The prevalence of chronic renal insufficiency (CRI) is increasing due to the greater longevity of the population and the ability to treat nephropathies. It has been estimated that 13% of the Spanish population suffer from some degree of renal infection, a certain percentage of which will progress until they require renal replacement therapy (RRT). Every year there is a linear increase in the incidence and prevalence of patients who require this therapy. The age and the time patients remain in the dialysis programme have also increased. An estimated 89% of the patients who start RRT use haemodialysis (HD), compared with 10% who enter a peritoneal dialysis programme and 1% who receive a preventive kidney transplant. Thus, the need to create, maintain and repair vascular accesses (VA) is also increasing, by approximately 10% every year.
In many cases, it is possible to foresee the need for haemodialysis and plan a VA in advance for this to mature and even be repaired and reconstructed if necessary, by monitoring patients with CRI. This is an important objective, as it is estimated that currently almost 50% of the patients who start haemodialysis reach the unit late and require a temporary catheter as the VA is not mature. This leads to greater morbidity and mortality, a higher failure rate of VA and the patients’ perception of a lower quality of life. It is important, then, to try to guarantee the availability of a functioning VA in subsidiary HD treatment patients. This requires multidisciplinary management, with close collaboration between primary care physicians, nephrologists, vascular surgeons, radiologists and nursing staff.
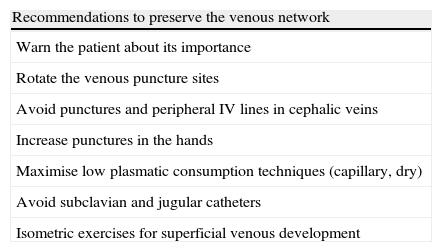
Care prior to the construction of a vascular accessThe early identification of renal patients and their referral and management in a nephrology unit permits: establishing a specific treatment, preserving the maximum renal function possible for as long as possible; treating other concomitant processes (nutritional state, nicotine dependence, high blood pressure, diabetes mellitus, anaemia, acidosis, alterations of the phosphocalcic metabolism), optimising the patient's general state and quality of life; educating the patient about his or her chronic disease, lifestyle, doctor's visits, etc., trying to achieve their collaboration and establishing a doctor-patient relationship that will probably be necessary for years. This education includes protecting the peripheral veins of the upper extremities, mainly the bilateral basilic and cephalic veins, which will be vital for the future construction of a VA, if necessary. Finally, the patient must be sent to the vascular surgeon when the renal function reaches terminal levels, sufficiently in advance to be able to guarantee, whenever possible, the availability of a mature VA, when starting the HD, thus avoiding the temporary emergency catheter. Some recommendations to preserve the venous network in renal patients are included in Table 1.
Recommendations to preserve the venous network in renal patients.
| Recommendations to preserve the venous network |
| Warn the patient about its importance |
| Rotate the venous puncture sites |
| Avoid punctures and peripheral IV lines in cephalic veins |
| Increase punctures in the hands |
| Maximise low plasmatic consumption techniques (capillary, dry) |
| Avoid subclavian and jugular catheters |
| Isometric exercises for superficial venous development |
A vascular access is a short-circuit created between an artery and a vein, either directly or through the interposition of a graft, in order to be able to extract and return a high blood flow to periodically filter it. A VA purports to: guarantee easy and repeatable access to the blood flow; obtain sufficient flows for the haemodialysis programme; permit outpatient management and guarantee the highest possible quality of life; and avoid complications. This requires: a donor artery with sufficient calibre and flow; permeable venous drainage, in continuity with the right atrium without stenosis in its trajectory; and distensibility of both to mature the access and adapt to the new haemodynamics of the system.
There are 3 types of VA: Autologous arteriovenous fistulae (AVF), which directly connect an artery and a peripheral vein, whose length is used for cannulation; prosthetic VA, where a graft is interposed between a native artery and vein, using the length of this graft for cannulation; and a central venous catheter (CVC), which is inserted into a jugular, subclavian or femoral vein, with no arterial manipulation.
The autologous AVF comes the closest to the requirements of an ideal VA. Its percutaneous puncture is simple and repeatable in a shorter or longer trajectory, it normally obtains venous flows of more than 350ml/sec, it reaches high long-term permeability rates (75% after 5 years), with low incidence of infections and other complications. It is a more comfortable VA for patients and develops the venous network of the extremity, preserving the possibility of other VAs in the future. A disadvantage is that it requires at least one month's maturation, which may extend up to 3 months. And a certain percentage of autologous AVF never develop and fail early on, with rates that vary between 10% and 30% for radiocephalic (RC) AVF.
