Advanced oxidation protein products (AOPPs) are used as a marker to estimate oxidative stress in plasma proteins. Oxidative stress is considered a factor of cardiovascular risk (CVRF) related to increased blood pressure, and dyslipidaemia. The aim of this study was to evaluate the association between plasma AOPPs and CVRF in apparently healthy young adults.
MethodsA prospective cross-sectional study was conducted on 120 students of the Faculty of Chemical-Pharmacobiology of the UMSNH. Body mass index (BMI) and blood pressure were determined. A blood specimen was also collected to quantify AOPPs, glucose, total cholesterol, lipoproteins (high, low, and very low density), and triglycerides.
ResultsDifferences were observed in the groups with and without CVRF, with significant differences in BMI, waist, body fat (p<0.05), and lipid profile (p<0.0001). AOPPs were higher in the group of young people with three and four CVRF (F: 4.651; p=0.002). A negatively correlation was found between AOPPs and LDL cholesterol (r=−0.364; p=0.0001).
ConclusionsIt was observed that AOPPs concentrations are increased as CVRF increase in young adults. Thus, this could be considered an important risk factor, because their deposition in the atherosclerotic plaque favours the atherogenic process, and thus the development of cardiovascular disease. Quantification of AOPPs contributes to the indirect determination of oxidative status in the body. The study of metabolic and oxidative state of apparently healthy young adults is important in the prevention of cardiovascular disease in later life. More longitudinal studies are required to study its evolution.
Los productos avanzados de oxidación proteica (PAOP) son un marcador para estimar estrés oxidativo en proteínas plasmáticas. El estrés oxidativo se considera un factor de riesgo cardiovascular (FRCV), relacionado con el aumento de presión arterial y la dislipidemia. Este trabajo tuvo por objetivo evaluar la asociación entre las concentraciones plasmáticas de PAOP y los FRCV en adultos jóvenes aparentemente sanos.
MétodosEstudio transversal comparativo prospectivo en 120 estudiantes de la Facultad de Químico Farmacobiología de la UMSNH, a los que se les determinó IMC, presión arterial, así como PAOP, glucosa, colesterol total, lipoproteínas (de alta, baja y muy baja densidad) y triglicéridos.
ResultadosLos grupos de jóvenes con y sin FRCV presentaron diferencias significativas respecto a IMC, cintura, grasa corporal (p<0,05) y perfil lipídico (p<0,0001). Se presentaron cifras más altas de PAOP en el grupo de jóvenes con 3 y 4 FRCV (F: 4,651; p=0,002). Los PAOP correlacionaron negativamente con el colesterol LDL (r=–0,364; p=0,0001).
ConclusionesSe identificó que las concentraciones de PAOP se ven incrementadas conforme aumentan los FRCV en los jóvenes, por lo que estos podrían considerarse un factor importante de riesgo debido a que su depósito en la placa de ateroma favorece el proceso aterogénico y así el desarrollo de enfermedades cardiovasculares. La cuantificación de PAOP contribuye a la determinación indirecta del estado oxidativo en el organismo. El estudio del estado metabólico y oxidativo de jóvenes de aspecto saludable es de importancia en la prevención de enfermedades cardiovasculares en etapas posteriores de la vida, sin embargo, se requieren estudios longitudinales para estudiar su evolución.
