The concept of prebiotics, probiotics, and symbiotics and their use in different situations of daily clinical practice related to clinical nutrition is reviewed, as well as their role in the treatment/prevention of diarrhea (acute, induced by antibiotics, secondary to radiotherapy), inflammatory bowel disease (ulcerative colitis and pouchitis), in colonic health (constipation, irritable bowel), in liver disease (steatosis and minimum encephalopathy), and in intensive care, surgical, and liver transplantation. While their effectiveness for preventing antibiotic-induced diarrhea and pouchitis in ulcerative colitis appears to be shown, additional studies are needed to establish recommendations in most clinical settings. The risk of infection associated to use of probiotics is relatively low; however, there are selected groups of patients in whom they should be used with caution (as jejunum infusion).
Se revisa el concepto de prebióticos, probióticos y simbióticos y su empleo en diferentes situaciones de la práctica clínica diaria relacionados con la nutrición clínica. Se analiza su papel en el tratamiento y/o prevención de la diarrea (aguda, por antibióticos, rádica), en la enfermedad inflamatoria intestinal (colitis ulcerosa y reservoritis), sobre la salud colónica (estreñimiento, intestino irritable), hepatopatías (esteatosis y encefalopatía mínima), en pacientes de cuidados intensivos, quirúrgicos y sometidos a trasplante hepático. Si bien parece demostrada su eficacia en la prevención de la diarrea por antibióticos y en la reservoritis en la colitis ulcerosa, son necesarios más estudios para poder establecer recomendaciones en la mayoría de escenarios clínicos. El riesgo de infección asociado al uso de probióticos es relativamente bajo; no obstante, existen grupos seleccionados de pacientes en los que se recomienda emplearos con cautela (como la infusión a nivel yeyunal).
The luminal surface of the bowel contains billions of living microorganisms in a number approximately ten times higher than the number of cells in an adult person. Most of them are located in the colon, where certain bacterial species reside. The human bowel is, therefore, a true ecosystem essential for the efficient absorption of nutrients and for the maintenance of health in general. In a Persian version of the Old Testament, the longevity of Abraham is attributed to the consumption of “sour milk”. As early as 76 AD, the Roman historian Pliny recommended fermented dairy products for the treatment of gastroenteritis. In 1908, the Nobel Prizewinner Elie Metchnikoff attributed the longevity of some Balkan populations to their regular consumption of fermented dairy products containing lactobacilli that “reduce the toxins produced by intestinal bacteria, promoting health and prolonging life”. At the beginning of the 20th century, it was reported that the bacterium Lactobacillus acidophilus was able to survive in the human bowel.
It appears that hominids started to use lactic acid fermentation of plant foods approximately 1.5 million years ago. This was a common practice in Europe until the industrial revolution, and continues to be regularly used by various African communities because it is a safe and simple way to preserve food. The intake of fermented dairy products by humans possibly started more recently (some 10,000 years ago). Over time, the hominid gastrointestinal tract gradually adapted to a generally high daily provision of live lactic acid bacteria. This type of food stopped being eaten in industrialized countries during the 20th century, which may have caused different gastrointestinal and immunological problems. In the 1980s, it was postulated that some indigestible components of the diet could promote the growth of certain bacterial strains present in the bowel which are associated with benefits for health.1
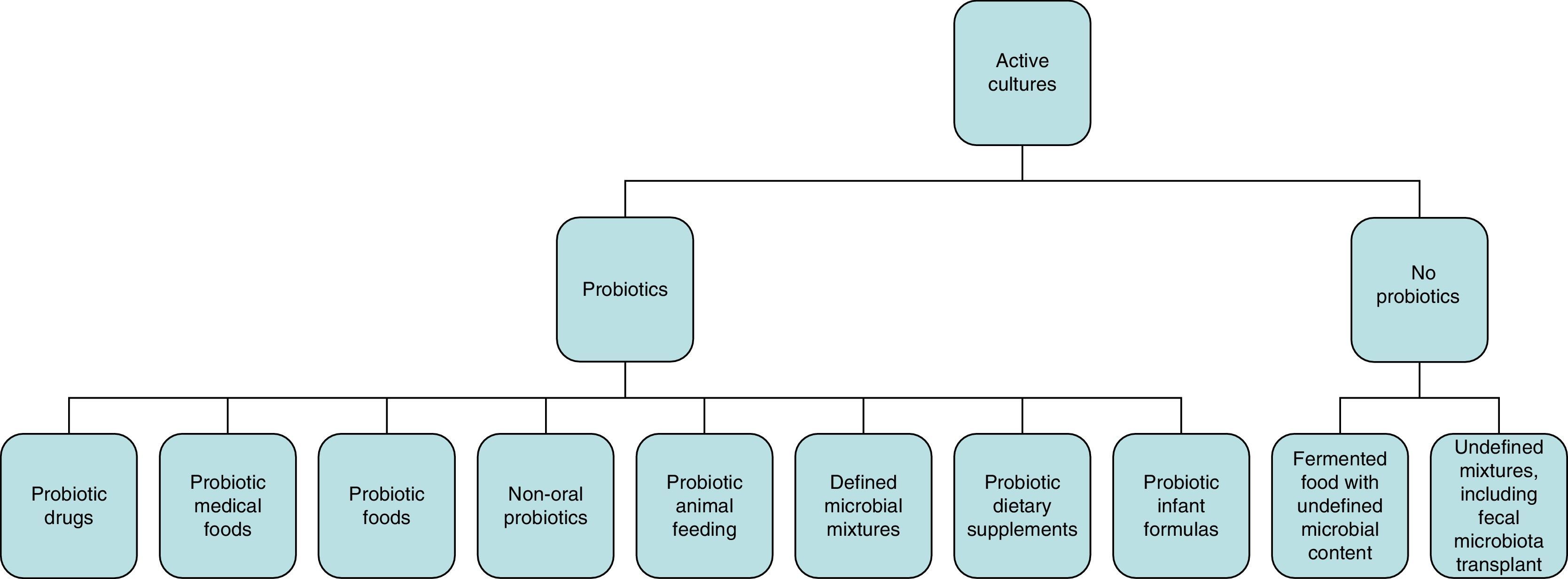
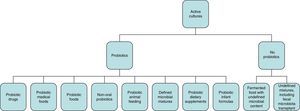
Concept of probiotics, prebiotics, and symbioticsWhile the initial definition of probiotics proposed in 1965 referred to substances secreted by microorganisms that stimulate the growth of others (in contrast to “antibiotics”),2,3 the WHO definition of “probiotic” refers to live microorganisms which, when administered in adequate amounts, have a beneficial effect on the health of the host.4 According to the International Scientific Association for Probiotics and Prebiotics, the spectrum of products and preparations that may be considered as probiotics is very wide (Fig. 1) and extends from probiotic drugs (e.g. VSL#3), foods for special medical uses with probiotics (e.g. enteral nutrition with probiotics), probiotic foods (e.g. fermented milk with studies showing benefits for health), and infant formulas (e.g. milk powders) to non-orally administered probiotics (e.g. vaginal). To be considered a probiotic, studies should have been conducted in humans effectively showing the specific health benefits of specific strains (e.g. Lactobacillus rhamnosus GG; not only of the genus Lactobacillus or the species rhamnosus).3 Fermented food containing live organisms often does not meet the concept of probiotics if its effects have not been specifically studied and/or the amount it contains is not known. By contrast, some fermented food products such as yoghurt can be considered in some circumstances as probiotics based on some specific effects. For example, if there is evidence that they improve lactose digestion in subjects with lactose intolerance; the benefits do not only depend on the lower lactose content of the product, but also on the fact that probiotic bacteria can also increase lactase activity in the small bowel.