The prosthetic VA offers a long and easy surface for cannulation. It normally provides flows of over 650ml/sec, its maturation is quick (2-3 weeks, up to 1 month), and its initial failure rate is less than that of the autologous AVF. However, the incidence of late failures is much greater, as they usually develop intimal hyperplasia in their venous anastomosis, with possible occlusion, lasting on average 2 years. The different surgical techniques used to try to minimise this only manage to prolong the permeability a few months. It is calculated that this type of VA requires between 1.2 and 2.6 secondary interventions per patient and year to maintain the permeability. And their rate of infection rate and other complications is very high. Furthermore, this type of VA is not so comfortable for the patient.
The CVCs are placed percutaneously in a jugular, subclavian or femoral vein, permitting simple access to the central venous flow, and not requiring repeated punctures. They achieve adequate flows for the HD and do not require any maturation time. They are very useful in emergency situations and may be used immediately. They have minimal initial failure rate. However, they last on average less than 1 year, with a higher infection incidence than in the 2 previous types. They can also cause stenosis or even occlusion in a central vein (normally subclavian, possibly jugular, iliac or even superior vena cava), which invalidate the ipsilateral extremity for future VAs. They are not very comfortable, either, for the patient. They are usually the last resort in nephropathic patients.
Preoperative evaluationThe aim of the preoperative evaluation of a patient with advanced CRI who requires a VA is to guarantee the creation of a functioning and long-lasting VA and to minimise complications. It must be performed by an experienced surgical team. It includes a complete medical history, a thorough physical examination and, sometimes, supplementary imaging tests.
Medical historyThe complete medical history of these patients must include:
- •
Age, sex, cardiovascular risk factors.
- •
Comorbidity: Cardiac insufficiency can be worsened by the increase of preload entailed by the construction of an AVF; a previous stroke can affect the functionality and utility of an extremity for the construction of a VA. Atherosclerosis can be generalised and occasionally affect the upper extremities, with a greater risk of steal if a VA is constructed. Certain haematological disorders can lead to thrombosis or haemorrhage, which is important in the arteriovenous manipulation of a VA. An active neoplasia may limit the life prognosis, etc.
- •
The estimated life expectancy may determine the choice of one VA or another, giving priority to the need for a long-lasting access, or on the other hand, a quick and not very aggressive short-term access such as a permanent CVC.
- •
The functional situation may also determine the use of one extremity or theother.
- •
Usual medication, with special emphasis on antiplatelet or anti-coagulant drugs, in order to plan an elective surgical procedure, or corticoidsteroids or immunosuppressors that might affect the healing of the surgical wounds and the risk of infection.
- •
Allergies to medications, especially to antibiotics or local anaesthesia.
More specifically focused on the technical choice of the VA, we will include:
- •
The history of VA, including current and previous catheters. We are interested in knowing the location and type of VA, the approximate date of creation, duration, complications and reasons for failure. This provides us with a lot of information about the already exhausted and the remaining arterial and venous reserves, which we will later confirm with the physical examination and supplementary tests.
- •
Current or previous central venous catheterisation, by way of central conduits (for HD or other purposes), pacemakers or intrachamber defibrillators, entails a risk of stenosis or even thrombosis in jugular, subclavian, brachiocephalic trunk veins or even superior vena cava of up to 40%, that are often not very symptomatic. Performing a VA in this extremity would mean the immediate failure of the access and a painful and massive oedema in the arm, which could even endanger the extremity. Therefore, its detection would invalidate the extremity for a new VA or would require previous repair.
- •
The same applies to thoracic surgery and to previous traumas in the scapular girdle or in the upper extremities, which may have harmed the central and/or peripheral veins or even the main arteries.
- •
A background of subclavian-axillary deep-vein thrombosis (DVT) may cancel the possibility of ipsilateral VA, as we have already mentioned. Repeated punctures by medical processes or parenteral drug addiction with or without clinical phlebitis may have cancelled the peripheral veins of one or both upper extremities (UE) for the construction of a VA.
- •
Whenever possible, the non-dominant upper extremity of the patient should be used to construct the VA, increasing the patient's comfort.
The medical history must be complemented with a thorough physical examination of the arterial and venous network of both upper extremities (UE), and occasionally of the lower extremities (LE) An examination of the arterial tree is performed by feeling the pulses all along the UE (axillary, humeral, radial and cubital), and by auscultating subclavian murmurs. The Allen test is performed on the radial and cubital arteries of the wrist to verify the integrity of the palmar arches and the dependence on digital perfusion of one artery or another. A positive Allen test may contraindicate a distal AVF. The symmetry of the blood pressure in both UE is also verified, ruling out stenosis or proximal arterial occlusions that would also invalidate the extremity or require previous repair to guarantee the functionality of the VA. The perfusion of both hands is observed.