In the past decade, cardiovascular diseases (CVD) have increased alarmingly, constituting the leading cause of death in Mexico.1 CVD have a clearly established multifactorial origin in which the role of various cardiovascular risk factors (CVRFs) have been described, including high blood pressure (HBP), high cholesterol, high low-density lipoprotein cholesterol (LDL-C) concentrations, low concentrations of high-density lipoprotein cholesterol (HDL-C), smoking and diabetes, in addition to those which have been confirmed more recently such as high triglycerides and obesity, which also play a significant role.2
Mediated by the mass media, which invite new generations to lead unhealthy lifestyles and eat unhealthily,3 the quantification of CVRFs in a young adult population has gained special relevance for developing prevention strategies, since this is a group more susceptible to changing behaviour patterns and establishing healthier lifestyle habits.4
Furthermore, it is well known that oxidative stress arises from an imbalance between the reactive oxygen species generated in the body's metabolic processes and deficiencies in antioxidant systems.5,6 Various prospective studies have established an association between this and CVD, suggesting that oxidative stress is an early event in the development of atherosclerotic plaques6 and subsequent CVD, since during the early stages of atherogenesis it promotes oxidative changes to the LDL-C trapped in the subendothelial space.7 Oxidative stress also has an adverse effect on cellular macromolecules, and because proteins make up the largest group of these molecules, the probability of protein oxidation is increased. In a state of oxidative stress, reactive oxygen species, in addition to chloraminated oxidants—mainly hypochlorous acid (HClO)—cause oxidative damage to plasma proteins, causing dityrosine cross-linking and giving rise to advanced oxidation protein products (AOPP). Witko-Sarsat et al.8 developed a spectrophotometric assay that, to date, can detect the formation of plasma AOPP, and proposed measurement of AOPP as a trustworthy marker to estimate oxidative damage to proteins.
The objective of this study was to evaluate the existing association between plasma AOPP concentrations and CVRFs in a population of apparently healthy young adults, and to estimate the frequency of dyslipidaemia and prediabetes in this population.
Materials/patients and methodsPatientsA comparative, cross-sectional study was conducted to study 120 apparently healthy young adults, students at the Faculty of Pharmacobiological Chemistry at Universidad Michoacana de San Nicolás de Hidalgo (UMSNH), in Morelia, Mexico. Young adults of both genders were included, between 18 and 30 years of age, who gave their written informed consent and were not taking drugs that could compromise the results of the study, such as oral hypoglycaemic agents, antihypertensives, lipid-lowering agents or weight loss medications. Young adults with a prior diagnosis of diabetes, high blood pressure, hypothyroidism or those who were pregnant were excluded.
Each patient was interviewed to research CVRFs, hereditary-familial history of chronic diseases such as type 2 diabetes, high blood pressure and kidney disease, as well as physical activity and smoking habits. Blood pressure (BP) was also measured with a calibrated mercury blood pressure sphygmomanometer, while seated, having not ingested caffeine-rich beverages or smoked in the 30min prior to the study. High blood pressure was determined with systolic BP (SBP) values ≥130mmHg, or diastolic BP (DBP) ≥85mmHg, according to the guidelines for high blood pressure management of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC).9
Bioelectrical impedance was performed (Tanita® TBF 300 GS, Tokyo, Japan) to measure their weight, height, BMI and body fat percentage (BF%). Cut off points for being overweight (≥25kg/m2) and obese (≥30kg/m2) were set in accordance with the values established by the WHO. Waist circumference (WC) was measured at the mid point between the lower border of the ribs and the iliac crest. A WC ≥102cm for men and a WC ≥88cm for women was considered abdominal obesity.10
Blood testsThe blood tests were scheduled after 12h of fasting. 10ml of blood was collected via venipuncture, 7ml of which were used for blood glucose measurement and lipid profile, while the remaining 3ml were used to quantify blood AOPP. Both serum and plasma were collected by centrifuging the sample at 3000rpm for 15min, the aliquots were stored at −30°C for later use, within a period no greater than 3 months. Serum concentrations of glucose, triglycerides, total cholesterol and high and low density lipoprotein cholesterol were measured with an automated clinical chemistry system (Vitros 5.1 Ortho Clinical Diagnostics®).
Abnormal baseline fasting glucose in young adults was considered to be ≥100mg/dl (according to the values established by the American Diabetes Association [ADA]11), and dyslipoproteinaemia according to the Adult Treatment Panel III (ATP-III) (triglyceride values ≥150mg/dl, total cholesterol ≥200mg/dl, LDL-C ≥100mg/dl and HDL-C <40mg/dl in men and <50mg/dl in women).12 Castelli's atherogenic index (AI)13 was calculated based on the total cholesterol and HDL-C values. The young adults in the study were grouped by the number of CVRFs.
