The aim of this study is to analyze the safety and efficacy of stent-graft endovascular treatment for visceral artery aneurysms and pseudoaneurysms.
MethodsMulticentric retrospective series of patients with visceral aneurysms and pseudoaneurysms treated by means of stent graft. The following variables were analyzed: Age, sex, type of lesion (aneurysms/pseudoaneurysms), localization, rate of success, intraprocedural and long term complication rate (SIR classification). Follow-up was performed under clinical and radiological assessment.
ResultsTwenty-five patients (16 men), with a mean age of 59 (range 27–79), were treated. The indication was aneurysm in 19 patients and pseudoaneurysms in 6. The localizations were: splenic artery (12), hepatic artery (5), renal artery (4), celiac trunk (3) and gastroduodenal artery (1). Successful treatment rate was 96% (24/25 patients). Intraprocedural complication rate was 12% (4% major; 8% minor). Complete occlusion was demonstrated during follow up (mean 33 months, range 6–72) in the 24 patients with technical success. Two stent migrations (2/24; 8%) and 4 stent thrombosis (4/24; 16%) were detected. Mortality rate was 0%.
ConclusionIn our study, stent-graft endovascular treatment of visceral aneurysmns and pseudoaneurysms has demonstrated to be safe and is effective in the long-term in both elective and emergent cases, with a high rate of successful treatment and a low complication rate.
El objetivo de este estudio fue analizar la eficacia y la seguridad del tratamiento de los aneurismas y pseudoaneurismas de arterias viscerales mediante la utilización de stenstents recubiertos.
MetodosSerie retrospectiva multicéntrica de pacientes intervenidos por aneurisma o pseudoaneurisma mediante stents recubiertos. Las variables estudiadas fueron: edad, sexo, tipo de lesión (aneurisma/pseudoaneurisma), localización, tasa de éxito terapéutico, tasa de complicaciones durante el procedimiento (según la clasificación SIR) y en el seguimiento. El seguimiento fue realizado mediante evaluación clínica y radiológica.
ResultadosSe trató a 25 pacientes (16 hombres) con una edad media de 59 años (rango 27-79). Diecinueve pacientes presentaban aneurismas y los restantes 6, pseudoaneurismas; la localización principal fue la arteria esplénica (12) seguida por las arterias hepática (5), renal (4), tronco celiaco (3) y gastroduodenal (1). La tasa de éxito fue del 96% (24/25 pacientes). Las complicaciones intraprocedimiento fueron del 12% (4% mayores; 8% menores). El tiempo medio de seguimiento fue de 33 meses (rango 6-72 meses) en los 24 pacientes con éxito técnico, con oclusión completa del aneurisma en todos los casos. En el seguimiento se observaron 2casos de migración (2/24; 8%) y 4de trombosis del dispositivo (4/24; 16%); en ninguno de estos pacientes se produjo daño isquémico del órgano. La mortalidad debida al procedimiento fue del 0%.
ConclusionesEn nuestro estudio, el tratamiento endovascular de los aneurismas y pseudoaneurismas viscerales mediante stents recubiertos demuestra ser seguro y eficaz a largo plazo, tanto de forma electiva como en urgencia, con una alta tasa de éxito terapéutico y una baja tasa de complicaciones.
Visceral artery aneurysms are saccular or spindle-shaped dilatations in the celiac trunk and its branches, superior or inferior mesenteric arteries and renal arteries. They are classically subdivided into false (also called pseudoaneurysms) and true. The walls of true aneurysms consist of the 3 layers of the arterial wall and, in general, are considered as such in the presence of a focal dilatation of the vessel greater than 1.5 times the caliber of the healthy segment of the affected vessel.1 The prevalence of true aneurysms in the adult population is estimated at around 0.1%–2%,1 while the incidence of pseudoaneurysms is difficult to calculate. True aneurysms can occur as a result of an underlying arterial disease, such as atherosclerosis, fibromuscular dysplasia, and arteritis, and generally remain asymptomatic. False aneurysms are ruptures of the arterial wall that are contained between the adventitia or the perivascular tissues.1,2 Pseudoaneurysms are usually the result of direct trauma, inflammation, or wall infection. In recent years, we have witnessed an increase in the diagnosis of aneurysmal disease as a result of the greater demand for imaging tests (increasingly better spatio-temporal resolution) and a surge in percutaneous, laparoscopic and endoscopic procedures in the urinary and biliary tracts, which increase the incidence of pseudoaneurysms.3
The most common site of aneurysms and pseudoaneurysms is the splenic artery and its branches (45%–70% of cases),1,4,5 followed by a smaller percentage in the hepatic artery (approximately 19%–20% of cases),4,6,7 renal artery (15%–25%),1,4,8 and celiac trunk (4%–5%).1,4,9 Finally, the least frequently involved vessels are the superior mesenteric, gastroduodenal and pancreatic arteries.