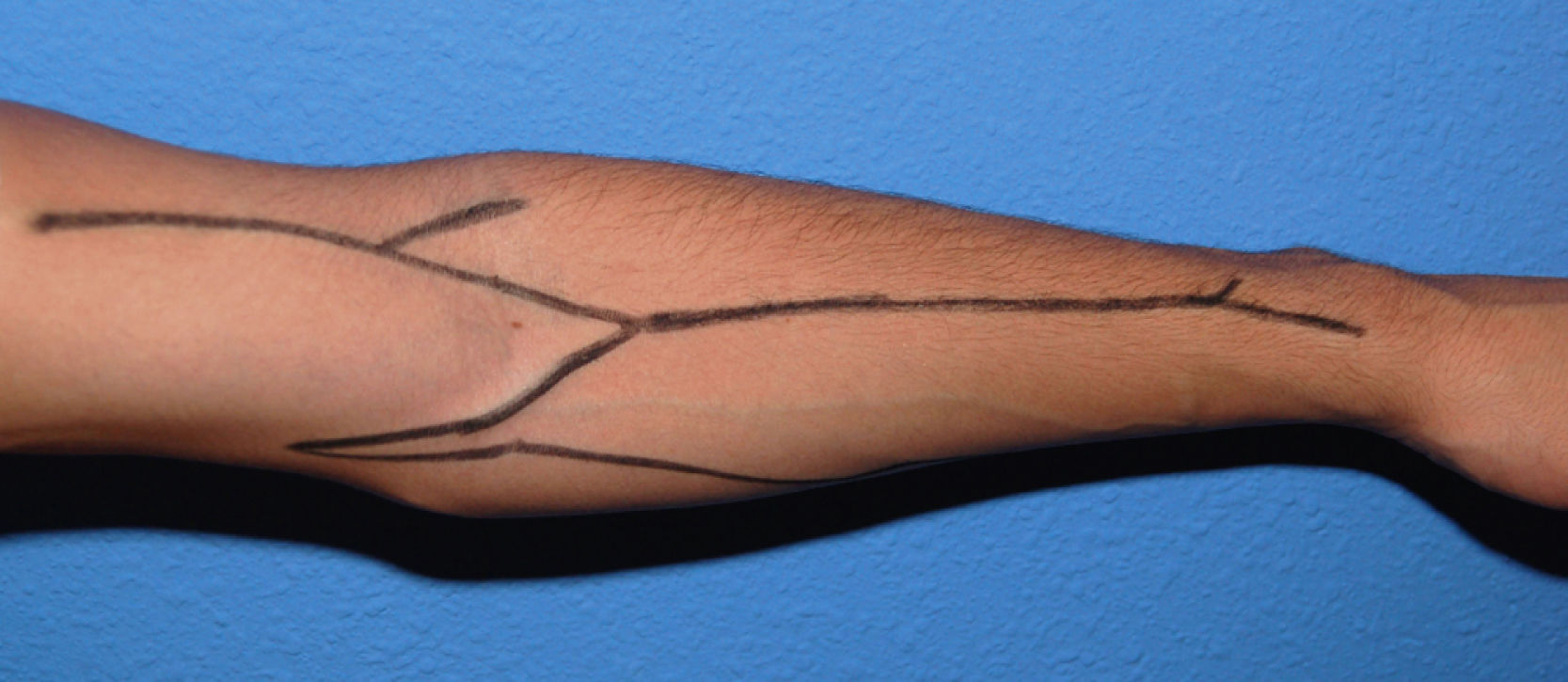
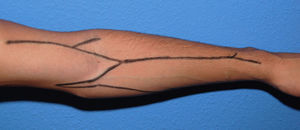
The examination of the superficial venous system must be carried out in a warm room, with the patient seated and his or her arm hanging down. A compressor is used to view the venous network better and determine its distension capacity. It is placed on the forearm to first evaluate the distal region of the arm and the carpus, and then on the proximal third of the arm to view the network around the elbow and proximal arm. The entire path of the cephalic and basilic veins is explored in both arms and forearms (Fig. 1), taking into account their permeability, calibre, twisting, distensibility, signs of previous punctures, scars from previous interventions or segments fibrosed by prior phlebitis along the trajectory. In multi-punctured patients, the thrombosis rate may reach 38%, affecting the cephalic veins in more than half the cases. In general, a vein is considered adequate for a VA when it is visible or clearly palpable through the skin, applying a tourniquet or without it. The presence of collateral circulation in the shoulder or the oedema of an extremity may indicate stenosis or occlusion of the subclavian-axillary venous axis, with the development of compensating subcutaneous collateral circulation and drainage deficit. This needs to be confirmed or ruled out by means of an imaging test. A large fat build-up in an upper extremity may hide the presence of useful veins for the construction of a VA (before ruling out the possibility of an autologous AVF in these patients, a more conclusive imaging test must be carried out), but it may also make it difficult or even prevent punctures of a functioning access.