Plasma AOPP concentrations were determined in accordance with the spectrophotometric assay described by Witko-Sarsat et al.8 The AOPP concentration was expressed in micromoles per litre (μmol/l) of chloramine-T equivalents and, given that there is no cut-off point to establish high values, the 75th percentile value was used, at a concentration of 48μmol/l. Intra- and inter-assay coefficients of variation (CV) obtained with this method in our laboratory were smaller than 5%.
Statistical analysisThe data were collected and analysed using the statistics package SPSS version 23.0 (SPSS Inc., Chicago, Illinois, USA). The Kolmogorov–Smirnov test was conducted to evaluate the distribution normality of the research parameters. The data were expressed as mean±standard deviation. An ANOVA was performed on one factor to compare the variables to the number of CVRFs. Also, Pearson's correlation coefficient was calculated to determine the association between continuous numeric variables. The linear regression analysis was performed using the consecutive steps method to evaluate the association between CVRFs and AOPP as dependent variable. Differences with p<0.05 were considered significant.
Results120 apparently healthy young adults were analysed who were students at the Universidad Michoacana de San Nicolás de Hidalgo, of which 46 were men and 74 were women. They were divided into 5 groups, classified by the number of CVRFs present.
Table 1 shows the general characteristics (clinical, anthropometric and biochemical) of the apparently healthy young adults by the number of CVRFs. It was observed that they were similar in height and BP (p>0.05). However, significant differences were noted in obesity indicators, both abdominal [circumference] and BMI and BF% (p<0.0001). Significant differences were also found upon analysing the biochemical parameters, both for glucose (p=0.008) and for the lipid profile and AI (p=0.0001). In