The greatest complication of visceral aneurysms is rupture, which can occur in approximately 20% of cases of true aneurysms and in 70% of pseudoaneurysms, which results in an associated mortality ranging from 25% to 100%.10–12 The main indication for the treatment of visceral aneurysms is size: those larger than 2cm generally require treatment, regardless of the location, whereas in smaller ones a watch-and-wait approach is suitable, with follow-up imaging methods.1,13,14 The factors that can lead to a change in therapeutic management are: the presence of associated symptoms,15 the growth of more than 0.5cm per year,16 pregnancy (maternal and fetal mortality in case of rupture is higher),14,17 and the presence of certain associated systemic vasculopathies (e.g., type IV Ehlers–Danlos).18 Location also seems to influence the risk of rupture: aneurysms of the hepatic artery, pancreatic arteries and superior mesenteric artery are most related with rupture.19,20 Regarding pseudoaneurysms, there is broad consensus in the literature about the need for treatment, regardless of location and size, due to the high risk of rupture.18,21,22 However, in the pediatric population or in some cases of adults with pseudoaneurysms less than 5mm in size, active follow-up with imaging techniques has been preferred, providing resolution of the disease without the need for invasive treatment.23,24
For many years, conventional surgical treatment has been considered the treatment of choice for this entity, with morbidity and mortality in elective cases of 1.3%–5% in the case of aneurysms2,14,25 and 9.4%25 in pseudoaneurysms. In urgent treatments, mortality varied depending on the location, reaching 25% in the splenic artery and up to 100% in the celiac trunk.2,13,26,27 In recent years, thanks to the important advances in endovascular techniques, a range of possibilities has been created for the minimally invasive treatment of visceral aneurysms: embolization with metallic coils, injection of liquid embolic agents (such as thrombin, N-butyl cyanoacrylate [NBCA] or ethylene vinyl alcohol dissolved in dimethyl sulfoxide), gelfoam (Pfizer, Puurs, Belgium), stents (coated or uncoated combined with coils), plugs or vascular occlusion devices such as the Amplatzer Vascular Plug (St. Jude Medical, St. Paul, Minnesota, USA) or the Micro Vascular Plug (Covidien, Irvine, California, USA), or the combination of two or more of these techniques. In cases where there is no surgical indication (either due to patient characteristics, or because the treatment of the aneurysm or pseudoaneurysm and its cause is not feasible), endovascular treatment is the treatment of choice. Of these, the most frequently chosen involves occlusion of the pathological vessel with coils. This treatment has proven to be safe and effective, although it is at the expense of sacrificing the treated vessel. The placement of coated stents is an alternative that allows potentially preserving vascular permeability and, therefore, distal perfusion, while excluding vascular dilatation.
There are few papers published in the medical literature about the endovascular treatment of visceral aneurysms and pseudoaneurysms using coated stents.12,28,29 Our objective is to analyze the long-term safety and efficacy of the treatment of visceral aneurysms and pseudoaneurysms with coated stents by means of a multicenter retrospective analysis.
MethodsWe conducted a retrospective multicenter analysis of the cases collected in databases with prospective patient introduction from February 2006 to October 2015. Patients included had visceral aneurysms (larger than 2cm) or visceral pseudoaneurysms of any size (including splenic, hepatic, renal, gastroduodenal, mesenteric and celiac arteries) treated endovascularly with coated stent placement.
Informed consent was obtained for the procedure in all patients. At the main medical center of this study, the Ethics Committee did not require authorization for retrospective studies. At both hospitals, informed consent included the approval of patients to anonymously utilize their clinical data for research and teaching purposes, in accordance with the provisions of the corresponding Spanish Personal Data Protection Law.
For each patient, the following variables were collected: age, sex, type of vascular lesion (aneurysm or pseudoaneurysm), location of the lesion (hepatic, splenic, gastric, celiac, superior mesenteric, gastroduodenal or renal artery) and symptoms at presentation (incidental, hemorrhage, abdominal pain).
The pseudoaneurysms differed from true aneurysms in their clinical criteria (history of vessel trauma, bile duct manipulations, inflammatory state or retroperitoneal or intraabdominal neoplasms) and imaging findings (focal alteration in the context of a healthy artery, signs of inflammation in the periphery of the lesion, marked aneurysm wall irregularities).
Procedure TechniquePrior to the interventional procedure, CT angiography was performed to evaluate and confirm the diagnosis and viability of the endovascular procedure. The CT study was done at the first hospital with a 16-slice Philips MDT MX 8000 multidetector helical CT (Philips Healthcare, Cleveland, OH, USA) and at the second center with a 64-slice multidetector helical CT, the Siemens SOMATOM Sensation 64 (Siemens, Erlangen, Germany). The acquired native images were assessed in a workstation with multiplanar reconstructions and maximum intensity projection. The measurements of the aneurysm and afferent/efferent vessels were done with digital subtraction angiography.
As an imaging guide for the treatment, 2 angiographic devices were used: Digital Innova 2000 (GE, New York, NY, USA) and Siemens AXIOM Artis (Siemens, Erlangen, Germany).
Depending on the hemodynamic situation of the patient, the procedure was performed under local anesthesia and sedation or general anesthesia (preferable in noncompliant patients or in cases where intensive monitoring and treatment were necessary during the procedure due to the hemodynamic instability of the patient). Prior to stent placement, the protocol required systemic heparinization to prevent thromboembolic complications (100IU/kg up to a maximum of 5000IU). If the procedure lasted for more than 60min after the initial bolus, a second bolus of 1000IU sodium heparin was administered every hour. In cases of bleeding secondary to aneurysm or pseudoaneurysm, according to the clinical situation of the patient, the risk of heparinization was assessed. In no case with correct excision of the lesion was it considered necessary to reverse heparin to decrease the risk of acute stent occlusion. All patients received a broad-spectrum antibiotic prior to the procedure to prevent infections due to possible stent contamination or infections secondary to necrotic tissue in case of distal organ infarction.