Spectrum of preparations and administration forms that meet the criterion of probiotics for use in clinical practice.
Fecal transplant or foods with dead microorganisms are not considered as probiotics.2
To produce the beneficial effects in the host, probiotics do not need to colonize the target organ, but it should be reached by a sufficient number of live microorganisms so as to affect its microecology and metabolism. Thus, most probiotic strains are able to reach the colon alive (in a variable percentage) after passing through the upper gastrointestinal tract, and their viability depends on many factors: intrinsic probiotic factors on the one hand and, on the other, host-dependent factors such as, for example, stomach acidity, the length of acid exposure, the concentration and duration of exposure to bile salts, and others.5 To give an example, the strains Lactobacillus casei Shirota, L. rhamnosus GG (ATCC 53103), Lactobacillus johnsonii LA1, and L. acidophilus NFCB 1748 have been shown to be safe and to have benefits for health in humans, and also to have high stability in acid media and to be resistant to bile acids; however, only the last three strains are able to adhere to the mucosa and only L. rhamnosus GG and johnsoniiLA1 induce colonic colonization.6
The term “prebiotic” refers to selectively fermented ingredients which result in specific changes in composition and/or the activity of gastrointestinal flora, with consequent health benefits for the host.3 This definition overlaps in part with that of dietary fiber, but adds the selectivity of prebiotics for some specific microorganisms (e.g. the intake of fructooligosaccharides and inulin selectively favors bifidobacteria).
“Dietary fiber” is a broader term that refers to various carbohydrates and lignin, which resist hydrolysis by human digestive enzymes, but may be fermented by colonic microflora and/or partly excreted in feces. This definition includes within the concept of fiber non-starch polysaccharides (celluloses, hemicelluloses, pectins, gums, and mucilages), inulin, fructooligosaccharides, galactooligosaccharides, and resistant starch (starch and starch degradation products, which are not digested in the small bowel of healthy individuals).7 Some of these components of fiber strictly meet the criteria necessary for them to be considered as prebiotics (inulin, fructooligosaccharides, galactooligosaccharides, soy-derived oligosaccharides, xylooligosaccharides, pyrodextrins, and isomaltooligosaccharides). Other components of fiber are difficult to classify. To give an example, guar gum, a type of fermentable soluble fiber, partly promotes the growth of probiotic bacteria, but also acts as a general (non-specific) substrate for colonic bacteria (“fermentable colonic food”), and cannot, therefore, be defined as “prebiotic” in the strict sense of the word. Similarly, some fractions of resistant starch specifically act as prebiotics, while others simply act as “fermentable colonic food” for saccharolytic bacteria. This review will mainly focus on the use of prebiotics (in the strict sense of the word), although some references will be made to the effects of (fermentable) fiber in specific conditions.
Indigestible carbohydrates are fermented in the colon to short chain fatty acids (SCFAs), mainly acetate, propionate and butyrate, and many other metabolites and gases. SCFAs acidify luminal pH, which suppresses the growth of some pathogens and has an influence on bowel motility. On the other hand, they are absorbed by colonic mucosa and contribute to the provision of energy to the host. Acetate is mainly metabolized in muscle, kidneys, heart, and brain. Propionate undergoes metabolism in the liver and is a neoglucogenic substrate that may inhibit cholesterol synthesis and regulate lipogenesis in adipose tissue. Butyrate is mainly metabolized by the colonic epithelium, where it acts as a preferential substrate and regulates cell growth and differentiation by different mechanisms. Among other effects, it can, for example, reduce colon cancer by stimulating apoptosis and improving inflammation in inflammatory bowel disease. In vivo measurement of SCFAs is difficult. There are, however, in vitro models that allow SCFA production to be assessed. For example, some wheat dextrins produce less gas than hydrolyzed guar gum and inulin upon fermentation, which suggests that dextrins may be better tolerated in vivo than the other two fibers. By contrast, inulin appears to produce significantly more butyrate than modified maltodextrins, while guar gum has an intermediate behavior.8 Some prebiotic fibers could therefore be preferentially used, at least in theory, as treatment for some conditions, based on their colonic metabolism.
The term “symbiotic” refers to products that contain both probiotics and prebiotics. Strictly speaking, this term should be reserved for products in which the prebiotic component selectively favors the probiotic component (e.g. oligofructose and bifidobacteria, but not oligofructose with Lactobacillus (L) casei; however, if synergy is widely understood, this last combination is possible).1
Table 1 lists the main probiotics, prebiotics, and symbiotics used in clinical practice. Table 2 shows the trade names and manufacturers of the main prebiotics marketed worldwide. We will preferentially focus on studies conducted with probiotics, prebiotics, and symbiotics (especially randomized, controlled studies) in which outcome variables are clinically important (studies assessing pathophysiological aspects will not be discussed, with some exceptions) and which are related (directly or indirectly) to clinical nutrition and dietetics (i.e. any potential effects on the immune and clinical response in atopy or vaginitis will not be addressed). Table 3 summarizes the main effects sought with the use of prebiotics, probiotics, and symbiotics in clinical practice.
