The aim was to analyse the stoma reversal rate after surgery for complicated acute diverticulitis (CAD), and more specifically the end-stoma-reversal, as well as the delay, feasibility, complications and risk factors for stoma maintenance.
MethodsA multicentre retrospective study of patients who had undergone urgent surgery for CAD with stoma formation in 10 hospitals during a period of 6 years. The frequency of reversal over time and the factors affecting the decision for reversal were analysed.
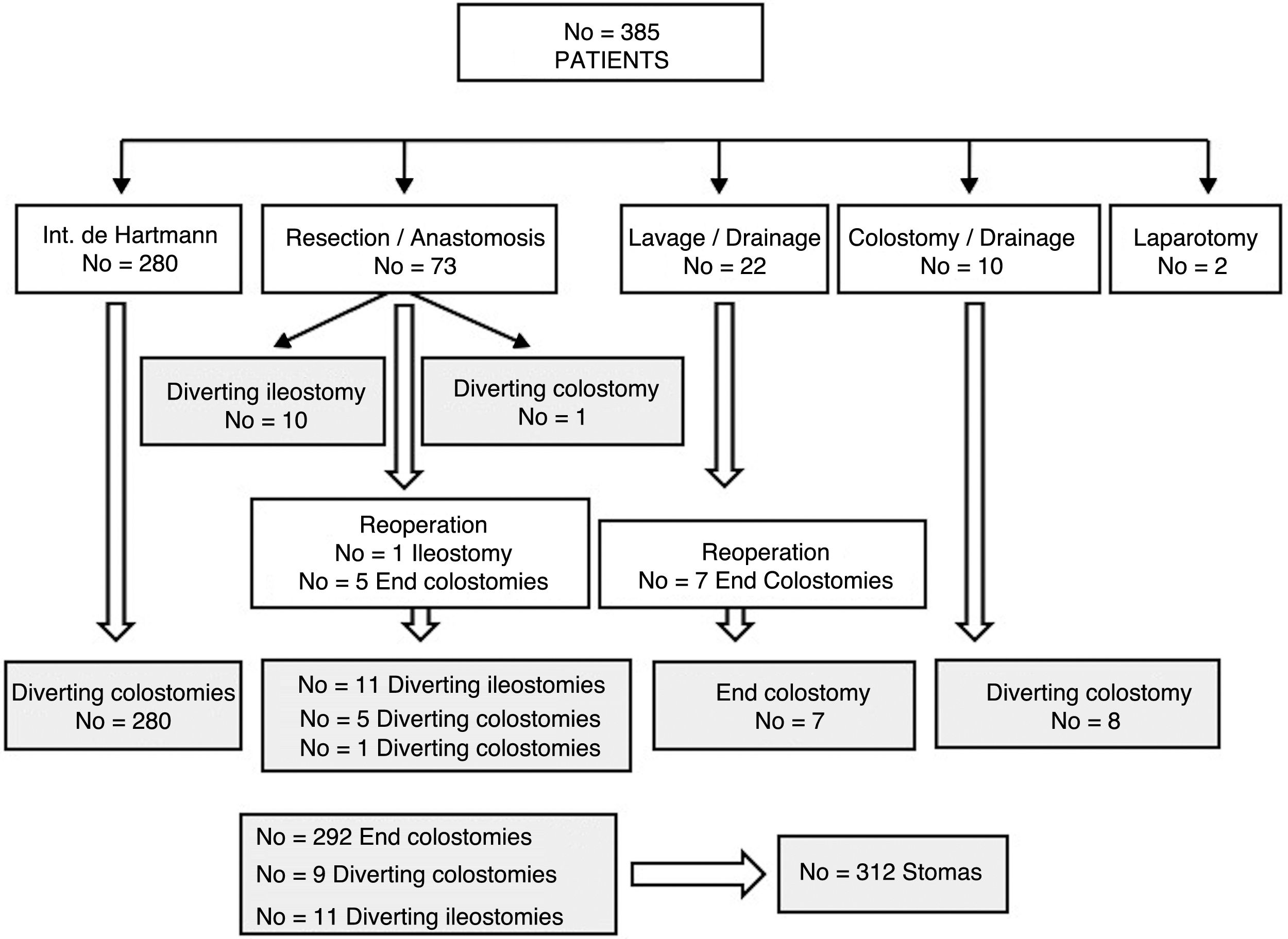
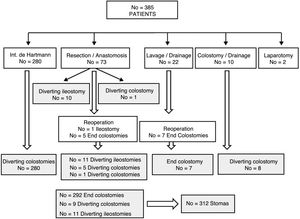
ResultsOut of 385 patients operated for CAD, 312 underwent stoma creation: 292 end colostomies and 20 diverting stomas. During follow-up, stoma reversal surgery was performed in 161 patients (51.6%) after a median of 9 months. The main causes for not performing stoma reversal were comorbidities and the death of the patient. Advanced age was an adverse factor in the multivariate analysis, and the actuarial rate of reversal was higher in men and in patients with no previous Hartmann's operation. Stoma reversal surgery was completed in all but 1 patient, and a loop ileostomy was associated in 4. Morbidity and mortality rates were 35.7% and 1.9%, respectively. A total of 8.4% of patients underwent re-operation, and 6% experienced an anastomotic leak. Twelve patients remained with a stoma after the attempted reconstruction surgery.