After the procedure, a double antiplatelet regimen was established for 30–60 days (clopidogrel 75mg/day and AAS 100mg/day), and mono-antiplatelet therapy was maintained with AAS 100mg/day, indefinitely. According to the location of the lesion, different vascular accesses (femoral, axillary or humeral) were used to minimize technical difficulties for accessing the affected artery. Guiding catheters (6F) or long introducer sheaths (6 to 9 Fr) were used to introduce the stent to the release site and avoid displacement. Six different types of self-expanding and balloon-expandable coated prostheses were used: Jostent Grafmaster (Abbott Vascular, Rangendingen, Germany), Jomed (Jomed International AB, Helsingborg, Sweden), Symbiot (Boston Scientific, Natick, MA, USA), Advanta (Atrium Medical Corporation, Hudson, NH, USA), Viabhan (WL Gore, Flagstaff, AZ, USA) and Be-Graft (Bentley Innomed GmbH, Hechingen, Germany). In some cases, if the lesion was not correctly sealed due to the stent length, more than one stent was placed telescopically or in different branches (in cases of vascular division) with the intention of maintaining the permeability of all divisions. In the presence of collateral branches originating in the aneurysm, previous coil embolization was performed to avoid type II endoleaks.
During the procedure, safety variables included complications related with the arterial puncture site (arterial perforation/dissection), rupture of the aneurysm/pseudoaneurysm, stent migration or acute thrombosis, ischemic damage to the distal organ, and death. Intraprocedural complications were classified as minor or major according to the Society of Interventional Radiology (SIR) classification30,31 (Table 1).
SIR Grading System for Complications According to Prognosis.
| Minor complications |
| A. No treatment or consequences |
| B. Maintenance therapy, with no consequences; one night of observation |
| Major complications |
| C. Requires treatment, minor hospitalization (48h) |
| D. Requires major treatment, unplanned increase in level of care, prolonged hospitalization (48h) |
| E. Permanent sequelae |
| F. Death |
The technique was considered a success when the stents were correctly placed in the planned location. The procedure was considered effective when stent placement in the planned situation demonstrated the following characteristics: no endoleak, stent permeability and exclusion of the circulatory aneurysm.
Patient follow-up entailed clinical and radiological evaluation using CT angiography, MR angiography or color Doppler plus CT angiography. Long-term effectiveness in the clinical-radiological follow-up was defined as: exclusion of the aneurysm from circulation, with absence of endoleak and with a permeable stent. Long-term safety was also assessed by collecting the following variables: emergence of new symptoms, stent migration or thrombosis, ischemic damage to the distal organ, and 30-day mortality related to the procedure.
Statistical AnalysisWe performed a descriptive statistical study that included the frequencies and percentages for categorical variables and the mean, standard deviation and median (interquartile range) for the continuous variables. The analysis was performed with the SPSS version 20.0 statistical package (SPSS, Chicago, IL, USA).
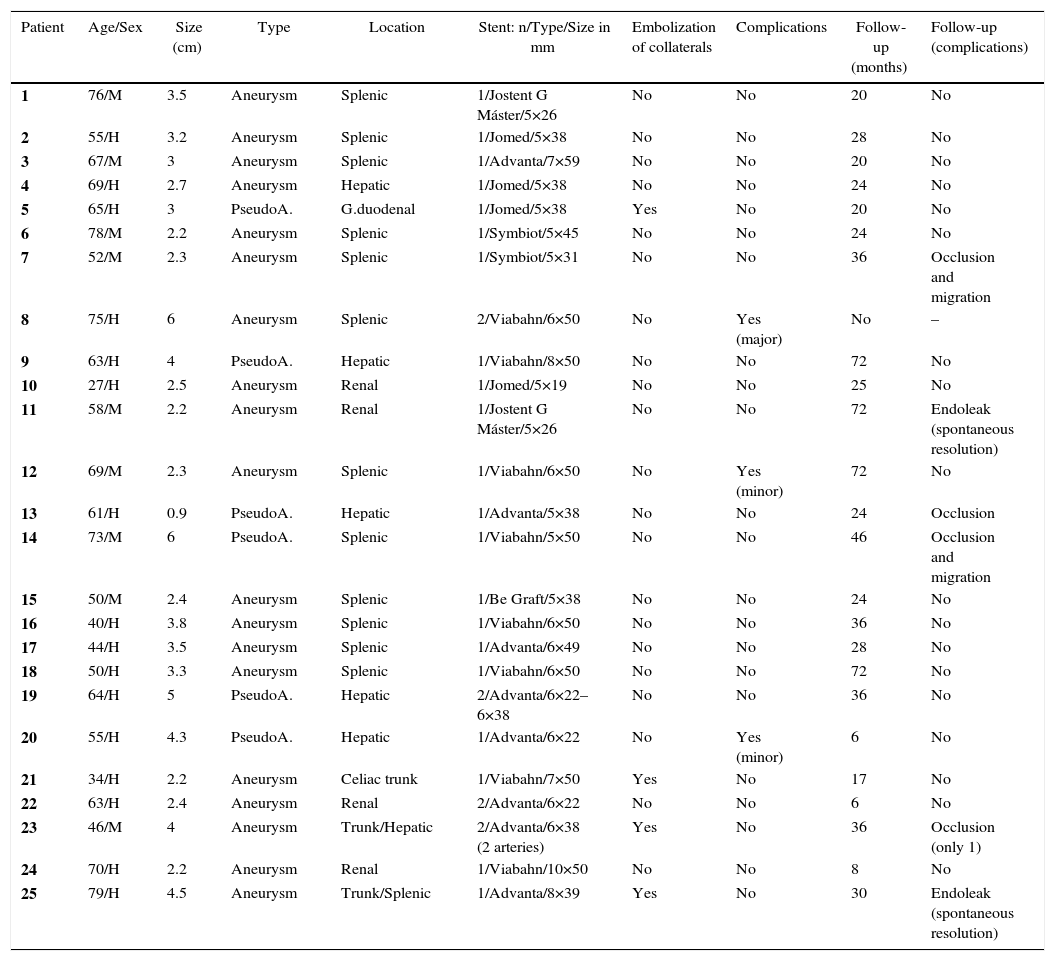
ResultsTwenty-five patients (16 men and 9 women) were identified with 25 lesions diagnosed as visceral aneurysms (n=19) or visceral pseudoaneurysms (n=6), with a mean age of 59 years (range 27–79). To treat the 25 lesions, a total of 29 stents were placed. The location of the lesions was: splenic artery (n=12), hepatic artery (n=5), renal artery (n=4), celiac artery (n=3) and gastroduodenal artery (n=1). Table 2 summarizes the patient data (patients 1, 2 and 3 have already been reported in another series).22
Summary of Patients Treated in Our Series.