Main probiotics, prebiotics, and symbiotics used in clinical studies.
| Main single-strain probiotics used in clinical studies |
| • Saccharomyces boulardii |
| • Lactobacillus rhamnosus GG |
| • Bifidobacterium bifidum |
| • Lactobacillus plantarum 299 |
| • Lactobacillus sporogens |
| • Enterococcus SF68 |
| • Bifidobacterium lactis BB12 (L) |
| • Lactobacillus reuteri |
| • Lactobacillus casei (L) |
| • Bifidobacterium longum BB 536 (L) |
| • Lactobacillus acidophilus LA1 |
| • Escherichia coli Nissle 1917 (serotype 06:K5:H1) |
| Main multistrain probiotics used in clinical studies |
| • Lactobacillus acidophilus and L. bulgaricus |
| • Lactobacillus acidophilus and Bifidobacterium lactis |
| • Lactobacillus acidophilus and Bifidobacterium infantis |
| • Bifidobacterium longum BB 536+L. acidophilus NCFB 1748 (L) |
| • Bifidobacterium lactis Bb12 (BB12) and Lactobacillus rhamnosus GG |
| • Bifidobacterium bifidum+Streptococcus thermophilus |
| • Bifidobacterium lactis and Streptococcus thermophilus (L) |
| • Lactobacillus acidophilus+L. bulgaricus+Streptococcus thermophilus (L) |
| • VSL#3: 4 lactobacillus strains (Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus delbrueckii subspecies bulgaricus and Lactobacillus plantarum), 3 bifidobacterium strains (Bifidobacterium longum, Bifidobacterium infantis, Bifidobacterium breve) and Streptococcus salivarius subspecies thermophilus |
| • TREVIS: Streptococcus acidophilus, LA5, Bifidobacterium lactis BP12, Streptococcus thermophilus and Lactobacillus bulgaricus |
| • Ecologic 641: 4 lactobacilli (Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus salivarius and Lactococcus lactis) and 2 bifidobacteria (Bifidobacterium bifidum and Bifidobacterium lactis) |
| • Ergyphilus: 1010Lactobacillus rhamnosus GG, Lactobacillus casei, Lactobacillus acidophilus and Bifidobacterium bifidus |
| • Jinshuangqi: Bifidobacterium longum>10UFC, Lactobacillus bulgaricus>10CFU, and Streptococcus thermophilus>10CFU |
| Main prebiotics used in clinical studies |
| • Fructooligosaccharides (FOS) |
| • Galactooligosaccharides (GOS) |
| • Inulin |
| • Trans-galactooligosaccharides (TOS) |
| • Beneo Synergy 1 (SYN1): oligofructose-inulin |
| • Lactulose |
| • Oat fibera |
| • Germinated barley (rich in hemicellulose)a |
| • Hydrolyzed guar guma |
| • Resistant starcha |
| • Plantago ovataa |
| • Beta glucana |
| • Pectina |
| Main symbiotics used in clinical studies |
| • Lactobacillus plantarum 299 and 10g of oat fiber |
| • Lactobacillus sporogens+Fructooligosaccharides |
| • Synbiotic 2000: 101CFU of each of the following: Pediococcus pentoseceus 5–33:3, Leuconostoc mesenteroides 32–77:1, Lactobacillus paracasei sp. paracasei 19, Lactobacillus plantarum 2362 and 2.5g each of beta glucans, inulin, pectin, and resistant starch |
| • Synbiotic 2000 Forte: 101CFU of each of the following: Pediococcus pentoseceus 5–33:3, Leuconostoc mesenteroides 32–77:1, Lactobacillus paracasei sp. paracasei 19, Lactobacillus plantarum 2362 and 2.5g each of inulin, oat fiber, pectin, and resistant starch |
| • Oligofructose+inulin (SYN1)+Lactobacillus rhamnosus GG and Bifidobacterium lactis Bb12 |
| • Golden Bifid: Bifidobacterium bifidum, Lactobacillus bulgaricus and Streptococcus thermophilus with FOS |
L: these are probiotics provided in dairy products.
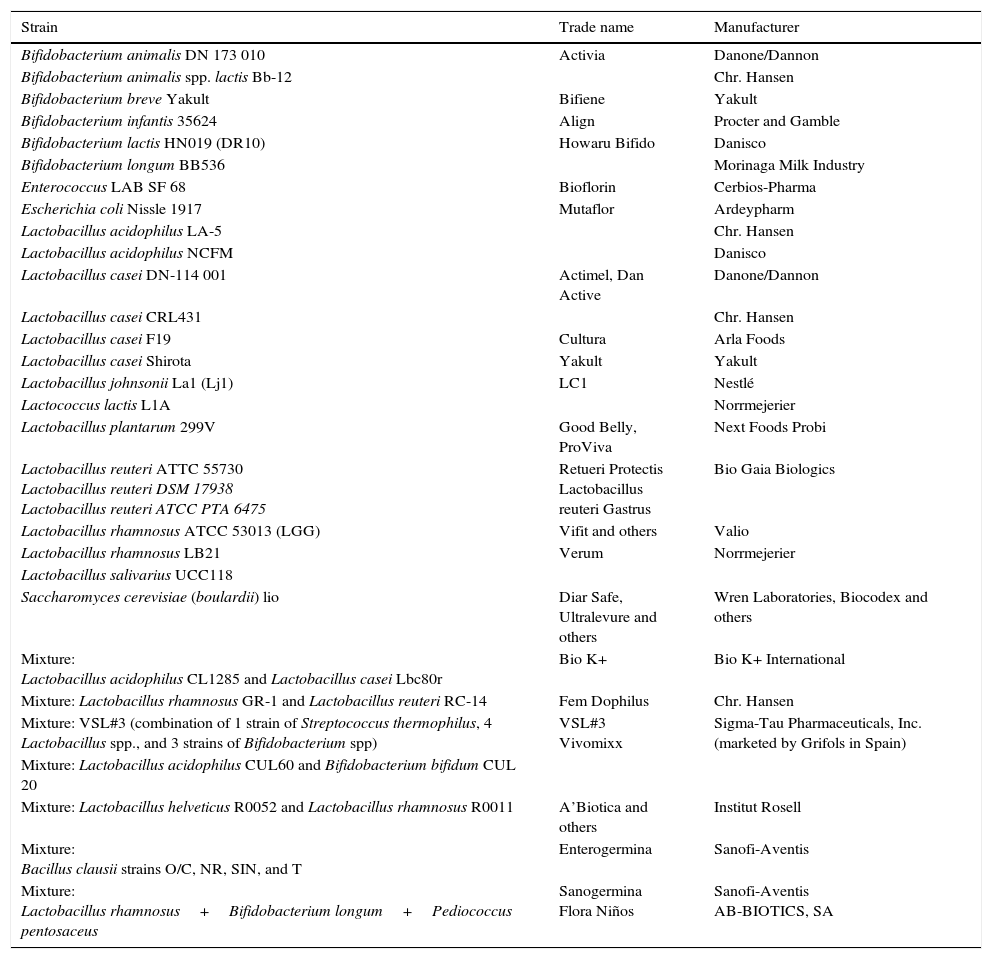
Examples of trade names and manufacturers of probiotics.