the group of subjects with two (n=25) and three (n=19) CVRFs, average BMI values (26.01±3.40 and 26.07±3.82kg/m2, respectively) indicative of being overweight were observed, while in the group with four CVRFs (n=18), BMI values (30.02±3.98kg/m2) consistent with obesity were observed, with high AI (6.21±3.30) and LDL-C values (111.94±32.26mg/dl) and low HDL-C concentrations (37.94±16.31mg/dl). No young adults with five CVRFs were found.
General characteristics of young adults by the number of cardiovascular risk factors.
| Variables | 0 CVRFs (n=28) | 1 CVRFs (n=30) | 2 CVRFs (n=25) | 3 CVRFs (n=19) | 4 CVRFs (n=18) | p* |
|---|---|---|---|---|---|---|
| Age (years) | 21.68±1.96 | 22.67±2.59 | 22.12±2.33 | 22.74±2.42 | 25.29±3.26 | 0.0001 |
| Weight (kg) | 57.45±10.04 | 63.33±11.72 | 73.79±12.11 | 70.79±10.75 | 79.38±13.64 | 0.0001 |
| Height (m) | 1.65±0.10 | 1.59±0.31 | 1.68±0.08 | 1.65±0.12 | 1.63±0.11 | 0.434 |
| BMI (kg/m2) | 20.97±2.41 | 23.39±3.59 | 26.01±3.40 | 26.07±3.82 | 30.02±3.98 | 0.0001 |
| WC (cm) | 74.39±6.05 | 77.20±8.95 | 84.56±8.13 | 85.21±7.58 | 95.47±11.18 | 0.0001 |
| BF (%) | 18.65±8.85 | 23.16±7.63 | 28.17±9.05 | 28.31±7.88 | 33.48±8.11 | 0.0001 |
| SBP (mmHg) | 107.04±7.80 | 108.20±10.66 | 111.36±10.83 | 111.26±18.48 | 113.53±13.67 | 0.379 |
| DBP (mmHg) | 63.43±6.58 | 68.57±6.39 | 70.24±7.90 | 71.37±13.07 | 74.94±12.15 | 0.080 |
| Glucose (mg/dl) | 70.38±12.0 | 72.40±14.39 | 74.65±8.76 | 79.42±18.27 | 85.14±16.71 | 0.008 |
| Triglycerides (mg/dl) | 97.78±19.90 | 106.78±47.17 | 103.53±37.21 | 139.60±72.23 | 178.89±59.83 | 0.0001 |
| Cholesterol (mg/dl) | 112.84±31.82 | 117.99±36.20 | 152.50±56.87 | 163.42±51.18 | 182.67±28.98 | 0.0001 |
| HDL-C (mg/dl) | 59.31±6.33 | 56.39±7.64 | 47.43±14.59 | 49.92±17.80 | 37.94±16.31 | 0.0001 |
| LDL-C (mg/dl) | 46.61±26.23 | 48.62±31.82 | 93.75±48.66 | 91.17±52.55 | 111.94±32.26 | 0.0001 |
| Atherogenic index | 1.92±0.57 | 2.14±0.73 | 3.80±2.35 | 4.09±2.78 | 6.21±3.30 | 0.0001 |