| Patient | Age/Sex | Size (cm) | Type | Location | Stent: n/Type/Size in mm | Embolization of collaterals | Complications | Follow-up (months) | Follow-up (complications) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 76/M | 3.5 | Aneurysm | Splenic | 1/Jostent G Máster/5×26 | No | No | 20 | No |
| 2 | 55/H | 3.2 | Aneurysm | Splenic | 1/Jomed/5×38 | No | No | 28 | No |
| 3 | 67/M | 3 | Aneurysm | Splenic | 1/Advanta/7×59 | No | No | 20 | No |
| 4 | 69/H | 2.7 | Aneurysm | Hepatic | 1/Jomed/5×38 | No | No | 24 | No |
| 5 | 65/H | 3 | PseudoA. | G.duodenal | 1/Jomed/5×38 | Yes | No | 20 | No |
| 6 | 78/M | 2.2 | Aneurysm | Splenic | 1/Symbiot/5×45 | No | No | 24 | No |
| 7 | 52/M | 2.3 | Aneurysm | Splenic | 1/Symbiot/5×31 | No | No | 36 | Occlusion and migration |
| 8 | 75/H | 6 | Aneurysm | Splenic | 2/Viabahn/6×50 | No | Yes (major) | No | – |
| 9 | 63/H | 4 | PseudoA. | Hepatic | 1/Viabahn/8×50 | No | No | 72 | No |
| 10 | 27/H | 2.5 | Aneurysm | Renal | 1/Jomed/5×19 | No | No | 25 | No |
| 11 | 58/M | 2.2 | Aneurysm | Renal | 1/Jostent G Máster/5×26 | No | No | 72 | Endoleak (spontaneous resolution) |
| 12 | 69/M | 2.3 | Aneurysm | Splenic | 1/Viabahn/6×50 | No | Yes (minor) | 72 | No |
| 13 | 61/H | 0.9 | PseudoA. | Hepatic | 1/Advanta/5×38 | No | No | 24 | Occlusion |
| 14 | 73/M | 6 | PseudoA. | Splenic | 1/Viabahn/5×50 | No | No | 46 | Occlusion and migration |
| 15 | 50/M | 2.4 | Aneurysm | Splenic | 1/Be Graft/5×38 | No | No | 24 | No |
| 16 | 40/H | 3.8 | Aneurysm | Splenic | 1/Viabahn/6×50 | No | No | 36 | No |
| 17 | 44/H | 3.5 | Aneurysm | Splenic | 1/Advanta/6×49 | No | No | 28 | No |
| 18 | 50/H | 3.3 | Aneurysm | Splenic | 1/Viabahn/6×50 | No | No | 72 | No |
| 19 | 64/H | 5 | PseudoA. | Hepatic | 2/Advanta/6×22–6×38 | No | No | 36 | No |
| 20 | 55/H | 4.3 | PseudoA. | Hepatic | 1/Advanta/6×22 | No | Yes (minor) | 6 | No |
| 21 | 34/H | 2.2 | Aneurysm | Celiac trunk | 1/Viabahn/7×50 | Yes | No | 17 | No |
| 22 | 63/H | 2.4 | Aneurysm | Renal | 2/Advanta/6×22 | No | No | 6 | No |
| 23 | 46/M | 4 | Aneurysm | Trunk/Hepatic | 2/Advanta/6×38 (2 arteries) | Yes | No | 36 | Occlusion (only 1) |
| 24 | 70/H | 2.2 | Aneurysm | Renal | 1/Viabahn/10×50 | No | No | 8 | No |
| 25 | 79/H | 4.5 | Aneurysm | Trunk/Splenic | 1/Advanta/8×39 | Yes | No | 30 | Endoleak (spontaneous resolution) |
Out of the 19 aneurysms, 16 were incidentally detected and 3 presented abdominal pain (one of which was hemorrhaging at the time of diagnosis). The 6 pseudoaneurysms had hemorrhage or abdominal pain. All pseudoaneurysms and aneurysms that were symptomatic or larger than 5cm were considered urgent.