Other additional details of the physical examination include possible motor and/or sensory deficits or joint limitations of an extremity that may hinder their use for the VA.
In extraordinary cases, the option of constructing a VA in a lower extremity is considered in patients with a history of multiple non-functioning vascular accesses, where the possibilities of new accesses in UE have been exhausted. In these patients, the examination will continue in the LE, with arterial evaluation by pulse palpation (femoral, popliteal, posterior tibial and pedis), auscultation of murmurs and the evaluation of perfusion in both feet. The ankle-brachial index (ABI) provides an objective quantification of distal perfusion. A LE with objective signs of distal perfusion deficit contraindicates the construction of a VA as the unavoidably associated arterial steal may speed up a gangrenous process and endanger the extremity. The trajectory of the internal saphenous vein is examined, and if varicose it is not suitable for an autologous AVF. Varicose veins, perimaleolar oedema, hyperpigmentation and other distal skin problems indicate superficial and/or deep venous insufficiency, which would be seriously worsened by the venous overload represented by a VA. Scars on the extremity may indicate the previous extraction of the internal saphenous veins for bypass, varicose vein operations, arterial ischemia or traumatic lesions that may also have compromised the venous system of the extremity.
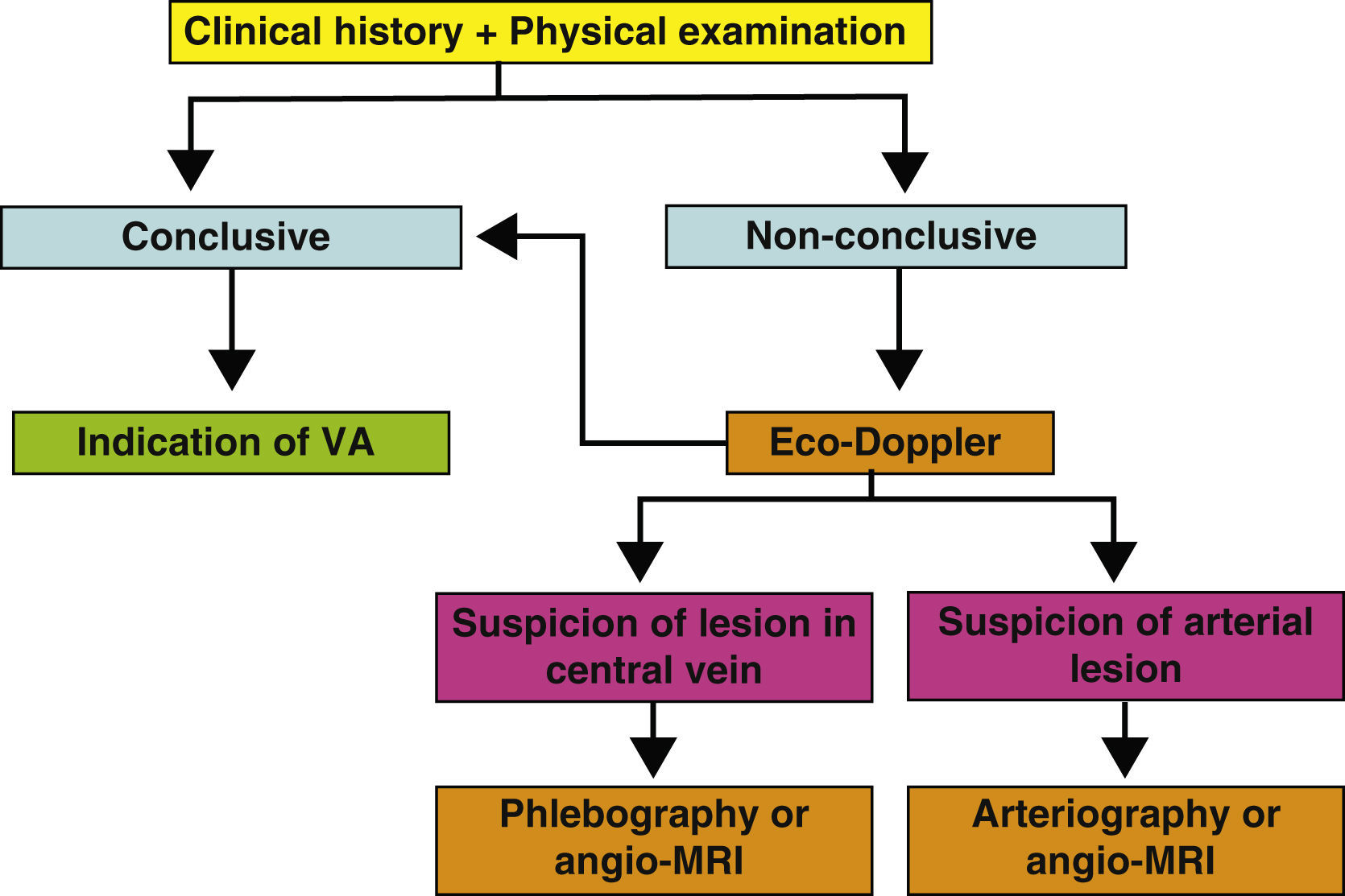
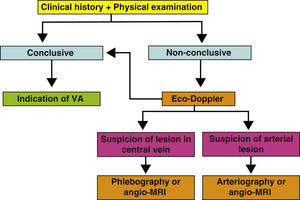
Supplementary testsThe medical history and the physical examination are essential in the preoperative evaluation of a VA. In many cases, they will suffice to establish a successful surgical indication of the VA. But, in others, it will not be possible to ascertain the receiving vein of an autologous or prosthetic access, or there will be signs of a possible deficient arterial network. In these cases, several imaging tests may give us key information about the state of the arterial and venous networks of the extremities (Fig. 2). Their objective is to identify peripheral veins that can be used for a VA (although we must remember that if the vein is not visible or palpable during the physical examination, it will probably not be very distensible or very deep and the possibility of generating a useful VA will be very unlikely), and to confirm the permeability of the central venous system (the subclavian-axillary axis up to the superior vena cava) to guarantee the drainage of an autologous AVF and the functionality of a prosthetic VA.