The data are shown as average±standard deviation. ANOVA test.
BF, body fat; BMI, body mass index; CVRF, cardiovascular risk factors; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; WC, waist circumference.
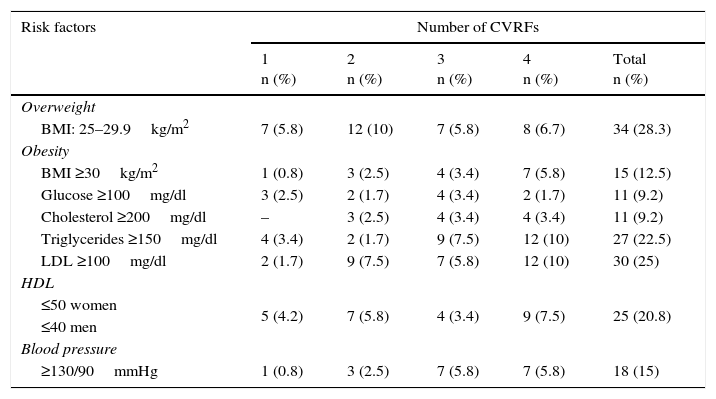
The frequency of CVRFs in the young adults in the study are show in Table 2. 40.8% of subjects were found to be overweight or obese, followed by LDL ≥100mg/dl (25%), hypertriglyceridaemia (22.5%) and low levels of HDL (20.8%).
Frequency of cardiovascular risk factors in apparently healthy young adults.
| Risk factors | Number of CVRFs | ||||
|---|---|---|---|---|---|
| 1 n (%) | 2 n (%) | 3 n (%) | 4 n (%) | Total n (%) | |
| Overweight | |||||
| BMI: 25–29.9kg/m2 | 7 (5.8) | 12 (10) | 7 (5.8) | 8 (6.7) | 34 (28.3) |
| Obesity | |||||
| BMI ≥30kg/m2 | 1 (0.8) | 3 (2.5) | 4 (3.4) | 7 (5.8) | 15 (12.5) |
| Glucose ≥100mg/dl | 3 (2.5) | 2 (1.7) | 4 (3.4) | 2 (1.7) | 11 (9.2) |
| Cholesterol ≥200mg/dl | – | 3 (2.5) | 4 (3.4) | 4 (3.4) | 11 (9.2) |
| Triglycerides ≥150mg/dl | 4 (3.4) | 2 (1.7) | 9 (7.5) | 12 (10) | 27 (22.5) |
| LDL ≥100mg/dl | 2 (1.7) | 9 (7.5) | 7 (5.8) | 12 (10) | 30 (25) |
| HDL | |||||
| ≤50 women | 5 (4.2) | 7 (5.8) | 4 (3.4) | 9 (7.5) | 25 (20.8) |
| ≤40 men | |||||
| Blood pressure | |||||
| ≥130/90mmHg | 1 (0.8) | 3 (2.5) | 7 (5.8) | 7 (5.8) | 18 (15) |
BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
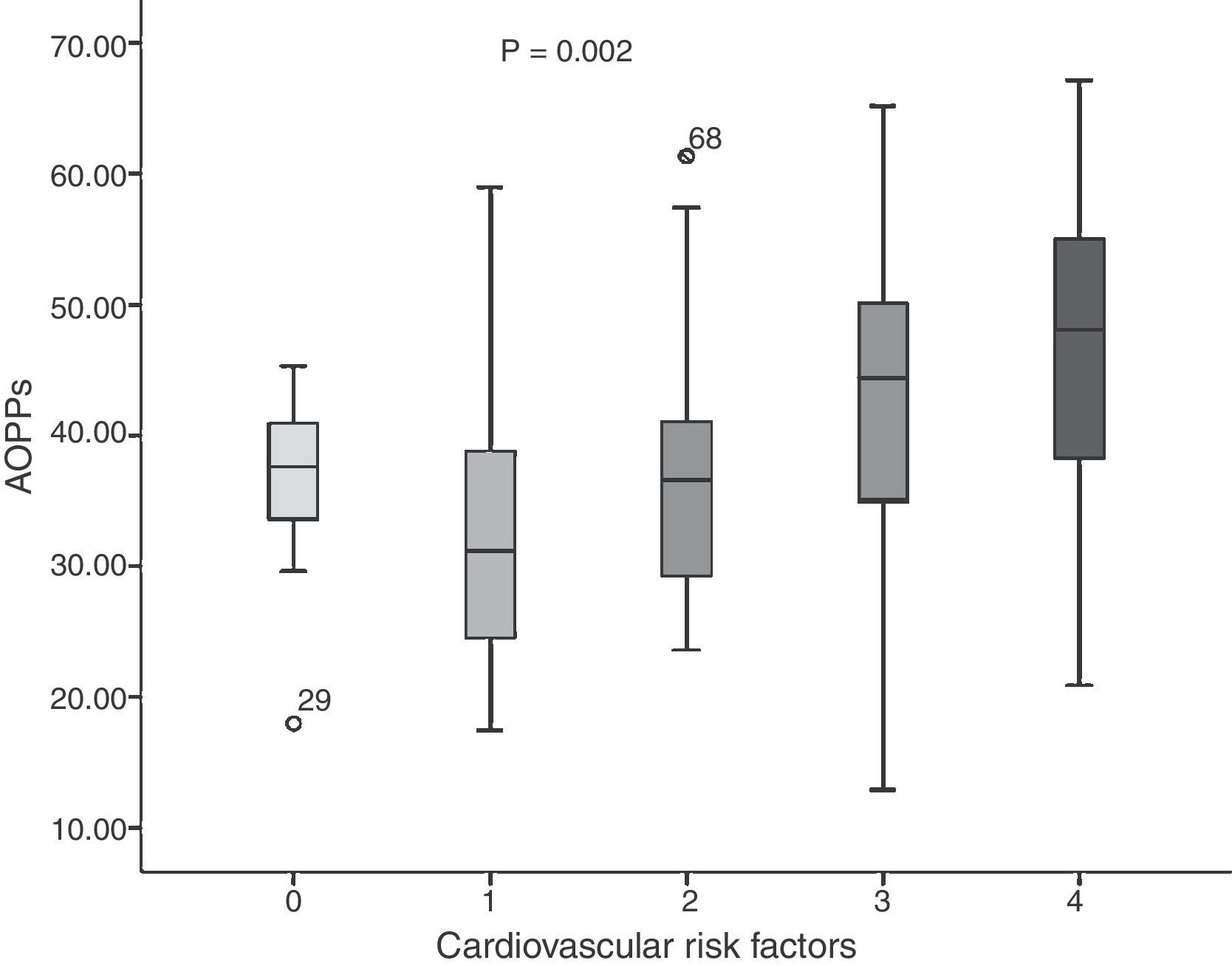
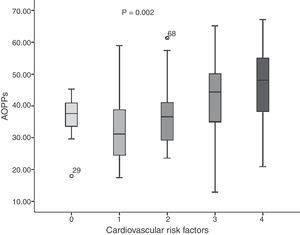
The average concentration of AOPP in the young adults was 37.89±11.19μmol/l. When comparing risk factors (Fig. 1), we found that in the group without CVRFs, the average was 36.90±5.94μmol/l; one CVRF: 32.84±10.62μmol/l; two CVRFs: 36.55±10.14μmol/l; three CVRFs: 42.11±14.70μmol/l, and four CVRFs: 45.47±11.80μmol/l (F=4.651; p=0.002). Nevertheless, higher rates were present in the group with three and four CVRFs.