The procedure was technically successful in 24 patients (27 of the 29 stents), with no endoleak in the immediate postoperative follow-up and adequate stent permeability. The efficacy rate was 96%, with immediate exclusion of the aneurysm in 24 of the 25 cases, as verified in the final angiographic series (Fig. 1). In one case with a common celiac artery/hepatic artery aneurysm, 2 stents were placed in parallel: one from the common trunk to the splenic artery and the other from the common trunk to the left gastric artery. The procedure was completed with coil embolization of the hepatic artery distal to the aneurysm and proximal to the gastroduodenal artery, preserving the distal hepatic flow and excluding the aneurysm from circulation. In another 4 cases, prior to stent placement, collateral branches originating at the aneurysm were embolized to avoid type II endoleaks. In 3 patients, 2 stents were placed telescopically. One of these cases, a splenic artery aneurysm, was the patient in whom the procedure could not be completed (upon placing the second stent, there was poor positioning of the distal end and it fell into the aneurysmal sac. In the arteriography series, extravasation of contrast medium was observed, so it was decided to embolize the aneurysm and the proximal artery with coils; the aneurysm was correctly excluded, although the patient presented complicated splenic ischemia with an abscess). In addition to this intra-procedural major complication (type C), another 2 minor complications (type B) were detected related with the arterial puncture site: 2 pseudoaneurysms (one femoral and one axillary), which were both controlled with manual compression and without the need for transfusion or other supportive measures. The overall percentage of complications was 12% (3/25), 4% of which (1/25) were major complications and 8% (2/25) were minor.
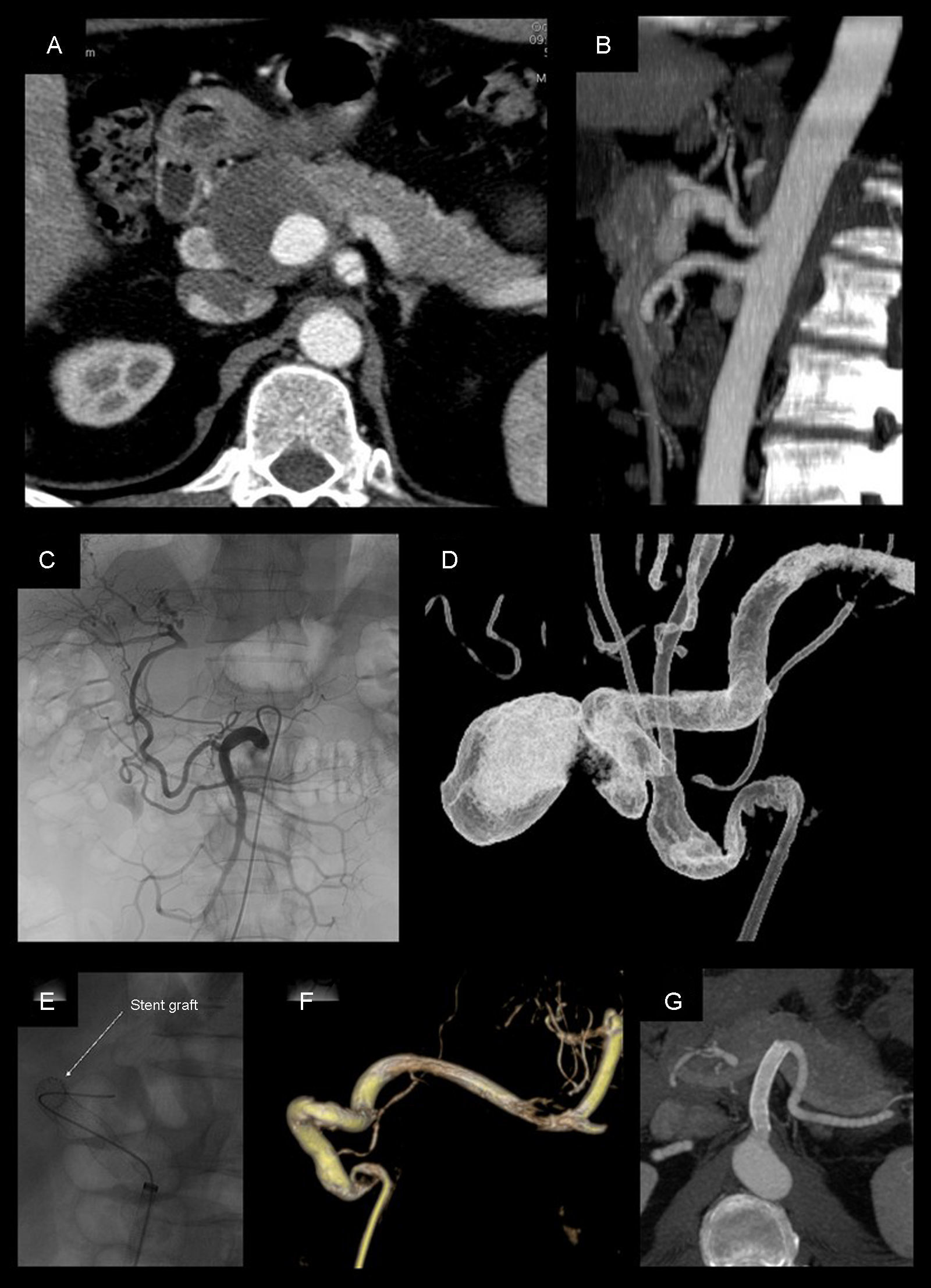
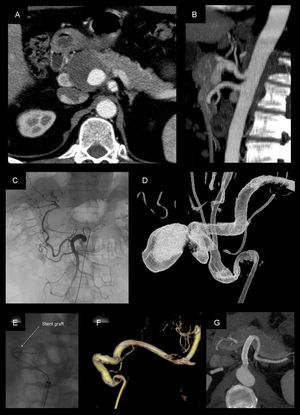
Pseudoaneurysm of the hepatic artery measuring 4cm, treated with coated stent placement (Viabahn 8×50mm): (A and B) CT angiogram prior to treatment; (C) selective angiogram from the superior mesenteric artery, where repermeabilization is observed of the hepatic artery proper through the gastroduodenal artery; (D) rotational angiography done from the celiac trunk; (E–F) placement and angiographic follow-up after positioning the stent, which shows complete exclusion of the pseudoaneurysm with permeability; (G) CT angiogram 72 months after the procedure, showing evidence of stent permeability and absence of endoleaks.