| Strain | Trade name | Manufacturer |
|---|---|---|
| Bifidobacterium animalis DN 173 010 | Activia | Danone/Dannon |
| Bifidobacterium animalis spp. lactis Bb-12 | Chr. Hansen | |
| Bifidobacterium breve Yakult | Bifiene | Yakult |
| Bifidobacterium infantis 35624 | Align | Procter and Gamble |
| Bifidobacterium lactis HN019 (DR10) | Howaru Bifido | Danisco |
| Bifidobacterium longum BB536 | Morinaga Milk Industry | |
| Enterococcus LAB SF 68 | Bioflorin | Cerbios-Pharma |
| Escherichia coli Nissle 1917 | Mutaflor | Ardeypharm |
| Lactobacillus acidophilus LA-5 | Chr. Hansen | |
| Lactobacillus acidophilus NCFM | Danisco | |
| Lactobacillus casei DN-114 001 | Actimel, Dan Active | Danone/Dannon |
| Lactobacillus casei CRL431 | Chr. Hansen | |
| Lactobacillus casei F19 | Cultura | Arla Foods |
| Lactobacillus casei Shirota | Yakult | Yakult |
| Lactobacillus johnsonii La1 (Lj1) | LC1 | Nestlé |
| Lactococcus lactis L1A | Norrmejerier | |
| Lactobacillus plantarum 299V | Good Belly, ProViva | Next Foods Probi |
| Lactobacillus reuteri ATTC 55730 Lactobacillus reuteri DSM 17938 Lactobacillus reuteri ATCC PTA 6475 | Retueri Protectis Lactobacillus reuteri Gastrus | Bio Gaia Biologics |
| Lactobacillus rhamnosus ATCC 53013 (LGG) | Vifit and others | Valio |
| Lactobacillus rhamnosus LB21 | Verum | Norrmejerier |
| Lactobacillus salivarius UCC118 | ||
| Saccharomyces cerevisiae (boulardii) lio | Diar Safe, Ultralevure and others | Wren Laboratories, Biocodex and others |
| Mixture: Lactobacillus acidophilus CL1285 and Lactobacillus casei Lbc80r | Bio K+ | Bio K+ International |
| Mixture: Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 | Fem Dophilus | Chr. Hansen |
| Mixture: VSL#3 (combination of 1 strain of Streptococcus thermophilus, 4 Lactobacillus spp., and 3 strains of Bifidobacterium spp) | VSL#3 Vivomixx | Sigma-Tau Pharmaceuticals, Inc. (marketed by Grifols in Spain) |
| Mixture: Lactobacillus acidophilus CUL60 and Bifidobacterium bifidum CUL 20 | ||
| Mixture: Lactobacillus helveticus R0052 and Lactobacillus rhamnosus R0011 | A’Biotica and others | Institut Rosell |
| Mixture: Bacillus clausii strains O/C, NR, SIN, and T | Enterogermina | Sanofi-Aventis |
| Mixture: Lactobacillus rhamnosus+Bifidobacterium longum+Pediococcus pentosaceus | Sanogermina Flora Niños | Sanofi-Aventis AB-BIOTICS, SA |
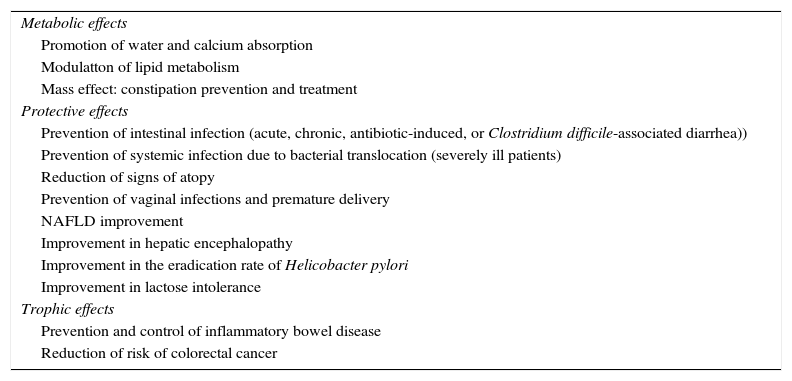
Expected effectsa of prebiotics, probiotics, and symbiotics in clinical practice.
| Metabolic effects |
| Promotion of water and calcium absorption |
| Modulatton of lipid metabolism |
| Mass effect: constipation prevention and treatment |
| Protective effects |
| Prevention of intestinal infection (acute, chronic, antibiotic-induced, or Clostridium difficile-associated diarrhea)) |
| Prevention of systemic infection due to bacterial translocation (severely ill patients) |
| Reduction of signs of atopy |
| Prevention of vaginal infections and premature delivery |
| NAFLD improvement |
| Improvement in hepatic encephalopathy |
| Improvement in the eradication rate of Helicobacter pylori |
| Improvement in lactose intolerance |
| Trophic effects |
| Prevention and control of inflammatory bowel disease |
| Reduction of risk of colorectal cancer |
The consensus of the International Scientific Association for Probiotics and Prebiotics includes a number of potential mechanisms of action, ranging from some which are common to most probiotics studied to very rare mechanisms specific to only some strains:
Very common mechanisms (shared by most probiotics)Resistance to colonization.
Production of short chain fatty acids and acidification of the medium.
Regulation of gastrointestinal transit.
Normalization of microbiota.
Increased enterocyte regeneration.
Competitive exclusion of pathogens.
Vitamin synthesis.
Direct antagonism of other bacteria.
Reinforcement of intestinal barrier.
Bile salt metabolism.
Enzymatic activities.
Neutralization of carcinogens.
Neurological effects.
Immunological effects.
Endocrine effects.
Production of bioactive substances.