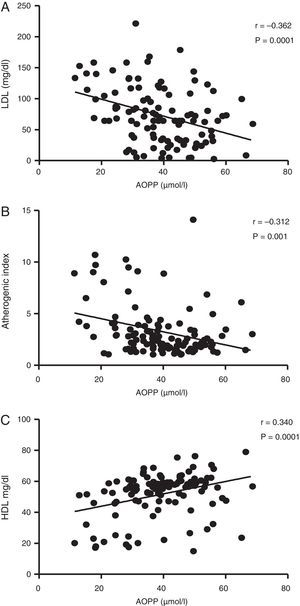
Fig. 2 shows the correlations between plasma concentrations of AOPP and LDL-C (r=−0.362; p=0.0001), AI (r=−0.312; p=0.001) and HDL (r=0.340; p=0.0001). No significant correlation was found between AOPP and serum glucose concentrations (r=−0.065; p=0.494), uric acid (r=0.035; p=0.714), triglycerides (r=0.079; p=0.405), BMI (r=−0.015; p=0.878) and WC (r=0.014; p=0.884).
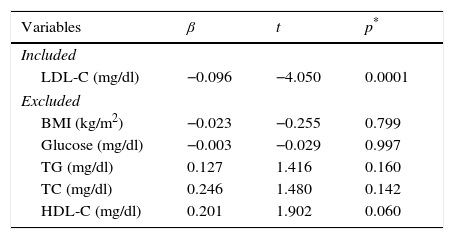
Table 3 shows the linear regression model of the variables that explain the AOPP concentrations. This analysis was adjusted for age and gender; the variable that most explains the AOPP concentration was LDL-C (R2=0.131, F=16.405; p=0.0001). The variables excluded from the model were: BMI, glucose, total cholesterol, triglycerides and HDL-C.
Regression analysis of cardiovascular risk factors that predict AOPP concentrations.
| Variables | β | t | p* |
|---|---|---|---|
| Included | |||
| LDL-C (mg/dl) | −0.096 | −4.050 | 0.0001 |
| Excluded | |||
| BMI (kg/m2) | −0.023 | −0.255 | 0.799 |
| Glucose (mg/dl) | −0.003 | −0.029 | 0.997 |
| TG (mg/dl) | 0.127 | 1.416 | 0.160 |
| TC (mg/dl) | 0.246 | 1.480 | 0.142 |
| HDL-C (mg/dl) | 0.201 | 1.902 | 0.060 |
AOPP, advanced oxidation protein products; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides.
This study reports AOPP concentrations in apparently healthy young adults and their increase as the number of CVRFs increases, and confirms the association between plasma AOPP concentrations and LDL-C as CVRFs in apparently healthy young adults.
In recent decades, young adults’ lifestyles have changed, leading to an increase in CVRFs in this population. This study found that being overweight and obese were the most common risk factors (40.8%) in apparently healthy young adults, followed by high concentrations of LDL-C (25%), which are associated with the development of atherosclerosis14–16 and which in the future could lead to onset of CVD.