These tests can be indicated when doubts arise about the permeability of the cephalic, basilar and/or central axis during the physical examination. In other words, when the basilic and cephalic veins are not obvious, such as in obese patients, or the quality proves not to be sufficient in the physical examination. Suspicious physical signs of stenosis or central venous occlusion, and a past history of venous catheterisation or trauma, or surgical procedures in the scapular girdle also require confirmation of use of the extremity for VA. Multi-punctured cephalic and perhaps basilic trajectories and past history of phlebitis also indicate an imaging test that will study them along the whole length. Signs of peripheral arteriopathy in an UE (reduced or no pulses, proximal murmurs, pressure difference >15mm Hg between both UE) usually invalidate the extremity as an arterial donor for a VA and also require the study to be completed. Finally, a history of previous failed VA requires a careful study of the remaining arterial and venous reserves.
The Eco-Doppler is a non-invasive, fast and available test that can be carried out in outpatients and is operator-dependent, which is able to perfectly evaluate superficial venous systems (SVS) and deep venous systems (DVS) in the arm, but it is unable to visualise the proximal subclavian vein or its central drainage due to acoustic shadows caused by the collar bone, the rib cage and the lung. It only obtains indirect haemodynamic data about proximal obstruction of the venous return, which require confirmation with traditional phlebography or angio-MRI. More and more centres are routinely using this system in the preoperative evaluation of AVF and it appears to improve the identification of adequate diameter veins, increasing the number of autologous AVF carried out compared with prosthetic VA (in general, it is estimated that using an imaging venous mapping protocol in the preoperative evaluation increases the construction of autologous accesses by 10-40%, although these figures vary greatly depending on the series), with a lower rate of associated complications. They also reduce the number of failed attempts. The systematic examination with Eco-Doppler includes:
- ∘
Arterial examination - This enables measuring the diameter of the arteries of interest, the intima-media thickness of their wall, their flow and peak systolic velocity, the hyperaemic response and the resistance index. It also helps identify stenotic or occluded segments.
- ∘
Venous mapping – This enables measuring the diameter of the superficial and deep veins of the extremity, their depth and distensibility after placing a proximal tourniquet, their continuity and flow. It also helps identify stenotic or occluded segments. It obtains indirect haemodynamic data about the central venous drainage.
