Treatment of hypothyroid pregnant women is usually calculated based on weight (1μg/kg/day) and TSH levels. This study assessed the usefulness of treating these women with a fixed dose of 75μg/day.
Patients and methodsAll women with pregnancy diagnosed from January to August 2012 in the Vigo Health Area (Spain) without previous diagnosis of thyroid disease or thyroxine treatment and with TSH levels over 4.5mUI/mL were enrolled by consecutive sampling. All 116 women in the sample were treated with a fixed daily dose of thyroxine 75μg-thyroxine levels were measured at two, four, and six months, and thyroxine dose was modified if TSH level was lower than 0.3 or higher than 4.5mUI/mL.
ResultsA woman had a TSH level less than 0.3mUI/mL in a test; reduction of thyroxine dose to 50μg/day allowed for maintaining TSH level within the desired range until delivery. Six women had TSH levels over 4.5mUI/mL in one test; in all of them, increase in thyroxine dose to 100μg/day allowed for maintaining the level within the desired range until delivery.
ConclusionsFixed daily doses of thyroxine 75μg allowed for achieving goal TSH levels in most of our pregnant women with subclinical hypothyroidism, irrespective of their weight and baseline TSH level.
Los métodos habituales de cálculo de la dosis inicial de tiroxina en el tratamiento de gestantes hipotiroideas usan el peso de las pacientes (1μg/kg/día) o la concentración plasmática de TSH. Este estudio analiza la idoneidad de tratar a estas mujeres con una dosis fija de 75μg/día de la hormona.
Pacientes y métodosSe seleccionaron mediante un muestreo consecutivo a todas aquellas mujeres diagnosticadas de gestación en el área sanitaria de Vigo entre enero y agosto de 2012, sin antecedentes de tiroidopatía y con una concentración de TSH superior a 4,5mUI/ml y T4L normal. Las 116 gestantes de la muestra resultante recibieron tratamiento con 75μg/día de tiroxina, y se les hizo un análisis a los 2, 4 y 6 meses tras la instauración del tratamiento, modificándose la dosis de la hormona si la concentración de TSH era inferior a 0,3 o superior a 4,5mUI/ml.
ResultadosUna de las pacientes tuvo, en un análisis, una concentración de TSH inferior a 0,3mUI/ml; el descenso de la dosis de tiroxina a 50μg/día permitió mantener dicha concentración en el rango deseado hasta el parto. Seis tuvieron en un análisis una concentración de TSH superior a 4,5mUI/ml; en todas ellas el aumento de la dosis de tiroxina a 100μg/día permitió mantener dicha concentración en el rango deseado hasta el parto.
ConclusionesUna dosis de tiroxina 75μg/día permitió conseguir los objetivos de concentración de TSH de nuestro estudio en la mayoría de las gestantes con hipotiroidismo subclínico, independientemente de su peso y de su concentración inicial de TSH.
Diagnosis and treatment of hypothyroidism in pregnant women are frequently controversial.1 Most, but not all,2 studies show decreased plasma levels of thyroid-stimulating hormone (TSH) during pregnancy,3–7 and both the American thyroid Association (ATA) and the American Association of Clinical Endocrinologists (AACE) recommend use of the following specific normal ranges for pregnant women: first trimester, 0.1–2.5mIU/mL; second trimester, 0.2–3.0mIU/mL; and third trimester, 0.3–3mIU/mL, instead of the standard range used in non-pregnant women (0.3–4.5mIU/mL).
Unlike in frank hypothyroidism, there is no agreement on the indications for treatment in pregnant women with subclinical hypothyroidism (increased TSH levels with normal FT4). The ATA and AACE recommend that only patients with peroxidase antibodies (anti-TPO) or thyroglobulin antibodies (anti-TG) are treated, based on a study which showed increased complications of pregnancy in these patients.3 By contrast, other guidelines advocate treatment of all pregnant women with subclinical hypothyroidism, regardless of plasma levels of thyroid antibodies.4 The goal of treatment, if administered, is to achieve normal TSH levels for each trimester of pregnancy.
There is no agreement either on how to calculate the starting thyroxine dose. Some authors suggest that dose is calculated based on patient weight (1μg/day),5 while others recommend that dosage is based on TSH levels at diagnosis: 25μg/day if TSH level is 4–8mIU/mL; 50μg/day if TSH level is 8–12mIU/mL; 75μg/day if TSH level is greater than 12mIU/mL.6 A recent report by our group showed that a fixed dose of 50μg thyroxine in pregnant women with subclinical hypothyroidism allowed for maintaining TSH levels ranging from 3.0 to 4.5mIU/mL in approximately 80% of women, regardless of weight and baseline TSH level.7 This study subsequently analyzed the convenience of treating subclinical hypothyroidism in pregnant women with a daily dose of 75μg of thyroxine.
Subjects and methodsA consecutive sample of women with plasma TSH levels higher than 4.5mIU/L was obtained from all women diagnosed with pregnancy in the Vigo health area between January and August 2012. Women with plasma FT4 levels less than 0.93ng/100mL (frank hypothyroidism), those who had ever been treated with thyroxine, and those previously diagnosed with any thyroid disease (including hypothyroidism, hyperthyroidism, goiter, and thyroid nodule) were excluded.