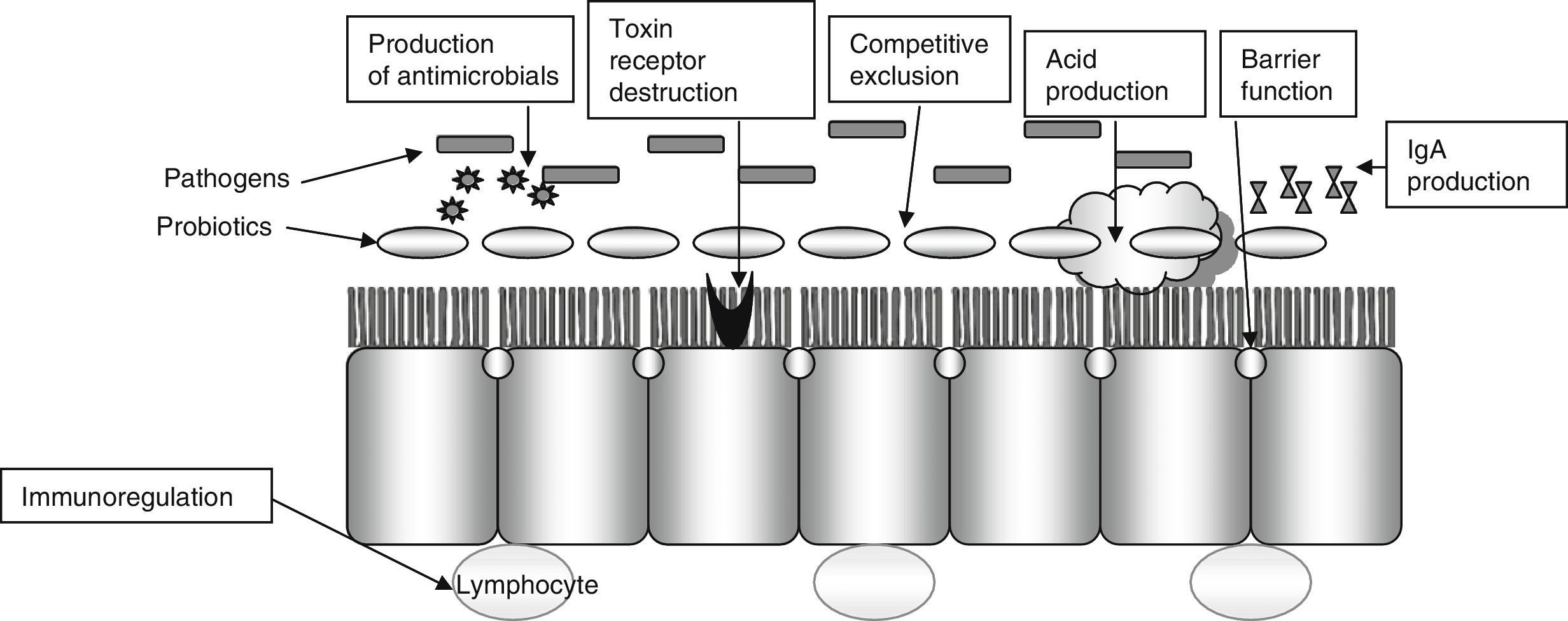
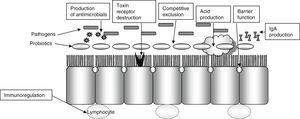
Diarrhea induced by antibiotics is a very common complication in the hospital setting (13–60%). Disease caused by Clostridium difficile is also a significant cause of nosocomial diarrhea and colitis that prolongs the hospital stay by 3–7 days and increases the risk of new nosocomial infections (20–65%), costs, and mortality (2- or 3-fold depending on studies). In these patients, the use of probiotics is intended to restore intestinal microflora, increase immune response, compete with pathogenic bacteria, and remove their toxins (Fig. 2).
Saccharomyces boulardii has been one of the most widely studied probiotics. In a recent meta-analysis of 21 studies (4780 patients), the administration of S. boulardii decreased the risk of antibiotic-induced diarrhea in both children and adults from 19% to 8.5%, with a relative risk of 0.47. The number that needed to be treated in order to prevent one case was 10 people. In some studies, it also decreased diarrhea induced by Clostridium difficile, but significance was only found in children9. Its use may thus be strongly recommended, based on a moderate level of evidence. S. boulardii should be started at the same time as antibiotic treatment at doses ranging from 250 to 1000mg in adults (maximum 500mg in children) and maintained at least until antibiotic therapy is completed (it is not clear whether it should be continued for some time after its conclusion).
In another meta-analysis of 82 randomized trials using different species (usually Lactobacillus, alone or combined with bifidobacteria, enterococci, or S. boulardii), a decreased risk of antibiotic-induced diarrhea was also found, with a relative risk of 0.58, and 13 subjects needed to be treated in order to prevent one case. Although positive results were generally seen with all the probiotics used, it is recognized that there is a significant heterogeneity in the studies, so that the evidence is not sufficient to state whether the effect varies systematically depending on the population (adults or children), the type or duration of the antibiotic used, or even the probiotic preparation given. The need to better define the optimum dose and time for each probiotic preparation is also stressed.10
In randomized, controlled studies where a probiotic drink fermented with L. casei DN-114001, Lactobacillus bulgaricus, and Streptococcus thermophilus was administered twice daily, the incidence of diarrhea induced by antibiotics and C. difficile was reduced,11 and some consensuses therefore recommend this drink, although the grade of recommendation is weak, with a low level of evidence.12
Patients who receive tube enteral nutrition could benefit from the use of probiotics to prevent or treat associated diarrhea. The results have not been consistent, however, and there is also a wide variability in the tested strains and in the nutritional formulas used (with or without added fiber and with different types of fiber, including prebiotics)13. For example, different strains have been compared to placebo, including L. acidophilus+L. bulgaricus 3g/day; VSL#3 (9×1011 bacteria/day); S. boulardii (2g/day); L. rhamnosus GG (2×1010 bacteria/day)+inulin 560mg/day; Ergyphilus (2×1010 bacteria/day); Lactobacillus paracasei+Bifidobacterium longum+FOS+inulin+acacia gum; Bifidobacterium breve 1×108+L. casei Shirota 1×108+GOS 15g; a mixture of bifidobacteria with enteral nutrition with mixed fibers and other immunonutrients.14–21 Less than half of the studies found significant benefits in terms of diarrhea reduction, and the number of patients studied with each strain was small. Thus, although this is a very attractive field, evidence-based recommendations for the use of these strains in enteral nutrition cannot currently be made. In fact, the 2015 Canadian clinical practice guidelines on nutrition in critically ill patients assessed the use of probiotics and symbiotics in ICU patients receiving tube enteral nutrition (both gastric and jejunal) and concluded that the use of probiotics had no impact on diarrhea.22
As regards the primary prevention of disease caused by C. difficile in patients treated with antibiotics, probiotics also decrease the incidence of such disease, especially when strains of S. boulardii, and possibly other Lactobacillus, such as GG, are administered.12,23 However, additional studies are needed. By contrast, a recent meta-analysis concluded that only four probiotic strains (not including Lactobacillus GG) have been shown to significantly decrease the incidence of diarrhea induced by C. difficile: S. boulardii (2×1010 colony-forming units [CFU] day), L. casei DN114001 (probiotic drink twice daily), a mixture of L. acidophilus and Bifidobacterium bifidum (2×1010CFU/day), and a mixture of L. acidophilus, L. casei, and L. rhamnosus).24 Other probiotics may be effective, but no conclusions may be drawn because of the scarcity of studies. For the secondary prevention of recurrent C. difficile infection, species such as S. boulardii and LGG, with low levels of evidence, may also be used,12 although not all authors agree on this.24
For the treatment of acute gastroenteritis in children, the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) recommends, in addition to rehydration, the use of L. rhamnosus GG (at doses ≥1010CFU daily for 5–7 days) and S. boulardii (250–750mg daily for 5–7 days) with a strong grade of recommendation and a low level of evidence, and Lactobacillus reuteri (at doses of 108 1–4 times daily for 5–7 days) with a weak grade.25 A recent Cochrane review reached similar conclusions, and only recommended the use of L. rhamnosus GG and S. boulardii at doses ranging from 5 to 40 million CFUs.26
Similarly, probiotics appear to decrease the risk of nosocomial diarrhea by rotavirus in children with Lactobacillus GG but not with other species, such as L. reuteri or Bifidobacterium lactis.
