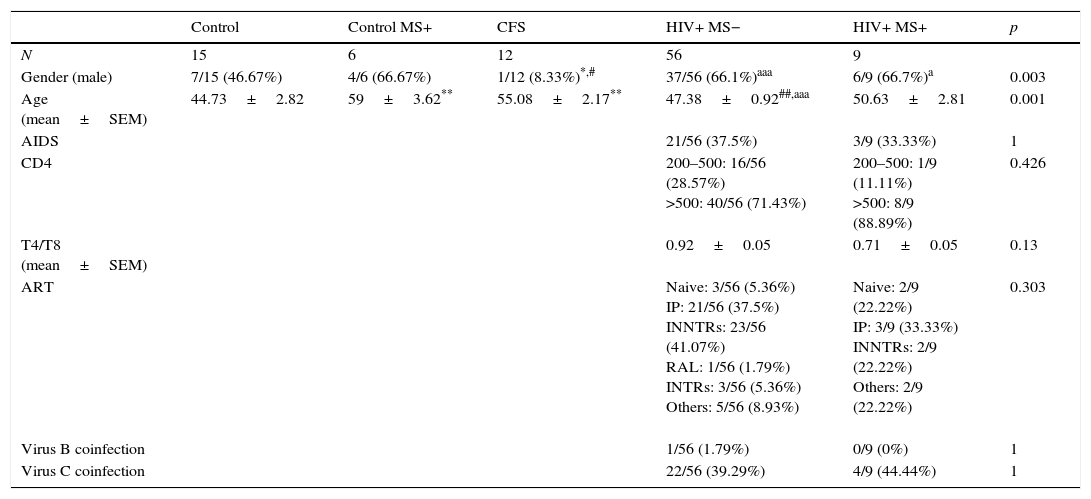
Subjects, materials and methodsStudy subjectsA total of 98 participants were included in this study. A stool sample was collected from each one. All participants were informed of the purpose of the study and a written consent was obtained from all of them. Demographic and epidemiological data, besides of a medical record, were obtained from their clinical history. Participants were divided into five groups (Table 1). The first one was composed of 15 healthy subjects (Control group, no pathologies associated). The second group consisted of six patients with metabolic syndrome (Control MS+). The third group was formed by 12 patients diagnosed of CFS. The used reference criteria28–30 were confirmed by personal interview by a specialized physician of the team. The last two groups were composed of 65 HIV-positive patients (viral load <50copies/mL). The HIV-positive patients of the fourth group (56 patients) did not have metabolic syndrome (HIV+; MS−), however, those in the fifth (n=9) were diagnosed of metabolic syndrome (HIV+; MS+). The diagnosis of MS was based on the definition provided by the NCEP-ATP III panel, which is most often used in clinical practice.31
Characteristics of the groups analysed in the study.
| Control | Control MS+ | CFS | HIV+ MS− | HIV+ MS+ | p | |
|---|---|---|---|---|---|---|
| N | 15 | 6 | 12 | 56 | 9 | |
| Gender (male) | 7/15 (46.67%) | 4/6 (66.67%) | 1/12 (8.33%)*,# | 37/56 (66.1%)aaa | 6/9 (66.7%)a | 0.003 |
| Age (mean±SEM) | 44.73±2.82 | 59±3.62** | 55.08±2.17** | 47.38±0.92##,aaa | 50.63±2.81 | 0.001 |
| AIDS | 21/56 (37.5%) | 3/9 (33.33%) | 1 | |||
| CD4 | 200–500: 16/56 (28.57%) >500: 40/56 (71.43%) | 200–500: 1/9 (11.11%) >500: 8/9 (88.89%) | 0.426 | |||
| T4/T8 (mean±SEM) | 0.92±0.05 | 0.71±0.05 | 0.13 | |||
| ART | Naive: 3/56 (5.36%) IP: 21/56 (37.5%) INNTRs: 23/56 (41.07%) RAL: 1/56 (1.79%) INTRs: 3/56 (5.36%) Others: 5/56 (8.93%) | Naive: 2/9 (22.22%) IP: 3/9 (33.33%) INNTRs: 2/9 (22.22%) Others: 2/9 (22.22%) | 0.303 | |||
| Virus B coinfection | 1/56 (1.79%) | 0/9 (0%) | 1 | |||
| Virus C coinfection | 22/56 (39.29%) | 4/9 (44.44%) | 1 |
Stool samples were collected from October 2013 through April 2014 in a sterile tube from each subject and processed in the first 24h for DNA extraction. DNA was frozen (−20°C) for the subsequent PCR analysis.
This study was performed following the Helsinki Declaration and was approved by the Committee for Ethics in Clinical Research of La Rioja (CEICLAR).