The resulting cohort consisted of 116 pregnant women, all of whom received (as usual in our health area) 200μg/day of potassium iodide at least from the time pregnancy was diagnosed. One of the patients moved to another town in the second trimester of pregnancy, and pregnancy did not reach its term in another four patients.
All pregnant women were informed of diagnosis of subclinical hypothyroidism and the need for treatment with thyroxine. The thyroxine dose of 75μg/day has become our standard since we found in a pregnant cohort that doses of 50μg/day is often inadequate and never excessive in this population.7 All pregnant women signed an informed consent.
All women were prescribed since diagnosis of hypothyroidism a daily dose of 75μg of thyroxine (Eutirox® 75) administered 30min before breakfast, regardless of weight, height, presence of thyroid antibodies, or plasma TSH levels. TSH and FT4 levels were tested in all pregnant women 2, 4, and 6 months after diagnosis. When TSH levels in any test were higher than 4.5mIU/mL, thyroxine dose was increased by 25μg/day, and when levels were less than 0.3mIU/mL, thyroxine dose was reduced in the same amount.
Plasma levels of thyroid peroxidase and thyroglobulin antibodies were tested in all pregnant women in the first trimester.
Plasma TSH levels (normal range in our laboratory: 0.3–4.5mcU/mL) were tested using a chemiluminescent immunometric assay (Cobas 6000®, Roche, Mannheim, Germany), FT4 levels (0.9–2ng/100mL) and TPO antibodies (0–34IU/mL) using a competitive electrochemiluminescence immunoassay (Cobas 6000®, Roche, Mannheim, Germany), and TG antibodies (0–115IU/mL) with a chemiluminescent immunometric assay (Immulite 2000®, Siemens, Germany).
Quantitative variables are given as mean±standard deviation. Differences between independent measurements of quantitative variables were analyzed using a Student's test for independent variables. Differences between repeated measurements of quantitative variables were analyzed using a Student's t test for related measurements. Differences with values of p<0.05 were considered significant.
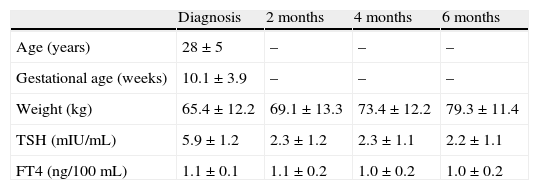
ResultsTable 1 shows the age, gestational age, and changes over time in weight and TSH and FT4 levels of women during pregnancy. Mean TSH level was significantly higher at diagnosis of hypothyroidism as compared to the other three test times (performed 2, 4, or 6 months after diagnosis). No differences were found in TSH levels at 2, 4, and 6 months. No significant differences were also found when mean FT4 levels during pregnancy were compared.
Age, gestational age, and hormone levels (mean±standard deviation) during pregnancy.
| Diagnosis | 2 months | 4 months | 6 months | |
| Age (years) | 28±5 | – | – | – |
| Gestational age (weeks) | 10.1±3.9 | – | – | – |
| Weight (kg) | 65.4±12.2 | 69.1±13.3 | 73.4±12.2 | 79.3±11.4 |
| TSH (mIU/mL) | 5.9±1.2 | 2.3±1.2 | 2.3±1.1 | 2.2±1.1 |
| FT4 (ng/100mL) | 1.1±0.1 | 1.1±0.2 | 1.0±0.2 | 1.0±0.2 |
The column “Diagnosis” shows the value of variables at diagnosis of hypothyroidism. All other columns give the value in the tests performed 2, 4, and 6 months after that time.
TSH, thyroid-stimulating hormone.
Six women (5% of the sample) had TSH levels higher than 4.5mIU/mL in one test, and three of them had thyroid antibodies. This occurred in three women at the month 2 test, in two women at the month 4 test, and in one woman at the month 6 test. In all these women, titration of thyroxine dose to 100μg/day allowed for maintaining TSH levels within the desired range until delivery.
A woman (0.9% of the sample) had a TSH level less than 0.3mIU/mL in the test performed at 4 months. Thyroxine dose reduction to 50μg/day allowed for maintaining her TSH levels within the desired range until delivery.
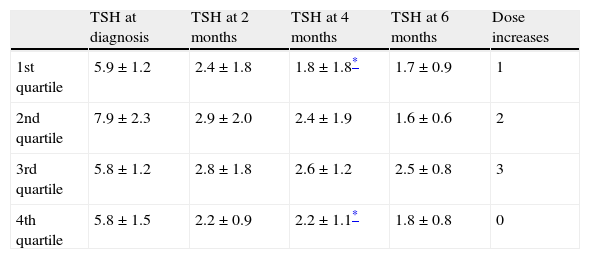
At diagnosis of hypothyroidism, weights of pregnant women in the first and fourth quartiles of weight were 53.3±2.9kg and 79±11kg, respectively (p<0.001). Plasma TSH levels of pregnant women in the first quartile of weight were not significantly different from those of women in the fourth quartile neither at diagnosis nor at the tests performed at 2 or 6 months. In the month 4 tests, plasma TSH levels were significantly lower in pregnant women in the first quartile of weight as compared to the fourth quartile (1.82 vs 2.22mIU/mL; p=0.04). One of the six women who required a higher thyroxine dose was in the first quartile of weight, while the remaining five women were in the second and third quartiles (Table 2).