In patients given antibiotics to eradicate Helicobacter pylori, studies have been conducted where probiotics were added to improve eradication rates and to prevent side effects such as antibiotic-induced diarrhea. Several meta-analyses showed that the addition of probiotics may increase the efficacy of eradication with an odds ratio (OR) ranging from 1.2 to 2 times as compared to the control group. Although additional studies are needed, it appears that the most effective strains are L. acidophilus (1.25×109CFU) (OR: 1.24), milk fermented with L. casei DN-114001 (2 packs daily) (OR: 1.47), yoghurt with Lactobacillus gasseri (OR: 1.19) (2 packs daily), and Bifidobacterium infantis (2×109) (OR: 1.21). By contrast, other strains such as S. boulardii are not as effective. Treatments vary in dose and duration, but are usually given for periods ranging from 7 days to 4 weeks. It has been noted that their effectiveness may be greater when antibiotic regimens achieve a lower eradication, i.e. in cases where antibiotic therapy is less effective. As in other clinical settings where antibiotics are used, probiotics also appear to decrease the incidence of diarrhea (with an OR ranging from 0.16 to 0.47).27,28 Recommendation of the use of probiotics for this indication (to prevent diarrhea) is weak, with a low level of evidence.27
The use of probiotics for the management of radiation enteritis has been tested in several placebo-controlled clinical trials using different species and strains of probiotics (pharmacological such as lactobacilli, bifidobacteria, VSL#3, or contained in fermented milk), and a decreased incidence of diarrhea was found in a meta-analysis. However, as in other clinical situations, the wide heterogeneity of studies does not allow for final conclusions to be drawn.12,29
As a reflection derived from the analysis of these studies, it should be noted that not all probiotics (or combinations of them) act in the same way, and their effects need therefore to be shown in well designed studies (with a homogeneous group of patients of an adequate size) for each clinical situation and with given strains, so that data cannot be extrapolated between them.
Several randomized, controlled studies have been conducted on the use of prebiotics (fructooligosaccharides, alone or combined with inulin) to prevent antibiotic-induced diarrhea in inpatients or outpatients. Although the use of prebiotics modified the count of bifidobacteria, the incidence of antibiotic-induced diarrhea was decreased in only a few cases.30
Constipation, bowel movements, and colonic healthPrebiotics, in general, have a positive but non-significant effect on the number and volume of bowel movements. Inulin may increase the frequency and consistency of bowel movements in chronic constipation.31 Fiber (especially the insoluble or poorly fermentable part) modestly (but significantly) increases the weekly number of bowel movements (1.4–1.5 movements per week on average). In irritable colon, studies published with both prebiotics and fiber (mixtures or preferably soluble) reported conflicting results.32 Theoretically and according to some studies, their use is associated with a worsening of symptoms of flatulence, so that a diet low in “fodmaps” (low in fermentable components such as oligosaccharides, disaccharides, monosaccharides, and polyoles, i.e. some prebiotics) could improve symptoms in some patients33; in other studies, however, fiber (e.g. hydrolyzed guar gum) appeared to improve symptoms and quality of life in patients with irritable colon and with predominant symptoms of constipation.34,35Plantago ovata seeds (ispaghula husk) may improve symptoms or abdominal pain in patients with irritable colon. The use of probiotics (bifidobacteria, lactobacilli or combinations of B. infantis B5624, Bifidobacterium animalis or VSL#3) may also decrease the associated symptoms, but further studies are needed before their routine use can be recommended.1,12,36
Based on multiple studies in animals, it has been suggested that some prebiotics, probiotics, and symbiotics could decrease the risk of colon cancer. In a randomized, placebo-controlled study using symbiotics (oligofructose+inulin [SYN1]+L. rhamnosus GG and Bifidobacterium lactis Bb12) in patients undergoing surgery for colonic polyps and cancer, improvements occurred in both fecal flora and various biomarkers (genetic, cellular, inflammatory, and immunological), decreasing the theoretical risk of colon cancer.37 In various epidemiological studies, the intake of food rich in fiber (mixed [fermentable or not]), especially fresh fruit and vegetables, was clearly associated with a probable decrease in the risk of colon and rectal cancer7. However, randomized clinical studies of the secondary prevention (of colonic polyps) conducted to date with a diet rich in fiber or supplemented (not with prebiotics) have not achieved the results expected; the follow-up and supplementation time or patient selection may possibly have influenced these findings.1
In preterm infants, probiotic supplementation could decrease the incidence of necrotizing enterocolitis and its associated mortality. While some meta-analysis reached this conclusion with a high level of evidence, others did not support it, and, therefore, its routine use in preterm infants is not recommended.38 In any case, although this cannot be stated in the prescribing information, in individual cases and taking the risks and benefits into consideration, combinations of L. acidophilus and B. infantis or Bifidobacterium bifidum or single strains of Lactobacillus LGG or Bb12 are more than adequate. By contrast, the effectiveness of isolated strains of Bifidobacterium breve, L. acidophilus, Escherichia coli Nissle 1917, or S. boulardii has not been adequately shown. Again, as in other conditions, the optimum dosage, the type of probiotic to be used (species, strain, a combination of several), and the duration of the supplementation and infant characteristics have yet to be elucidated. Extrapolating data from some studies to others would be inappropriate.39
Inflammatory bowel diseaseInflammatory bowel disease (IBD) is a recurrent chronic condition in which an abnormal interaction exists between intestinal flora and the host. Patients with IBD have an increased risk of colorectal cancer. In recent years, the use of probiotics, prebiotics, and symbiotics to restore intestinal microflora (ecomedicine) and decrease inflammation has been proposed.
Probitics have been used in many studies in animal models with promising results. In human studies, the use of a multistrain probiotic (VSL#3, containing 4 lactobacilli strains–L. acidophilus, L. casei, Lactobacillus delbrueckii sp. bulgaricus, and Lactobacillus plantarum; 3 bifidobacterial strains–B. longum, B. infantis, and Bifidobacterium breve; and Streptococcus salivarius sp. thermophilus) has been shown, with a high level of evidence, to decrease activity (the prevention of occurrence and the maintenance of remission) of pouchitis (a non-specific inflammation of the ileal pouch) in ulcerative colitis (UC) after ileal anastomosis.12,40 The recommendation for use in this indication is weak, with a moderate level of evidence, because of the low number of patients studied. The most common doses in pouchitis are 2–4 sachets daily (each sachet contains 450,000 million live bacteria 4.5×1011CFU; there are also capsules containing 112,000 million live bacteria).