ConclusionsSurgery for CAD is frequently associated with an end stoma, which will ultimately not be reversed in almost 50% of patients. Moreover, reversal surgery is frequently delayed and is associated with significant morbidity and mortality.
El objetivo del estudio es analizar la tasa de reconstrucción del estoma tras cirugía por diverticulitis aguda complicada (DAC), su demora, factibilidad, complicaciones y factores de riesgo de mantenerlo.
MétodosEstudio retrospectivo multicéntrico de pacientes intervenidos mediante cirugía urgente por DAC con realización de un estoma en 10 hospitales durante 6 años. Se analiza la frecuencia de reconstrucción del estoma, fundamentalmente de los terminales, y el tiempo en que se produce, así como los factores relacionados con ella.
ResultadosDe 385 pacientes intervenidos por DAC, a 312 (81%) se les realizó un estoma: 292 fueron colostomías terminales y 20 estomas derivativos. Durante el seguimiento, en 161 (51,6%), se intentó el cierre a una mediana de 9 meses. Las causas más frecuentes de no efectuarlo fueron la comorbilidad y el fallecimiento del paciente. La edad más avanzada se mostró factor adverso en el análisis multivariante y la tasa actuarial de reconstrucción fue mayor en hombres y en quienes no se realizó un Hartmann. La cirugía pudo completarse en todos menos en un paciente y en 4 se asoció un estoma derivativo. La morbimortalidad fue del 35,7 y 1,9%, respectivamente. Hubo un 8,4% de reintervenciones y un 6% de fallos de sutura, quedando 12 pacientes (7,9%) con un estoma tras el intento de reconstrucción.
ConclusionesLa cirugía de la DAC se asocia muy frecuentemente a la construcción de un estoma terminal, que en casi un 50% no se reconstruirá. Además, la intervención de reconstrucción tiene una demora notable y está asociada a una morbimortalidad nada despreciable.
Despite changes in the operative management of complicated acute diverticulitis (CAD),1 the Hartmann procedure remains the most widely used treatment.2,3 However, aside from the controversy over the choice of technique and its impact on the initial results, these patients will require a second surgery to restore intestinal continuity, which itself has technical difficulties and inherent risks. In addition, up to 20%–50% of patients who undergo a Hartmann procedure for any indication will never be reconstructed.4
Although there are case series that deal with reconstruction,5–7 the obvious difference between CAD and other indications, such as surgery for complicated colorectal cancer, ischaemia or trauma, to name just a few, makes a specific analysis interesting.
The objective of this study is to assess the stoma reconstruction rate, particularly for end colostomy reversal (ECT), after urgent surgery for CAD, its delay, feasibility and complications, as well as the risk factors for stoma maintenance.
MethodsWe conducted a multicentre retrospective study within the Valencian Society of Surgery. Inclusion criteria included patients who had undergone emergency or deferred emergency surgery related to the failure of a conservative treatment after urgent hospitalization, a diagnosis of CAD and the creation of a stoma during initial surgery or after a reoperation due to postoperative complications. The study period was from January 2004 to December 2009 and data were collected at the end of 2012. The results of this initial surgery for CAD were recently published.8 At each hospital involved, a surgeon in charge was given the study protocol and a computer file for data collection. The study was approved by the Clinical Research Ethics Committee at the General University Hospital of Valencia.