TSH levels (mIU/mL) by quartiles of weight at diagnosis of hypothyroidism and after 2, 4, and 6 months.
| TSH at diagnosis | TSH at 2 months | TSH at 4 months | TSH at 6 months | Dose increases | |
| 1st quartile | 5.9±1.2 | 2.4±1.8 | 1.8±1.8* | 1.7±0.9 | 1 |
| 2nd quartile | 7.9±2.3 | 2.9±2.0 | 2.4±1.9 | 1.6±0.6 | 2 |
| 3rd quartile | 5.8±1.2 | 2.8±1.8 | 2.6±1.2 | 2.5±0.8 | 3 |
| 4th quartile | 5.8±1.5 | 2.2±0.9 | 2.2±1.1* | 1.8±0.8 | 0 |
Given as mean±standard deviation. The last column represents the number of patients in whom thyroxine dose during pregnancy.
TSH, thyroid-stimulating hormone.
At diagnosis of hypothyroidism, plasma TSH levels of pregnant women in the first and fourth quartiles of TSH levels were 4.77±0.17 and 7.74±1.14mIU/mL, respectively (p<0.001). No significant differences were seen in plasma TSH levels between women in the first and fourth quartiles of TSH in the tests performed at 2 months (1.95 vs 2.66mIU/mL; p=0.08), 4 months (2.56 vs 2.48mIU/mL; p=0.78), or 6 months (2.31 vs 2.21mIU/mL; p=0.19) since diagnosis of hypothyroidism. Of the six patients in whom thyroxine dose was increased, one was in the first quartile of TSH levels at diagnosis, while three were in the third quartile and two in the fourth quartile.
In the tests conducted at 2 months, 29 women (27% of the sample) has TSH levels higher than recommended by the ATA-AACE but lower than 4.5mIU/mL, which were maintained until delivery. Fifty-four pregnant women (48% of the sample) had at least one TSH value prior to pregnancy. The mean±standard deviation of TSH levels was 3.3±0.9mIU/mL.
Forty-three patients (37% of the sample) had thyroid antibodies (thyroglobulin and/or peroxidase antibodies). Four of the six patients who required increased thyroxine doses during pregnancy belonged to this group.
Of the four women in whom pregnancy did not reach its term, three had thyroid antibodies. The last TSH level before termination of their pregnancies was not significantly different from the mean value in the sample at that time.
DiscussionThe range of TSH levels we have considered adequate in pregnant women in this study is not the same as recommended by the ATA-AACE. This is not an uncommon occurrence: a recent report showed that the upper limit for TSH levels in pregnant women used in different Spanish regions ranged from 2.63 and 4.75mIU/mL.8 Fear of overtreatment is a factor that limits use of stricter ranges in actual clinical practice. Uncertainty about the clinical impact of subclinical hypothyroidism on pregnancy has also prompted use of wider TSH ranges by clinicians. Adoption of stricter TSH ranges would also increase prevalence of hypothyroidism to an extent difficult to assume by already overburdened clinical departments.
We do not know whether or not intake of iron or multivitamin preparations by the group of women who required an increased thyroxine dose was similar to that of all other women. Three of the four women in whom pregnancy did not reach its term had thyroid antibodies (i.e. 75%, higher than the prevalence of these antibodies in the whole sample). Both circumstances may have led to bias limiting the conclusions of this study.
Our data suggest that in pregnant women with subclinical hypothyroidism in whom the goal is to achieve TSH levels ranging from 0.3 to 4.5mIU/mL, there is no need to calculate the initial thyroxine dose based on patient weight or TSH level. A daily dose of 75μg of thyroxine is adequate in most these patients.
In fact, the thyroxine dose calculated based on weight or TSH level appears to be low: administration of 1μg/kg/day to this cohort would result in a mean daily dose of 65μg; if TSH levels were used, only pregnant women with values higher than 12mIU/mL would receive 75μg/day of thyroxine.
Our data suggest that if the goal was a TSH level within the ranges recommended by the ATA-AACE, 75μg/day of thyroxine would often be an inadequate dose. A dose of 100μg/day would probably be more adequate under these conditions.
Despite the increase in weight over the course of pregnancy, the thyroxine dose needed by our cohort did not increase. This may be explained by the limited capacity of thyroxine to cross the placenta, so that its actual volume of distribution changes little over these months.9
We recommend clinicians use of 75μg/day as initial thyroxine dose in any pregnant woman with subclinical hypothyroidism–regardless of weight and TSH level–if the goal is to achieve a TSH level less than 4.5mIU/mL.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Penin M, Trigo C, López Y, Barragáns M. Tratamiento del hipotiroidismo subclínico en gestantes con una dosis fija diaria de 75μg de tiroxina. Endocrinol Nutr. 2014;61:347–350.