In our series, we did not detect dissection, rupture of the aneurysm/pseudoaneurysm, thrombosis or stent migration during the procedure. There was also no intraprocedural mortality. The mean hospitalization time in the true aneurysms was 40h, while in pseudoaneurysms it was 7 days.
In the 24 patients with coated stent placement, clinical/radiological follow-up was an average of 33 months long (range 6–72 months). Radiological testing showed adequate aneurysm exclusion in all cases, although stent occlusion was detected in 4 patients (long-term efficacy 86%). The patient with intraprocedural type C complication and coil embolization was excluded from follow-up to assess long-term efficacy. Radiological follow-up included CT angiogram in 20 patients and CT angiogram plus color Doppler ultrasound in 2 patients. In the remaining 2 patients, MR angiography was used because of their age (27 and 34 years).
During follow-up, 2 endoleaks were found, one after one month and one after 6 months. These endoleaks had no repercussions on the size of the aneurysmal sac and did not require any additional intervention, resolving spontaneously after 6 and 24 months, respectively. Out of the 4 patients who underwent prophylactic embolization to prevent type II endoleak, 3 had no endoleak in successive controls and the fourth was described previously with endoleak after one month that resolved spontaneously after 6 months. Deferred complications included 4 stent thromboses (4/24; 16%): one after 1 month in a pseudoaneurysm of the hepatic artery, one after 3 months in a pseudoaneurysm of the splenic artery and 2 after 6 months (one in a splenic aneurysm and another in a celiac artery aneurysm). In none of the cases was evidence found of any clinical symptoms or ischemic injury to the related organ.
In the 2 patients with thrombosis of the stent placed in the splenic artery, migration occurred (2/24; 8%). In one, the migration was proximal, probably because of an incorrect measurement due to overestimation of the treated artery, although there were no clinical repercussions or recanalization of the aneurysm. In the second case, a perforation of the gastric wall occurred 36 months after its placement. In the latter case, due to the oncological comorbidities of this patient resulting in a short life expectancy, the approach was noninvasive. The patient died 10 months later due to this baseline disease: adenocarcinoma of the head of the pancreas. This case was has already been reported in the literature.21
DiscussionSurgery is still the treatment of choice in patients with visceral aneurysms and pseudoaneurysms because in many instances it allows the surgeon to treat the vascular lesion and its cause in the same operation. In the last 10 years, however, interventional radiology has offered several treatment alternatives in aneurysmal and pseudoaneurysmal vascular lesions, with a general decrease in hospitalization times compared to conventional surgery and a mortality rate ranging from 0 to 10.5%.9,12,15,32–34 This is largely due to the fact that these techniques are minimally invasive and can be performed with local anesthesia and sedation, which makes their application possible in patients with high anesthesia risk. This fact is supported by the results of our series, in which we had a mortality of 0%. In our experience, the coated stent treatment had a technical success rate of 96%, with immediate efficacy of 100% and long-term efficacy of 86% in those patients with technical success. If we did not consider stent permeability as part of the efficacy variable (as in the case of embolization with plugs and coils) and only considered the exclusion of bleeding, the long-term efficacy would continue to be 100%.
The complication rate during the procedure was 12% (4% major, 8% minor). In the follow-up, complete occlusion of the aneurysm was confirmed in all cases, with a stent thrombosis rate of 16% (there was no ischemic organ injury in any of these patients), and the migration rate was 8%.
In our series, no deaths occurred due to the procedure. In the literature, the technical success of endovascular treatment including all techniques (coils, stents and coils, coated stents, etc.) was between 57.1% and 100%,12,13,29,32,35 depending on several factors, including: the morphology of the afferent/efferent vessels and the aneurysm itself; aneurysm location; presence of associated disease; and, operator experience. Our results, with a technical success of 96%, would fall within this range.