The 81 variables analysed included 40 related with the initial surgery for CAD, including demographics, comorbidity, surgical indication, findings and type of intervention that led to the stoma. The other 41 variables were related with the stoma reversal, particularly when it was terminal, the delay and results in terms of hospital stays and morbidity and mortality within 30 days after surgery using the modified Clavien-Dindo classification,9 as well as factors related with both the closure and its complications.
Statistical AnalysisThe data were analysed with SPSS (version 20) statistical software for Windows (SPSS Inc, Chicago IL, USA). The Mann–Whitney U or Kruskal–Wallis tests were used for independent data, and the categorical variables were analysed with the Chi-squared and Fisher's tests. We used binary logistic regression to predict the influence of variables with a significance of P<.1 in the univariate study about the stoma closure or its morbidity and mortality. The actuarial maintenance of the stoma was calculated with the Kaplan–Meier method, and the corresponding tables were created, analysing the previously significant variables by means of the log-rank test (Mantel–Cox). A P value <.05 was considered statistically significant.
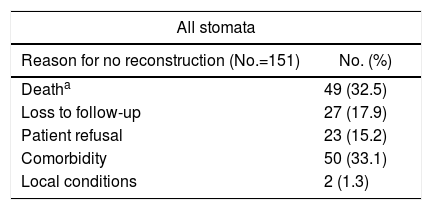
ResultsOut of 385 patients operated on for CAD in 10 hospitals, 312 had a stoma created: 292 (93.6%) end colostomies and 20 (6.4%) diverting stomata (Fig. 1). After a median follow-up of 32 (9–60) months, in 161 (51.6%) patients closure was attempted after a median of 9 (7–13.7) months (range: 0.5–48). Excluding the 45 deaths in the immediate postoperative period of the first intervention, the stoma closure rate was 60.3%. The specific rate for Hartmann reversal was 49.3%, and 52% for all end colostomies. Comparing the 292 end with the 20 derivative stomata, 152 (52%) were reconstructed versus 13 (65%); P=.157. Likewise, 154 (51.2%) colostomies and 7 (63.6%) ileostomies were reversed; P=.308. The reasons for not carrying out the reconstruction are shown in Table 1.
Reasons for no Reconstruction of the Intestinal Tract.
| All stomata | |
|---|---|
| Reason for no reconstruction (No.=151) | No. (%) |
| Deatha | 49 (32.5) |
| Loss to follow-up | 27 (17.9) |
| Patient refusal | 23 (15.2) |
| Comorbidity | 50 (33.1) |
| Local conditions | 2 (1.3) |
The data are numbers, with percentages in parentheses.
When specifically analysing the ECT, closure was attempted in 108 men and 44 women, with a mean age (SD) of 56.6 years, which was lower in males: 53.5 (13.1) versus 64 (14.2) in women; P<.0001. 64% of the patients underwent mechanical bowel preparation and were operated on by a colorectal surgeon in 40.8% of the cases, who performed a laparoscopic approach in only 5. In 14%, the resection of the affected sigmoid colon was completed, and the anastomosis was created in 80.3% of patients in the area of the sacral promontory, with an associated diverting stoma in 4. Only in one case was ECT not feasible. During surgery, 20 incisional hernias were repaired simultaneously, and 5 cholecystectomies and 2 inguinal hernia repairs were performed. Mean surgical time (SD) was 205 (82)min.
The overall morbidity rate for ECT was 35.5%, and the operative mortality rate was 2%. The most common complication was infection of the surgical wound in 28 cases (18.4%), with complications of Grade III or higher in 14.5% (Table 2). There were 13 (8.4%) reoperations, most of them due to suture dehiscence (No.=7)—6 of which required a new end colostomy—or evisceration (No.=4). Other reasons were necrosis of the colon and severe rectal bleeding. The 3 deaths were due to multiple organ failure after an acute myocardial infarction, necrosis of the colon and medullary aplasia. The type of initial surgery and immunosuppression were related to postoperative mortality in the univariate analysis (Table 3) and anastomotic dehiscence did not correlate with any factor. In total, 12 (7.9%) patients once again had a stoma after surgery (4 derivative ileostomies and 8 end colostomies).