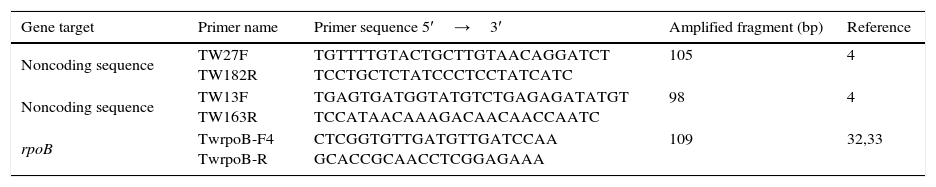
Real time PCR for T. whipplei from stoolDNA extraction of the samples was obtained according with the manufacturer's recommendations for the QIAamp DNA MiniKit (Qiagen). The three pairs of primers used and their conditions are shown in Table 2. Two noncoding sequences repeated several times in the genome of the bacterium were tested as targets4: primers TW27F and TW182R which amplify 105pb and primers TW13F and TW163R which amplify 98pb. To amplify a 109pb segment of the rpoB gene of T. whipplei, primers TwrpoB-F4 and TwrpoB-R were used.32,33 All the samples were analysed in duplicate in an Applied Biosystems 7300 fast real time PCR system. Negative controls containing water instead of template DNA as well as a positive control of T. whipplei were included in all PCR assays. Amplification products were sequenced, and nucleotide sequences were compared with those available in GenBank by using a Basic Local Alignment Search Tool (BLAST) search (http://www.ncbi.nlm.nih.gov/blast). Only samples were considered positive if, at least, two of the targets were positive.
PCR primer pairs used in this study.
| Gene target | Primer name | Primer sequence 5′→3′ | Amplified fragment (bp) | Reference |
|---|---|---|---|---|
| Noncoding sequence | TW27F TW182R | TGTTTTGTACTGCTTGTAACAGGATCT TCCTGCTCTATCCCTCCTATCATC | 105 | 4 |
| Noncoding sequence | TW13F TW163R | TGAGTGATGGTATGTCTGAGAGATATGT TCCATAACAAAGACAACAACCAATC | 98 | 4 |
| rpoB | TwrpoB-F4 TwrpoB-R | CTCGGTGTTGATGTTGATCCAA GCACCGCAACCTCGGAGAAA | 109 | 32,33 |
To compare the positive samples obtained from each group, a Pearson Chi-Square or Fisher's Exact Test were used. Kruskal Wallis and T-student analyses have been used to analyse the differences of the quantitative parameters of the characteristics of the studied groups (Table 1). SPSS 17.0 software has been used for these statistical analyses.
ResultsCharacteristics of the studied groups are shown in Table 1. A total of 98 stool samples from 98 subjects were analysed. Twenty-five stool samples (25.51%) of the total had a positive result for T. whipplei. The analysed sequences showed highest identity (98–100%) with sequences of T. whipplei available in GenBank (GenBank accession no. AE014184). Of 33 HIV-negative subjects, 3 (9.09%) were positive compared with the HIV-positive group in which 22 of 65 (33.8%) were positive (Fig. 1A). When comparing the positive samples in both groups (HIV negative and positive, respectively), a statistically significant difference was observed (p=0.008). In the control group, 3 of 15 samples (20%) showed positive results (Fig. 1B). However, in the Control MS+ group as well as in the CFS group, no positive results were observed (Fig. 1B). The HIV-positive patients were divided into two smaller groups depending on the presence or absence of metabolic syndrome. Comparing those HIV-positive patients without metabolic syndrome (VIH+ MS− group), 17 of 56 (30.35%) had a positive result, while if metabolic syndrome was associated (VIH+ MS+ group), the percentage of positive samples increased: five of nine (55.5%) (Fig. 1B). The group of HIV+ MS+ presented the higher percentage of positive samples and a significant difference was observed when comparing this percentage with the absence of positive samples in the Control MS+ and CFS groups (p=0.04 and p=0.006, respectively). A slightly difference was also observed between the HIV+ MS+ group and the control subjects (p=0.07).
Prevalence of T. Whipplei. (A) Positive and negative samples from the total of HIV-positive patients and HIV-negative patients. (B) Positive and negative samples in the HIV-negative group (healthy subjects), in HIV-negative patients with metabolic syndrome (control MS+), CFS patients, HIV-positive patients without metabolic syndrome associated (VIH+ MS− group) and HIV-positive patients with metabolic syndrome (VIH+ MS+ group). **p<0.01 and &p=0.07 vs. HIV-negative subjects. ap<0.05, aap<0.01 vs. HIV+ MS+ subjects.