An Eco-Dopplier is currently recommended for any patient in their preoperative study, whenever it is available. If the information it provides is insufficient or requires an additional evaluation of the central veins or of the arterial axis, it will be complemented with one of the following tests:
- •
The phlebography is an invasive test that uses radiation and iodinated contrast. It is potentially allergenic and nephrotoxic and is not recommended in patients with terminal CRI in predialysis, who still preserve a residual renal function that may be affected by this test. It can be used with greater freedom in patients who have already begun treatment with dialysis. But the injection of intravenous contrast into the studied extremity may cause superficial phlebitis that also limits the useful veins for VA. One of its greatest advantages is that it is able to integrally visualise the cephalic and basilic axis and the deep veins up to the drainage into the right atrium. It evaluates their permeability, trajectory, diameter, anatomic variants and possible stenotic or occluded segments. It also enables simultaneous repairs of possible venous lesions via transluminal angioplasty +/- stent (for example, in the subclavian or superior vena cava). It is still the gold standard of venous imaging.
- •
The angio-MRI offers more and more diagnostic and therapeutic possibilities and is becoming more and more accessible and less expensive. It is not invasive, it does not use ionising radiation or iodinated contrast, although the use of gadolinium requires preparation and coordination by the nephrologists due to the risk of precipitating nephrogenic systemic fibrosis. It visualises the DVS and the central thoracic veins especially well. It can visualise peripheral veins of ≥2mm, down to the elbow, although it is still not the imaging technique of choice to evaluate these.
- •
The arteriography is used exceptionally to define an arterial pathology detected in the physical examination and/or Eco-Doppler. It is an invasive test that offers morphological images and the possibility of simultaneously repairing the arterial afflux in some cases. This test is not risk-free, and when available, the arterial angio-MRI of the UE provides similar information with much greater comfort and safety for the patient.
The choice of the right moment to start HD determines the type of initial VA. And the type of VA available at the start of the HD determines the efficiency of the blood purification and thus, the patients’ morbidity and mortality. As we have mentioned in the introduction, this is greater if the first VA is a CVC rather than a AVF.
Some guidelines (DOQI, SNC, VAS, SEN-SEAC) aim to establish patterns and protocols for the indication, construction, maintenance and monitoring of the VA for HD. Their main objective is to obtain VAs that satisfy the requirements of durability, availability and low complication rate, especially considering that the complications derived from the VA are the main cause of hospital admission of patients in HD, with the subsequent impairment of their quality of life and greater economic cost. In all these guidelines, autologous AVF are the first choice, due to the characteristics that we have described above. The intention is for at least 50% of the new VA to be autologous AVF (DOQI). The consensus protocols have managed to increase the number of autologous AVF and CVC over the last few years, in Spain too, and decrease that of prosthetic VA.
There are some general criteria about the indication of VA that can be considered unanimous: the preferential location in UE rather that LE, given that there are fewer complications in the former, with greater long-term permeabilities; distal better than proximal, as the former preserve the most proximal venous network for future VA, should they become necessary, and reduce the possibility of arterial steal, affecting the extremity; non-dominant extremity, for the patient's greater comfort; easy-to-puncture and long trajectory, in order to rotate the puncture points, and more comfortable for the patient during the HD; autologous AVF rather than prosthetic VA, due to greater long-term permeability and fewer complications; and foresee that the VA usually have a limited useful lifetime and that, if the patient remains in an HD programme for many years, new accesses will be required over time, so it is interesting to preserve the greatest venous reserve possible for the future when planning the VA.
In general, the VA construction order starts with a radiocephalic AVF in the non-dominant arm, and then in the dominant arm. If this is no longer possible, the next option will be a humeral-cephalic (HC) AVF in the same order. Then, prosthetic VA in the forearm and basilic vein transpositions. Finally, there is the proximal prosthetic VA option in the arm. VAs in LE are the last resort when the options of performing new accesses in the UE have been exhausted, together with the CVC, given their low permeability and high complication rate (55% thrombosis, 35% infection). They must be indicated according to each individual case. There are some atypical VAs, such as those performed in the anterior wall of the thorax or in the abdomen, which are performed exceptionally in extreme cases, according to the patient's anatomy and the vascular surgeon's criterion.