Complications in the Closure of Terminal Stomata.
| Clavien-Dindo Classification9 | No. (%) |
|---|---|
| No. of complications | 98 (64.5) |
| I. Deviation of the postoperative course without any need for action; includes SSI | 22 (14.5) |
| II. Requires medical treatment, transfusion of blood products or parenteral nutrition | 10 (6.6) |
| III. Requires surgical, endoscopic or radiological intervention | 17 (11.2) |
| IIIa. Without need for general anaesthesia | 8 (5.3) |
| IIIb. Under general anaesthesia | 9 (5.9) |
| IV. Life-threatening dysfunction of one or more organs | 2 (1.3) |
| IVa. One organ | 1 (0.6) |
| IVb. Multiple organs | 1 (0.6) |
| V. Death of the patient | 3 (2) |
The data are numbers, with percentages in parentheses.
SSI: surgical site infection.
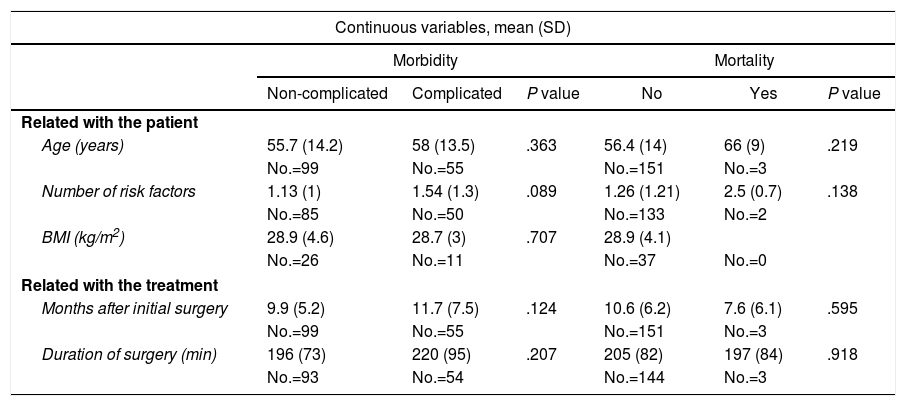
Risk Factors for Morbidity and Mortality of the ECT; Univariate Analysis.
| Continuous variables, mean (SD) | ||||||
|---|---|---|---|---|---|---|
| Morbidity | Mortality | |||||
| Non-complicated | Complicated | P value | No | Yes | P value | |
| Related with the patient | ||||||
| Age (years) | 55.7 (14.2) | 58 (13.5) | .363 | 56.4 (14) | 66 (9) | .219 |
| No.=99 | No.=55 | No.=151 | No.=3 | |||
| Number of risk factors | 1.13 (1) | 1.54 (1.3) | .089 | 1.26 (1.21) | 2.5 (0.7) | .138 |
| No.=85 | No.=50 | No.=133 | No.=2 | |||
| BMI (kg/m2) | 28.9 (4.6) | 28.7 (3) | .707 | 28.9 (4.1) | ||
| No.=26 | No.=11 | No.=37 | No.=0 | |||
| Related with the treatment | ||||||
| Months after initial surgery | 9.9 (5.2) | 11.7 (7.5) | .124 | 10.6 (6.2) | 7.6 (6.1) | .595 |
| No.=99 | No.=55 | No.=151 | No.=3 | |||
| Duration of surgery (min) | 196 (73) | 220 (95) | .207 | 205 (82) | 197 (84) | .918 |
| No.=93 | No.=54 | No.=144 | No.=3 | |||
| Categorical variables, No. (%) | ||||
|---|---|---|---|---|
| Morbidity, No. (%) | P value | Mortality, No. (%) | P value | |
| Related with the patient | ||||
| Age (years) | .731 | .282 | ||
| 50 or younger (No.=60) | 20 (33.3) | 0 | ||
| Older than 50 (No.=92) | 35 (37.2) | 3 (3.2) | ||
| Sex | 1 | .205 | ||
| Males (No.=108) | 39 (35.8) | 1 (0.9) | ||
| Females (No.=44) | 16 (35.6) | 2 (4.4) | ||
| ASA grade | .416 | .784 | ||
| I (No.=20) | 8 (40) | 0 | ||
| II (No.=66) | 23 (34.8) | 1 (1.5) | ||
| III (No.=27) | 9 (33.3) | 1 (1.5) | ||
| IV (No.=4) | 3 (75) | 0 | ||
| Immunosuppressiona | .073 | .014 | ||
| No (No.=132) | 46 (34.8) | 1 (0.7) | ||
| Yes (No.=13) | 8 (61.5) | 2 (15.4) | ||
| Related with the treatment | ||||
| Level of the hospital | .591 | .695 | ||
| Tertiary (No.=102) | 39 (38.2) | 2 (1.9) | ||
| District (No.=50) | 16 (32) | 1 (2) | ||
| Surgery for CAD | .697 | .034 | ||