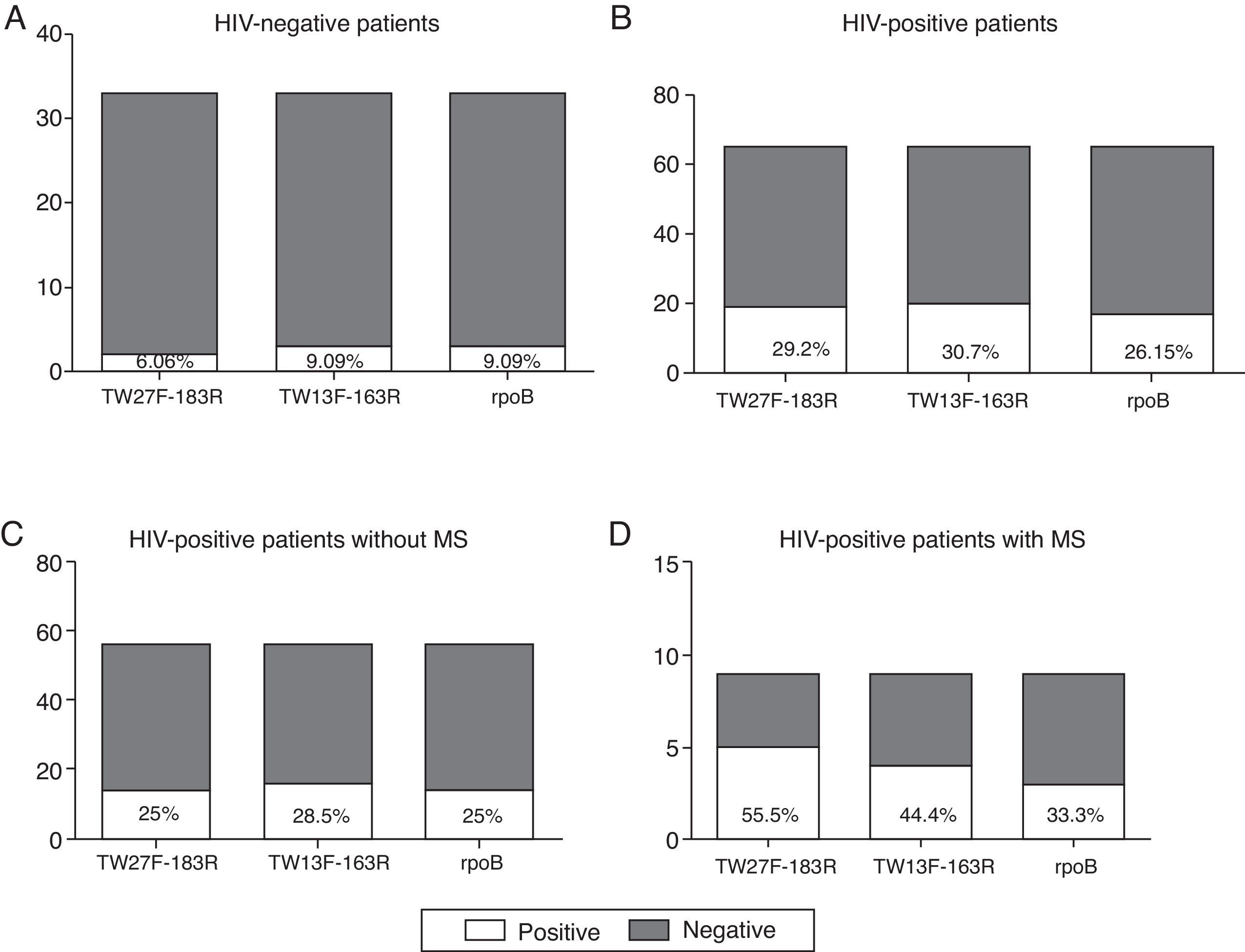
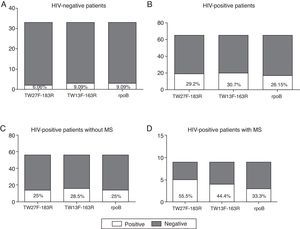
As can be observed in Fig. 2A–D, the number of positive samples varies depending on the pair of primers used, although not statistically significant differences were observed. Based on the positive results obtained in the non-HIV infected patients (Fig. 2A), both the TW13F-163R and the rpoB primers are more sensitive than the TW27F-183R primers, as they were able to identify more samples as positive (9.09% vs. 6.06%). However, in the HIV-infected population, the TW13F-163R pair seems to be the most sensitive (Fig. 2B–D) and, in contrast with the results obtained in the control group, the primers that amplify a region of the rpoB gene were the least sensitive.