Special recommendationsThe VA that is closest to the ideal VA today is the radiocephalic AVF. But there are some factors that are associated with a higher rate of early failure of this type of VA. These include: advanced age, which is accompanied by less arterial distensibility and greater endothelial dysfunction, which slows down the maturation of the VA; the female sex, whose vessels usually have a smaller calibre; obesity, which as we have mentioned above, means that the veins are located in a much deeper position, with difficulties for puncture, greater incidence of haematomas and access lesions; diabetes mellitus and high blood pressure, often associated with arterial calcification with greater technical difficulties to construct the access and lower effective maturation rates; low blood pressure, which increases the risk of early thrombosis; both ischemic and dilated cardiopathy, which also favour the failure of the fistula or may worsen with the increase of the cardiac overload due to the vascular short-circuit; peripheral arteriopathy, which reduces the donor afflux pressure to the AVF and increases the risk of distal arterial steal; the current or prior prolonged presence of CVC; thrombophylic haematological disorders; late referral to the vascular surgeon; previous VAs, with manipulation and possible lesion of the vascular networks; small calibre or bad quality radial artery and cephalic vein (radial artery with a calibre ≤1.5mm is associated with a high percentage of early failures, a cephalic vein with a diameter of >3.4mm is associated with access success in 99%); and the flow in the infraclavicular subclavian vein during non-forced inspiration <400ml/min.
Thus, although RC AVF is recommended generally as the first choice, in some specific cases the most recommendable VA may be another one. Elderly people usually present greater distal arterial lesions and frequently less distal venous development or exhaustion due to multiple previous punctures, with a shorter life expectancy, so an HC AVF may be indicated as the first choice of access. In women low calibre peripheral veins are very common, often not very useful in their distal segments, so in these patients an HC AVF as the first choice may also offer a greater chance of effective maturity, unless we find a good cephalic vein in the wrist with continuity in the forearm. Obese patients may often require superficialisation of the venous trajectories and, in the case of limited venous development, which occurs quite frequently, the best option may be a prosthetic VA. Dilated cardiopathy with episodes of congestive cardiac insufficiency requires the CVC to be considered. Significant arterial ischemia in UE and LE may contraindicate a VA, which could affect the feasibility of the extremity, making the CVC or even peritoneal dialysis safer options. A past history of stroke has been related to the lack of maturation of the accesses. Although not generally accepted and each case will have to be addressed individually, it is recommended to perform a VA in the paretic extremity, in order not to further limit the patient's quality of life, although the lack of mobility of the extremity may also affect the process of maturation and success of the access. In the case of active neoplasia, the patient's life expectancy must be assessed and maybe the CVC or a prosthetic VA in the arm should be chosen as first options, with good permeabilities during the first year. If only temporary treatment with haemodialysis is foreseen a CVC can be placed, without the need for a permanent VA. Haemostasis disorders must be treated specifically before the surgical intervention to try to guarantee the success of the access as much as possible and thus avoid complications. In the case of previous failed VA and/or use of CVC, the arterial axis and the venous reserve must be assessed comprehensively, indicating the most feasible VA in each individual case. Finally, children are a special population of renal patients. In general, they do not tolerate dialysis well and are usually candidates for kidney transplant within 6 to 12 months. If, they require RRT in the intermediate period, they usually receive peritoneal dialysis in two thirds of the cases, so long as they have a family environment that is able to adequately manage the devices. In the other third of children who require HD, it is common to use paediatric CVCs, which achieve good flows and permeabilities during the months when they are necessary, prior to the transplant, and they avoid repeated punctures that many children develop panic to, as well as possible haemorrhages. If they require HD on a more long-term basis, a more definite VA must be carried out, following the same general criteria as for the adults: distal rather than proximal, autologous rather than prosthetic, non-dominant extremity, upper extremity rather than lower extremity. The surgical techniques are very similar but much more difficult to perform due to the smaller size of the structures and their special tendency to vasospasm and thrombosis.
ConclusionsIn conclusion, the VAs must be planned and programmed sufficiently in advance to be available at the start of the haemodialysis. Achieving a functioning and adequate VA for the patient requires multi-disciplinary collaboration. The most important factors for the success of a VA are the characteristics of the chosen vessels and the correct staging of the procedures, as not only the first VA must be planned, but new ones in the medium and long term must also be foreseen. Autologous AVFs are generally the first choice, recommending prosthetic VA when the possibilities of autologous AVF in UE have been exhausted. The CVCs are indicated if there is acute renal failure, temporary HD and absence of maturation or impossibility or contraindication of another type of VA. The VAs in LE are used exceptionally in individualised cases. And finally, the clinical characteristics of each patient may affect the indication of the technique to be performed.