Other studies have reported modest improvements in the reduction of disease activity (associated with conventional treatment) in patients with UC, and mild to moderate involvement, with the use of VSL#3, Escherichia coli Nissle, Lactobacillus GG, or milk fermented with bifidobacteria and/or lactobacilli (whether or not compared to placebo or other treatments, such as mesalazine),41 but it is not clear whether a reduction of disease activity or the maintenance of remission is induced, and further studies are needed.12,42
However, trials with probiotics on remission induction or maintenance in Crohn's disease (using several strains such as Lactobacillus GG, VSL3, L. johnsonii LA1, Escherichia coli Nissle 1917, S. boulardii) have reported conflicting and usually less satisfactory results than in UC.12,40
Evaluation of the most effective strains and of how host factors (such as the genetic characteristics of patients) influence therapeutic response is also required.
The use of prebiotics alone or combined with probiotics (symbiotics) in IBD is also proposed because of their effects on the growth of lactobacilli and endogenous bacteria, promoting the production of short chain fatty acids (particularly butyrate, a preferential nutrient for enterocytes), the prevention of the adhesion of pathogenic bacteria, the production of antibiotics, and a decrease in luminal pH. The most widely studied prebiotics are inulin, resistant starch, oligosaccharides such as fructooligosaccharides (FOS) and galactooligosaccharides (GOS). Prebiotics and fiber especially have been used in UC. On the other hand, in the treatment of pouchitis in UC, inulin fiber and fiber from P. ovata seeds could also be useful in preventing its appearance. In pouchitis, inulin, as compared to placebo, may decrease the severity (endoscopic and histological) of the condition and improve the microbiological profile. Another option in IBD is the use of symbiotics, in an attempt to promote the synergy of both treatments to achieve greater effects than with the two products alone. An improvement of endoscopic and inflammatory parameters has been seen in some studies. Few studies are, however, so far available, and, consequently, no relevant conclusions may be drawn.43
Liver diseaseNon-alcoholic fatty liver disease (NAFLD) encompasses a wide range of pathological conditions, from simple steatosis to cirrhosis, through steatohepatitis and fibrosis. It has been suggested that probiotics (e.g. VSL#3) could modulate intestinal flora, influencing the bowel–liver axis and improving NAFLD. Some studies have shown that the use of probiotics and symbiotics (VSL#3, LGG, or lactobacilli, bifidobacteria, and oligosaccharides) provides benefits. Although there are studies reporting improvements in laboratory parameters in NAFLD (transaminases, lipid peroxidation), the level of evidence is still low.12,44,45
The use of probiotics, prebiotics, and symbiotics in cirrhotic patients with minimal encephalopathy has been shown to improve ammonium levels and encephalopathy in terms of some aspects of the quality of life, as well as intestinal ecology. By contrast, probiotics have shown no effects in established encephalopathy.45
A meta-analysis of four prospective studies in liver transplant patients comparing symbiotics (usually Lactobacillus plantarum 299 and 10g of oat fiber) to the prebiotic fiber contained in the preparation has been published. A significant decrease in the incidence of postoperative bacterial infections was seen in the group given the symbiotic as compared to the prebiotic fiber contained in the preparation alone (7% vs 35%). The number that needed to be treated in order to prevent an event was 3.6 patients.46 The days of stay at the ICU and the duration of antibiotic treatment also decreased. These promising results need to be confirmed by other studies.
Intensive care and surgical patientsDifferent randomized studies have been conducted in patients undergoing major abdominal surgery (for multiple trauma, cancer, gastric and colonic procedures, etc.). They have usually compared symbiotics added to enteral nutrition (most commonly, Symbiotic 2000 or Lactobacillus plantarum+oat fiber) to the probiotic fiber contained in the preparations and to another control group on parenteral or enteral nutrition (standard nutrition with fiber, peptides, or glutamine). In some studies, but not all, symbiotic preparations decreased the incidence of bacterial infections as compared to total parenteral nutrition or other enteral nutrition formulations, and with intermediate results as compared to prebiotic fiber.
A meta-analysis conducted in ICU patients evaluated the use of Synbiotic 2000 FORTE (a combination of B. longum+L. bulgaricus+S. thermophilus) and compared these patients with a control group (glutamine+fermentable fiber) for the prevention of ventilator-associated pneumonia (VAP). A significant decrease was found in thr incidence of VAP. However, the quality of evidence was again low, and because of the great heterogeneity of the results, no definitive conclusions may be drawn.47 Other recent meta-analysis also concluded that VAP incidence decreases when probiotics and symbiotics are given alone or in combination by the oral route, by nasogastric tube, or absorbed through the oropharyngeal mucosa. In addition, those critically ill patients who would possibly benefit the most from the use of probiotics (or symbiotics) would be multiple trauma and surgical patients, for whom the ICU stay would be shorter. However, unlike in other studies, no reductions were seen in other parameters such as infections, the incidence of diarrhea, overall hospital stay, or mortality.48
The 2015 Canadian clinical practice guidelines on the nutrition of critically ill patients recommended that the use of probiotics be considered for ICU patients based on a reduction of infections and a trend to decreases in VAP and length of ICU stay, with no effects on mortality. The results were highly heterogeneous. No evidence was found of an increased risk of mortality or side effects (unlike in the Propratia study in acute pancreatitis; see below), despite the use of jejunal (rather than nasogastric) infusion in many cases. Although no recommendations as to the type of probiotics to be used were made, it was, however, stressed that S. boulardii should not be used in critically ill patients because of the risk of fungal infection.22
Species, strains, doses, and the duration of treatment in each clinical condition need, therefore, to be better defined, in order that clear evidence-based recommendations can be made.22,49
Severe acute pancreatitisStarting in 2005, the Oláh group reported two studies conducted in 45 and 62 patients respectively with severe acute pancreatitis. They assessed the use of symbiotics (Lactobacillus plantarum or Symbiotic 2000) vs prebiotics alone (oat fiber or the fiber in Symbiotic 2000 respectively) as an infusion by nasojejunal tube.50 In the first study, the incidence of infected necrosis or abscess was clearly lower with symbiotics (4.5% vs 30%). In the second, there was a lower, non-statistically significant incidence of multiorgan failure, sepsis, and mortality. but a significant decrease was seen in the incidence of multiorgan failure and systemic inflammatory response syndrome when they were jointly assessed. However, the results of the PROPATRIA trial were reported in 2008. This well-designed study (double blind, randomized and with an adequate number of patients [296]), tested whether the probiotic Ecologic® 641 (containing six bacterial strains: L. acidophilus, L. casei, Lactobacillus salivarius, Lactococcus lactis, and two bifidobacteria: Bifidobacterium bifidum and Bifidobacterium lactis, with a total dose of 1010 live bacteria), administered together with an enteral formula with a fiber mixture (including probiotics, soy polysaccharide, arabic gum, resistant starch, inulin, alpha cellulose, and oligofructose), reduced infections in severe acute pancreatitis as compared to the infusion of the same enteral formula without probiotics. While there was no difference in the number of infections between the two groups, mortality was significantly higher in the probiotic group (16% vs 6%). Forty-one percent of patients with necrotizing pancreatitis died in the probiotic group, as compared to 15% of placebo patients. Nine patients experienced mesenteric ischemia in the probiotic group, and eight of them died. Both bacteremia and infection of necrosis, multiorgan failure, and mortality appeared to be associated with early intestinal barrier impairment during pancreatitis. In patients with multiorgan failure, the use of probiotics was shown to increase bacterial translocation.51 Based on these results, it has been suggested that the jejunal administration of probiotics with prebiotic fiber in severely ill patients (not only with severe acute pancreatitis) possibly has negative effects on intestinal perfusion, promoting multiorgan failure, bowel necrosis, and death. It appears wise, for the moment, not to infuse probiotics using this administration route in critically ill patients in standard clinical practice, and only to use them in the context of well designed, randomized studies. On the other hand, further understanding is needed of the impact of some probiotic strains on bowel integrity and interactions of endogenous flora and prebiotics and probiotics before the widespread use of this therapy, which is not as safe as was initially thought. In this regard, it should be emphasized again that not all probiotic strains have the same safety and efficacy profile in different clinical settings.