Comparative study of the primers used. (A) Positive and negative samples from the total of HIV-negative patients. (B) Positive and negative samples from the total of HIV-positive patients. (C) Positive and negative samples from HIV-positive patients without metabolic syndrome associated (VIH+ MS− group). (D) Positive and negative samples from HIV-positive patients with metabolic syndrome associated (VIH+ MS+ group).
This is the first study concerning the prevalence of T. whipplei in faeces carried out in Spain. The global prevalence of T. whipplei infection in our study was 25.51%. When only WD asymptomatic healthy patients were analysed (those with no pathologies associated, n=15), the prevalence was slightly lower, 20%. This percentage is quite similar than that observed in saliva in previous studies.1,34 However, other studies failed to find such prevalence and one of the reasons could be that the primers used in the saliva analyses also amplified Eikenella corrodens and Actinomyces odontolyticus DNA.4,35 However, the primers used in our study are similar than those used by Fenollar et al.,4,5 and despite this fact, the prevalence found in our study was still higher than the percentage observed in the French studies (4% and 2.3%, respectively). One plausible reason for that disparity could be the regional differences that have also been observed when compared countries from Africa against those from Europe. As no studies have been carried out in our country, it is very difficult to compare our results with other Spanish regions or with other countries from Europe except for France and Switzerland. It is also important to highlight that we obtained a prevalence of 9.09% when the control group also included patients diagnosed with CSF or MS. This number is closer to the French values and also to the prevalence observed in Switzerland.2 Thus, one explanation for the disparities in prevalence's could be due to the characteristics and recruitment of the subjects considered as “controls-healthy people”.
Until recently, T. whipplei had only been involved in classic WD, but the use of molecular tools has enabled to involve T. whipplei in other common acute disorders such as pneumonia and acute diarrhoea among others. It has also enabled to increase the number of endocarditis cases diagnosed.36 In this context and to our knowledge, this is the first survey carried out in stools from CSF patients in the world and one of the fewest carried out in HIV-positive patients. We have excluded false positive results since we have used different targets and, for accepting a positive result, the sample must have had necessary, at least, a positive result with two different targets and subsequent sequencing. Obviously, the objective of this study was not to analyse the sensitivity of the different primers used. However, one of the potential factors that could explain the different prevalence's of T. whipplei presence in asymptomatic subjects is the lack of a validated pair of primers for the specific amplification of T. whipplei. Thus, it would be of interest to check which one of the three targets used in this study is able to find out more number of positive samples for T. whipplei, in an attempt to complete the information herein showed. Although slightly differences were observed among the three pairs, no statistical differences were obtained, suggesting a similar utility for this purpose.
It is remarkable the high prevalence found in the HIV-positive group (33.8%). We know that microbiota is affected by several factors37 including HIV infection.38,39 We also know that antiretroviral agents (ARV) can disturb the microbiota.40,41 In our HIV studied series, all but four patients were on ARV treatment. The series is small for concluding that ARV treatment or HIV itself are the responsible for the high prevalence found, but it is a fact that HIV-positive patients under ARV treatment have a significant high prevalence. None of our HIV-positive patients have clinical symptoms related with WD. Metabolic syndrome associated with HIV-infection, but also MS in the general population, is very common27 and it is unknown if T. whipplei could be associated with this disorder. In our study, the prevalence of T. whipplei in HIV-positive patients with MS was higher (55%) than in HIV-positive patients without this syndrome (30.35%) or in comparison with healthy people with this syndrome (0%). Thus, could be T. whipplei infection a risk factor for developing metabolic syndrome in HIV patients? To date, we cannot answer this question yet and it will be necessary to perform new and larger studies to confirm this association, but it must be remembered that originally, George H. Whipple describes the illness of WD as deposits of fat and fatty acids in the intestinal and mesenteric lymphatic tissues.26 Finally, we have not found any relationship between CFS and T. whipplei infection.
In conclusion, there is a high prevalence of T. whipplei infection in healthy and overall in the collective of HIV-infected people. We do not know the role of T. whipplei infection in the development of metabolic syndrome in HIV patients but it is a fact that the prevalence is much higher than in other studied population. Further studies are needed in order to investigate the dynamics of T. whipplei in those patients with positive results in order to find out if the bacterium spontaneously disappear or, otherwise, the clinical consequences of its continued presence in faeces.
Conflicts of interestNone.
Ethical approvalAll procedures in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consentInformed consent was obtained from all individual participants included in the study.
The authors thank E. Recio-Fernández and M.J. Villanueva-Millán for their excellent technical help. This study has been supported by GILEAD Fellowship GLD13-00094, SEINORTE foundation (Spain) and Fundación Rioja Salud (Spain).