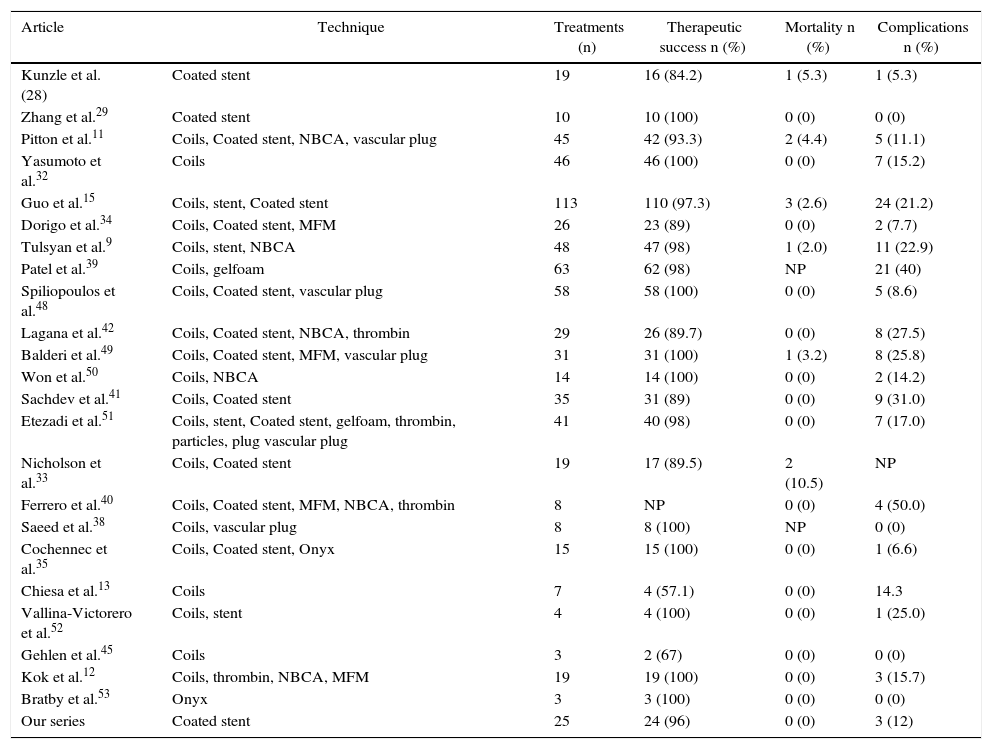
It should be noted that, as the technology used in interventional radiology has evolved, there has also been an improvement in the clinical results obtained in the procedures. Table 3 summarizes the main series of the literature that deal with the endovascular technique in comparison with ours. In a comparative study by Shukla et al.,4 the endovascular technique and conventional surgery did not present significant differences in terms of major periprocedural complications and 30-day mortality in the treatment of unruptured aneurysms. In the case of ruptured aneurysms, several studies have demonstrated lower morbidity and mortality in patients treated by the endovascular approach.4,36,37 It is important to emphasize the importance of patient selection for endovascular treatment to achieve adequate exclusion of the aneurysm with permeability of all the visceral arteries. Otherwise, there is a potential for severe ischemic damage and the surgical technique should be the treatment of choice. Likewise, it should be taken into account that patients treated with the endovascular method need retreatment more frequently than patients treated surgically. In patients with ruptured aneurysms, the retreatment rate in endovascular treatment is 15% compared to 8.2% of patients treated surgically, although these differences are not statistically significant.4
Studies About Endovascular Treatment of Visceral Aneurysms and Pseudoaneurysms.
| Article | Technique | Treatments (n) | Therapeutic success n (%) | Mortality n (%) | Complications n (%) |
|---|---|---|---|---|---|
| Kunzle et al. (28) | Coated stent | 19 | 16 (84.2) | 1 (5.3) | 1 (5.3) |
| Zhang et al.29 | Coated stent | 10 | 10 (100) | 0 (0) | 0 (0) |
| Pitton et al.11 | Coils, Coated stent, NBCA, vascular plug | 45 | 42 (93.3) | 2 (4.4) | 5 (11.1) |
| Yasumoto et al.32 | Coils | 46 | 46 (100) | 0 (0) | 7 (15.2) |
| Guo et al.15 | Coils, stent, Coated stent | 113 | 110 (97.3) | 3 (2.6) | 24 (21.2) |
| Dorigo et al.34 | Coils, Coated stent, MFM | 26 | 23 (89) | 0 (0) | 2 (7.7) |
| Tulsyan et al.9 | Coils, stent, NBCA | 48 | 47 (98) | 1 (2.0) | 11 (22.9) |
| Patel et al.39 | Coils, gelfoam | 63 | 62 (98) | NP | 21 (40) |
| Spiliopoulos et al.48 | Coils, Coated stent, vascular plug | 58 | 58 (100) | 0 (0) | 5 (8.6) |
| Lagana et al.42 | Coils, Coated stent, NBCA, thrombin | 29 | 26 (89.7) | 0 (0) | 8 (27.5) |
| Balderi et al.49 | Coils, Coated stent, MFM, vascular plug | 31 | 31 (100) | 1 (3.2) | 8 (25.8) |
| Won et al.50 | Coils, NBCA | 14 | 14 (100) | 0 (0) | 2 (14.2) |
| Sachdev et al.41 | Coils, Coated stent | 35 | 31 (89) | 0 (0) | 9 (31.0) |
| Etezadi et al.51 | Coils, stent, Coated stent, gelfoam, thrombin, particles, plug vascular plug | 41 | 40 (98) | 0 (0) | 7 (17.0) |
| Nicholson et al.33 | Coils, Coated stent | 19 | 17 (89.5) | 2 (10.5) | NP |
| Ferrero et al.40 | Coils, Coated stent, MFM, NBCA, thrombin | 8 | NP | 0 (0) | 4 (50.0) |
| Saeed et al.38 | Coils, vascular plug | 8 | 8 (100) | NP | 0 (0) |
| Cochennec et al.35 | Coils, Coated stent, Onyx | 15 | 15 (100) | 0 (0) | 1 (6.6) |
| Chiesa et al.13 | Coils | 7 | 4 (57.1) | 0 (0) | 14.3 |
| Vallina-Victorero et al.52 | Coils, stent | 4 | 4 (100) | 0 (0) | 1 (25.0) |
| Gehlen et al.45 | Coils | 3 | 2 (67) | 0 (0) | 0 (0) |
| Kok et al.12 | Coils, thrombin, NBCA, MFM | 19 | 19 (100) | 0 (0) | 3 (15.7) |
| Bratby et al.53 | Onyx | 3 | 3 (100) | 0 (0) | 0 (0) |
| Our series | Coated stent | 25 | 24 (96) | 0 (0) | 3 (12) |
Gelfoam: Pfizer, Puurs, Belgium; NBCA: N-butyl cyanoacrylate; NP: not published; Onyx: Covidien, Irvine, CA, USA; MFM: multilayer flow modulator stent; Tx: treatments.