Further reading- -
De Francisco ALM, Otero A: Epidemiología de la enfermedad renal crónica en España. Nefrología 2003;28:475-7
- -
López Revuelta K, Saracho R, García-López F, Gentil MA, Castro P, Castilla J, et al: Informe de diálisis y trasplante año 2001 de la Sociedad Española de Nefrología y registros autonómicos. Nefrología 2004;24:21-33
- -
Rodríguez JA, González Parra E: Accesos vasculares para hemodiálisis: preparación del paciente con insuficiencia renal crónica. Angiología 2005; 57(S2):S11-21
- -
Weiswasser JM, Sidawy AN: Estrategias de los accesos arteriovenosos para diálisis. En: Rutherford R: Cirugía Vascular. 6ª Edición. 2 Vol. Elsevier. Madrid, 2006. p.1669-76
- -
Stevick CA: Angioaccess. In: Scribner RG, Brown WH, Tawes RL: Decision making in vascular surgery. B.C. Decker Inc. Philadelphia, 1987. p.186-7
- -
Malovrh M: Approach to patients with ESRD who need an AV fistula. Nephrol Dial Trasplant 2003;18(Suppl 5):v50-2
- -
Del Río Prego A, Aparicio Martínez C, González García A: Accesos vasculares para hemodiálisis. En: SEACV: Tratado de las enfermedades vasculares. Viguera Editores. Barcelona, 2006. p.1255-67
- -
Rodríguez Hernández JA, González Parra E, Gutiérrez Julián JM: Guías SEN. Guías de acceso vascular en hemodiálisis. Nefrología 2005;25 (Supl 1): 1-97
- -
Collins A, Xia H, Ma J: Pre-ESRD VA insertion is associated with improved elderly patient survival. J Am Soc Nephrol 1997;8:230-5
- -
Górriz JL, Sancho A, Pallardó LM, Amoedo LM, Martín M, Sanz P, et al: Prognosis significance of unplanned start of dialysis. A Spanish multicentrical study. Nefrología 2001;22:49-59
- -
Moreno RM: Registro de actividades de la SEACV, año 2008. Angiología 2009;61:325-48
- -
White GH, Wilson SE: Planning and patient assessment for vascular access surgery. In: Wilson SE: Vascular access: principles and practice. 4th edition. Mosby, St. Louis 2002; p.7-13
- -
Fernández Heredero A, Martínez Aguilar E, March García JR, Acín García F: Momento idóneo de creación del AV desde el punto de vista técnico. Angiología 2005;57(S2):S47-54
- -
Davison JA: Access for dialysis: surgical and radiologic procedures. 2 ed. Georgetown, TX: Landes Bioscience;2002.p1-10
- -
Camblor Santervás LA, Menéndez Herrero MA, Carreño Morrondo JA, Llaneza Coto JM, Rodríguez Olay J: Estudio preoperatorio del paciente: examen físico y pruebas de imagen. Angiología 2005;57:S23-34
- -
Feldman HI, Joffe M, Rosas S, Burns JE, Knauss J, Brayman K: Predictors of successful AVF maturation. Am J Kidney Dis 2003;42:1000-12
- -
Wong V, Ward R, Taylor J, Selvakumar S, How TV: Factors associated with early failure of arterio-venous fistulae for hemodialysis access. Eur J Vasc Endovasc Surg 1996;12:207-13
- -
Brimble KS, Rabbat CG, Schiff D, Ingram AJ: The clinical utility of Doppler ultrasound prior to arteriovenous fistula creation. Semin Dial 2001;14:314-7
- -
Yerdel MA, Kesenci M, Yazicioglu KM, Doseyen Z, Turkcapar AG, Anadol E: Effect of haemodynamic variables on sugically created arteriovenous fistula flow. Nephrol Dial Transplant 1997;12:1684-8
- -
Riera Vázquez R, Cordobès Gual J, Lozano Vilardell P, Manuel Rimbau E, Corominas Roura C, Juliá Montoya J: Selección del tipo de AV en pacientes crónicos y agudos. Angiología 2005;57(Supl 2):S35-45
- -
Sociedad Española de Nefrología. Guías de acceso vascular en hemodiálisis, 2004. URL: http://www.senefro.org
- -
National Kidney Foundation. K/DOQI clinical practice guidelines for vascular access, 2000. Am J Kidney Dis 2001;37(Suppl 1): S137-81
- -
Nguyen N, Cinat ME: Vascular access in the neonatal and pediatric patient. In: Wilson SE: Vascular access: principles and practice. 4th edition. Mosby, St. Louis 2002; p.132-48