AOPP have been described as ultrasensitive markers of oxidative stress in addition to being a new class of inflammatory mediators,17 since they activate neutrophils, monocytes and T lymphocytes.18 It is well known that overproduction of reactive oxygen species under pathological conditions compromises endothelial functions, being considered a causing factor of vascular dysfunction,19,20 which is important in the pathophysiology of various CVDs including arteriosclerosis21,22 and others such as diabetes mellitus.23 This study showed that the higher the number of CVRFs present in apparently healthy young adults, the higher the concentrations of AOPP, which could be considered an additional risk factor for the young adults to develop CVD in the future. To our knowledge, this is the first study where the concentrations of AOPP in apparently healthy young adults are reported in relation to CVRFs.
Despite a high frequency of overweight and obese subjects in this study, no correlation was found between AOPP and BMI or WC. This finding is consistent with the study conducted by Codoñer-Franch et al. in obese children,24 where no correlation was found between AOPP and anthropometric measurements (BMI, WC). The authors explained that this was due to the extremely small range in WC values; however, in this study we had a wide range of both BMI and WC without any apparent correlation.
On the other hand, hyperlipidaemia is associated with oxidative stress and inflammation,25 which are considered CVRFs.26 It is well known that high LDL-C in young adults predicts the onset of CVD in later stages of life27; this study found a high LDL-C frequency of 25% in the young adults, possibly fostered by a diet rich in saturated fats and carbohydrates in addition to a sedentary lifestyle. One limitation in this study is that a reminder was not issued at least 24h in advance to estimate macronutrient intake in the study sample.
Liu et al.28 have reported that AOPP are an important component of the complex interaction between inflammation and oxidative stress with the atherogenic process. The formation of AOPP is mediated by hypochlorous acid (HClO) arising from the action of myeloperoxidase, the same compound that promotes oxidation of LDL-C (Ox-LDL),29 and it is well known that once oxidised, they are transported to the subendothelial space of artery walls, thus triggering the formation of atherosclerotic plaques. Also, Liu et al. report an accumulation of AOPP in atheromatous plaques, which promotes an oxidative environment and suggests participation in the formation of greater amounts of Ox-LDL. However, a limitation of this study was not having directly quantified the concentration of Ox-LDL.
Our study showed a negative association between LDL-C and AOPP, which could be attributed to the fact that AOPP increased as a result of metabolic changes such as being overweight, obesity, hypertriglyceridaemia, or a combination of the former. Also, it is known that LDL-C are responsible for transporting cholesterol to the tissues. Given the low frequency of hypercholesterolaemia in these apparently healthy young adults, we did not find elevated LDL-C levels.
Pirinccioglu et al.30 conducted a study in subjects with familial hypercholesterolaemia and reported a correlation between LDL-C and high levels of carbonylated proteins, in addition to a greater carotid artery intimal-medial thickness; however, the young adults included in this study stated that they did not know whether they had a family history of hypercholesterolaemia, which would be reflected in an increase in cardiovascular risk.
In conclusion, AOPP concentrations were observed to increase as CVRFs increase in young adults, therefore they could be considered a significant risk factor given that their deposit on atheromatous plaques promotes the atherogenic process and thus the development of CVD.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed adhere to the ethical standards of the responsible human experimentation committee and are to the World Medical Association and the Declaration of Helsinki.
Confidentiality of dataThe authors declare that they have followed their centre's protocols regarding the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis study did not receive direct funding.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We would like to thank the Centro de Investigación Biomédica de Michoacán [Michoacán Biomedical Research Centre] at the Instituto Mexicano del Seguro Social [Mexican Social Security Institute] for the facilities granted for this study.
Please cite this article as: Villalpando Sánchez DC, Alvarez Aguilar C, Gómez García A. Productos avanzados de oxidación proteica (PAOP) y su relación con los factores de riesgo cardiovascular en jóvenes aparentemente sanos. Clin Invest Arterioscler. 2017;29:209–215.