| Hartmann procedure (No.=141) | 49 (35.7) | 2 (1.4) | ||
| Resection and anastomosis (No.=5) | 1 (20) | 0 | ||
| Peritoneal lavage (No.=6) | 3 (50) | 1 (16.7) | ||
| Bowel preparation | .85 | 1 | ||
| No (No.=52) | 20 (38.5) | 1 (1.9) | ||
| Yes (No.=91) | 30 (33) | 1 (1.1) | ||
| Surgeon | .111 | .949 | ||
| Colorectal (No.=59) | 16 (27.1) | 1 (1.6) | ||
| Staff (No.=86) | 38 (44.2) | 2 (2.) | ||
| Resident (No.=1) | 0 | 0 | ||
| Surgical approach | .654 | .901 | ||
| Open (No.=142) | 52 (36.6) | 2 (2.1) | ||
| Laparoscopic (No.=5) | 1 (20) | 0 | ||
| Anastomosis | .587 | .766 | ||
| Manual (No.=28) | 12 (42.9) | 1 (3.6) | ||
| Linear stapler (No.=11) | 5 (45.4) | 0 | ||
| Circular stapler (No.=102) | 36 (35.3) | 2 (2) | ||
| Region of the anastomosis | .494 | .089 | ||
| Sigmoid colon (No.=7) | 4 (57.1) | 1 (14.3) | ||
| Rectum (promontory sacrum) (No.=101) | 40 (39.6) | 2 (2) | ||
| Rectum (under the promontory) (No.=19) | 6 (31.7) | 0 | ||
ASA: American Society of Anesthesiologists surgical risk classification; CAD: complicated acute diverticulitis; BMI: body mass index; ECT: end colostomy reversal.
The mean hospital stay (SD) was 12 (12.2) days (2–125). The sum of the hospital stays of the initial surgery by CAD and second intervention (stoma closure) when in the former an end stoma was associated, was a mean (SD) of 27.1 (17.5) days, and the mean number of complications (SD) was 1.3 (1.3). Meanwhile, resection and primary anastomosis, protected or not by a stoma, had a total hospital stay (SD) of 16.5 (18.7) days (P<.0001) and 0.84 (0.97) complications (P=.042).
If we divided all patients with stomata according to whether the tract was reconstructed or not and analysing the risk factors of not doing so, age, Peritonitis Severity Score (PSS)10 and the number of complications in the first surgery were lower in the group with tract reconstruction. There was great variability between hospitals, ranging from 25% to 69%, although this was not observed when we compared overall the tertiary/university hospitals with the district hospitals. Patients younger than 50 years and those without immunosuppression had a higher reconstruction rate: odds ratio (95% CI) 2.3 (1.99–2.77) and 2.3 (1.276–4.418), respectively. The same occurred with men versus women: OR (95% CI) 1.97 (1.53–2.55); and in those who did not have haemodynamic instability in the first surgery: OR (95% CI) 3.47 (1.84–6.55). Meanwhile, faecal peritonitis as well as the morbidity from the first surgery were adverse factors (Table 4). In the multivariate study, only age was predictive of stoma closure (P=.006).
Differences According to the Reconstruction or not of the Intestinal Tract (No.=312).