Calcium absorption and bone healthSome randomized, controlled studies have assessed calcium absorption after the administration of prebiotics (FOS, GOS, inulin, or combinations of them), which was increased in some patients. In a randomized study comparing inulin, administered for one year to adolescents, with placebo, improvements were seen in both calcium absorption and bone mineral density.52 These results, and those of other studies with probiotics in animal models showing improvements in different pathophysiological aspects in bone health, could be of interest as regards their application in clinical practice, but additional research is needed.53
Probiotics, prebiotics, and symbiotics in metabolic diseases, obesity, and diabetesBoth experimental studies in animals and observational studies in humans (and some intervention studies with fecal transplant) have shown a different composition of gut microbiota in obese and slim subjects, in diabetic and non-diabetic subjects, and in other conditions such as NAFLD or cardiovascular and renal diseases as compared to healthy subjects. Changes in the composition and/or activity of gut microbiota after the administration of nutrients with prebiotic or probiotic properties may modulate gene expression and host metabolism (at multiple levels, including adipose, muscle, and liver tissue, and even the modulation of satiety), and thus have an impact on related metabolic disorders. Some prebiotics and probioticos may counteract the metabolic changes associated with obesity and diabetes such as insulin resistance, hyperglycemia, inflammation, dyslipidemia or NAFLD, to give some examples.54 However, these hypotheses need to be confirmed in humans in well-designed, controlled studies. For example, the addition of probiotics (in studies preferentially conducted with various types of Lactobacillus) may modestly contribute to improvements in blood glucose control.55 Similarly, the use of prebiotics (such as GOS, inulin, FOS, etc.), probiotics, and symbiotics is associated with slight short- and mid-term improvements in lipid control,56,57 and they may, therefore, be considered as adjuvants to other treatments. Overall, however, many of the effects reported are still poorly relevant for clinical practice.58
Risks of probiotics in clinical practiceBecause of the rapid increase in the use of probiotics in recent years in very different circumstances, their safety is an important issue, especially when they are used for conditions where their efficacy is not supported by scientific evidence. The potential risk of jejunal infusion of probiotics has already been discussed. An additional issue is the potential risk of infection induced by probiotics. In this regard, we know that lactobacilli and bifidobacteria are abundant in both human diet and healthy bowel. Natural infections by these microorganisms may occur, even unrelated to their intake. A majority of the few case reports of bacteremia, sepsis, or endocarditis caused by lactobacilli were caused by L. rhamnosus GG or L. casei. Infections by bifidobacteria are rare in the literature, but bacteremia, sepsis, and cholangitis induced by Bacillus subtilis have been reported. Fungal sepsis caused by S. boulardii has also been reported. All systemic infections caused by probiotics have occurred in patients with severe underlying disease (diabetes mellitus, valve disease, preterm infants, hematological problems, AIDS, patients on intensive care, with parenteral nutrition, with jejunostomy, short bowel syndrome, transplant and cancer patients, etc.). Most of these infections resolved with antibiotic treatment, but some of them evolved into septic shock or even caused death. Different strains of probiotics may possibly have different safety profiles. However, no cases of sepsis induced by Lactobacillus have been reported in prospective, randomized studies conducted in immunosuppressed adults and children with HIV infection and in preterm newborns. Overall, the benefits appear to outweigh the risks, because the risk of infection from the use of probiotics is similar to that of infection by commensal strains and usually low, even in immunosuppressed patients. There are, however, selected groups of patients, especially some immunosuppressed patients, in whom probiotics should be used with caution. Boyle et al.,59 proposed a number of factors predisposing to sepsis induced by probiotics (Table 4). The risk of sepsis caused by these bacteria should be weighed against the risk of sepsis induced by other pathogenic bacterial species and against the risk of suffering from the disease one is trying to prevent (e.g. necrotizing enterocolitis in newborns).
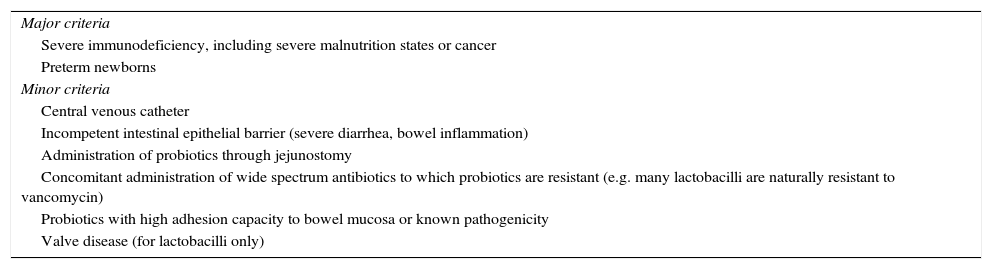
Criteria for assessing the risk of sepsis by probiotics in clinical practice.
| Major criteria |
| Severe immunodeficiency, including severe malnutrition states or cancer |
| Preterm newborns |
| Minor criteria |
| Central venous catheter |
| Incompetent intestinal epithelial barrier (severe diarrhea, bowel inflammation) |
| Administration of probiotics through jejunostomy |
| Concomitant administration of wide spectrum antibiotics to which probiotics are resistant (e.g. many lactobacilli are naturally resistant to vancomycin) |
| Probiotics with high adhesion capacity to bowel mucosa or known pathogenicity |
| Valve disease (for lactobacilli only) |
The use of probiotics, prebiotics, and symbiotics is emerging as a promising therapy which is generally safe in different clinical settings. While their efficacy for the prevention of antibiotic-induced diarrhea, the reduction of the incidence of necrotizing enterocolitis in preterm newborns, and the prevention and treatment of pouchitis in UC appears to be proven, further research in all other fields is still needed before any final recommendations can be made.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Olveira G, González-Molero I. Actualización de probióticos, prebióticos y simbióticos en nutrición clínica. Endocrinol Nutr. 2016;63:482–494.