The overall complication rate of 12% detected in our series is within the range of complication rates for the endovascular technique generally reported in other series (0%–50%).9,29,38–41 According to a recently published meta-analysis,12 the literature reports a rate of endoleaks-recanalization, including the different techniques, of 5.4% (ranging from 0% to 26% in cases of coiling with long-term follow-up).32,41,42 The need for retreatment ranges between 0 and 15%.4,13,34,35 In our experience, we had 2 cases of endoleaks during follow-up, which, due to the absence of increased size or symptoms, did not require treatment. In fact, in both cases spontaneous resolution was observed in the radiological follow-up with CT angiography at 6 and 24 months. In the follow-up of these patients, we had 4 cases of thrombosis after 1, 6 and 12 months, with no evidence of ischemic injury to the associated organ. Kunzle et al. also reported 2 cases of asymptomatic thrombosis during follow-up.28 Acute thrombosis was not reported in this series or in our series. Late thrombosis seems to be better tolerated thanks to collateral hemodynamic compensation, thereby avoiding ischemic damage to the affected organ. This is unlike coil embolization, in which acute and voluntary occlusion of the treated vessel may cause relevant ischemic injury.
In our series, in the case of technical failure, the splenic artery had to be embolized with coils, which caused complicated ischemia with a splenic abscess. Similarly in our series, thrombosis was associated with stent migration in 2 cases, one asymptomatic and one with fistulization to the stomach. In the literature, stent migration into splanchnic vessels usually occurs in association with the placement of EVAR, which are always intravascular migrations. In contrast, extravascular migrations are extremely rare events that appear to be due to the cytotoxic effects of chemotherapy43 or chronic local inflammatory conditions like pancreatitis, as in our case. In a mixed surgical and endovascular series, Ferrero et al. report one case of coated stent migration in a patient with a celiac trunk aneurysm and previous aorto-aortic bypass; the case was resolved with placement of a second stent.40
In the published series, embolization with platinum coils is the most commonly used technique.12 But, since it does not guarantee the distal permeability to the embolized vessel area, different complications related to this technique have been reported, such as sac perforation,1 postembolization syndrome (0%–30% of cases)9,29,35,44 and major ischemia of organs vascularized by said arteries (50% or more of the organ), which, according to the literature, can occur in 0 to 21% of cases.32,39,45 From a theoretical standpoint, which our experience endorses, the endovascular technique that would reduce the rate of this type of complications is the use of coated stents, since they preserve the permeability of the treated vessel (with a lower associated risk of perforation of the aneurysmal sac and distal embolus)46 while reducing the time of the procedure.29,45 Stent repair of aneurysms and pseudoaneurysms is particularly indicated in cases of wide neck aneurysms (2:1 ratio between the diameter of the aneurysm and the neck), situated in vessels proximal to the aorta or those with limited tortuosity (given the limitation of the profile of current stents that require a minimum size of 6 Fr), both electively and urgently. Similar to most groups, our preference is the use of balloon-expandable stents in cases where high precision is required when placing the device (non-embolizable branches near the sac, aneurysms very proximal to the aorta) and in areas where there is no risk of extrinsic compression. We prefer self-expandable stents in cases where greater flexibility of the device is more useful due to the tortuosity of the affected vessels. It is also important to remember that, in addition to various brands of stents, there are also differences in lengths, which should be considered when deciding on the device to be used.
Preventive embolization of collateral branches at the origin of the aneurysm is recommended to avoid endoleaks; the coil technique is the most widely used. Kunzle et al.28 reported one case of organ infarction out of 9 embolizations. In our series, embolization of the collateral branches was necessary in 4 cases, with no evidence of related ischemic damage. In another series of 10 patients, treated only with coated stents and limited to patients with celiac trunk aneurysm, the authors reported a 100% success rate without complications.29
The use of multilayer stents with flow redirection could be an alternative to the use of coated stents, with the potential advantage of the preservation of collateral branches. There are still few data in the literature concerning the use of multilayer stents, and these are limited to elective cases. One of the most extensive series is that of Ruffino et al. on 19 aneurysms treated with the Cardiatis Multilayer Flow Modulator stent (Cardiatis, Isnes, Belgium), which, after 6 months, presented aneurysm exclusion in 87.5% of the cases, with collateral preservation in all cases. Stent thrombosis occurred in 2 cases.47
Ours is the first multicenter study and the most numerous series published in the literature about the endovascular treatment of visceral aneurysms with coated stents. The study presents some limitations: it is retrospective, the number of patients is insufficient to perform a statistically significant analysis, and adequate stratification is not possible (due to the extreme variability of the characteristics of the aneurysm, the vessel involved and the type of stent used). Last of all, there are currently no clinical practice guidelines available for the treatment or follow-up of this condition.
In our study, the endovascular treatment of visceral aneurysms and pseudoaneurysms using coated stents was found to be safe and effective, in both elective and emergency situations, with a high rate of therapeutic success and a low rate of long-term complications.
Authorship/CollaborationsStudy design: MC, FG, JMF, MR.
Data collection: MC, FZ, GO, JM, FL, DB, MAT.
Analysis and interpretation of the results: MC, FZ, GO, JM, FL, DB, MAT, ALR, FG, JMF, MR.
Article composition: MC, FZ, ALR, FG.
Critical review: MC, FZ, GO, JM, FL, DB, MAT, ALR, FG, JMF, MR.
Approval of the final version: MC, FZ, GO, JM, FL, DB, MAT, ALR, FG, JMF, MR.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Cappucci M, Zarco F, Orgera G, López-Rueda A, Moreno J, Laurino F, et al. Tratamiento endovascular de aneurismas y pseudoaneurismas de arterias viscerales mediante stents recubiertos: análisis de resultados inmediatos y a largo plazo. Cir Esp. 2017;95:283–292.