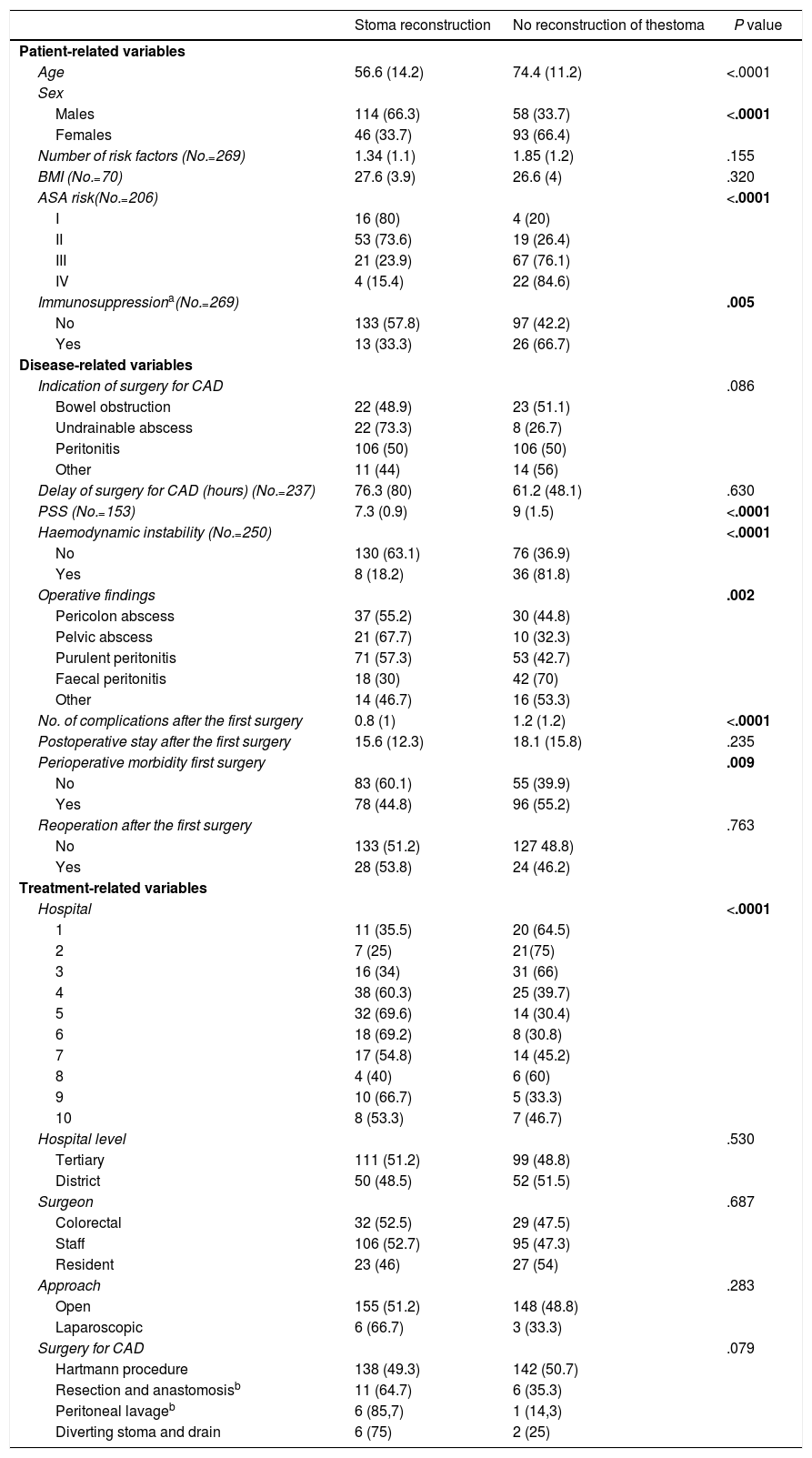
| Stoma reconstruction | No reconstruction of thestoma | P value | |
|---|---|---|---|
| Patient-related variables | |||
| Age | 56.6 (14.2) | 74.4 (11.2) | <.0001 |
| Sex | |||
| Males | 114 (66.3) | 58 (33.7) | <.0001 |
| Females | 46 (33.7) | 93 (66.4) | |
| Number of risk factors (No.=269) | 1.34 (1.1) | 1.85 (1.2) | .155 |
| BMI (No.=70) | 27.6 (3.9) | 26.6 (4) | .320 |
| ASA risk(No.=206) | <.0001 | ||
| I | 16 (80) | 4 (20) | |
| II | 53 (73.6) | 19 (26.4) | |
| III | 21 (23.9) | 67 (76.1) | |
| IV | 4 (15.4) | 22 (84.6) | |
| Immunosuppressiona(No.=269) | .005 | ||
| No | 133 (57.8) | 97 (42.2) | |
| Yes | 13 (33.3) | 26 (66.7) | |
| Disease-related variables | |||
| Indication of surgery for CAD | .086 | ||
| Bowel obstruction | 22 (48.9) | 23 (51.1) | |
| Undrainable abscess | 22 (73.3) | 8 (26.7) | |
| Peritonitis | 106 (50) | 106 (50) | |
| Other | 11 (44) | 14 (56) | |
| Delay of surgery for CAD (hours) (No.=237) | 76.3 (80) | 61.2 (48.1) | .630 |
| PSS (No.=153) | 7.3 (0.9) | 9 (1.5) | <.0001 |
| Haemodynamic instability (No.=250) | <.0001 | ||
| No | 130 (63.1) | 76 (36.9) | |
| Yes | 8 (18.2) | 36 (81.8) | |
| Operative findings | .002 | ||
| Pericolon abscess | 37 (55.2) | 30 (44.8) | |
| Pelvic abscess | 21 (67.7) | 10 (32.3) | |
| Purulent peritonitis | 71 (57.3) | 53 (42.7) | |
| Faecal peritonitis | 18 (30) | 42 (70) | |
| Other | 14 (46.7) | 16 (53.3) | |
| No. of complications after the first surgery | 0.8 (1) | 1.2 (1.2) | <.0001 |
| Postoperative stay after the first surgery | 15.6 (12.3) | 18.1 (15.8) | .235 |
| Perioperative morbidity first surgery | .009 | ||
| No | 83 (60.1) | 55 (39.9) | |
| Yes | 78 (44.8) | 96 (55.2) | |
| Reoperation after the first surgery | .763 | ||
| No | 133 (51.2) | 127 48.8) | |
| Yes | 28 (53.8) | 24 (46.2) | |
| Treatment-related variables | |||
| Hospital | <.0001 | ||
| 1 | 11 (35.5) | 20 (64.5) | |
| 2 | 7 (25) | 21(75) | |
| 3 | 16 (34) | 31 (66) | |
| 4 | 38 (60.3) | 25 (39.7) | |
| 5 | 32 (69.6) | 14 (30.4) | |
| 6 | 18 (69.2) | 8 (30.8) | |
| 7 | 17 (54.8) | 14 (45.2) | |
| 8 | 4 (40) | 6 (60) | |
| 9 | 10 (66.7) | 5 (33.3) | |
| 10 | 8 (53.3) | 7 (46.7) | |
| Hospital level | .530 | ||
| Tertiary | 111 (51.2) | 99 (48.8) | |
| District | 50 (48.5) | 52 (51.5) | |
| Surgeon | .687 | ||
| Colorectal | 32 (52.5) | 29 (47.5) | |
| Staff | 106 (52.7) | 95 (47.3) | |
| Resident | 23 (46) | 27 (54) | |
| Approach | .283 | ||
| Open | 155 (51.2) | 148 (48.8) | |
| Laparoscopic | 6 (66.7) | 3 (33.3) | |
| Surgery for CAD | .079 | ||
| Hartmann procedure | 138 (49.3) | 142 (50.7) | |
| Resection and anastomosisb | 11 (64.7) | 6 (35.3) | |
| Peritoneal lavageb | 6 (85,7) | 1 (14,3) | |
| Diverting stoma and drain | 6 (75) | 2 (25) | |
The data are numbers, either percentages or SD in parentheses.
ASA: American Society of Anesthesiologists surgical risk classification; CAD: complicated acute diverticulitis; SD: standard deviation; BMI: body mass index; PSS: Peritonitis Severity Score
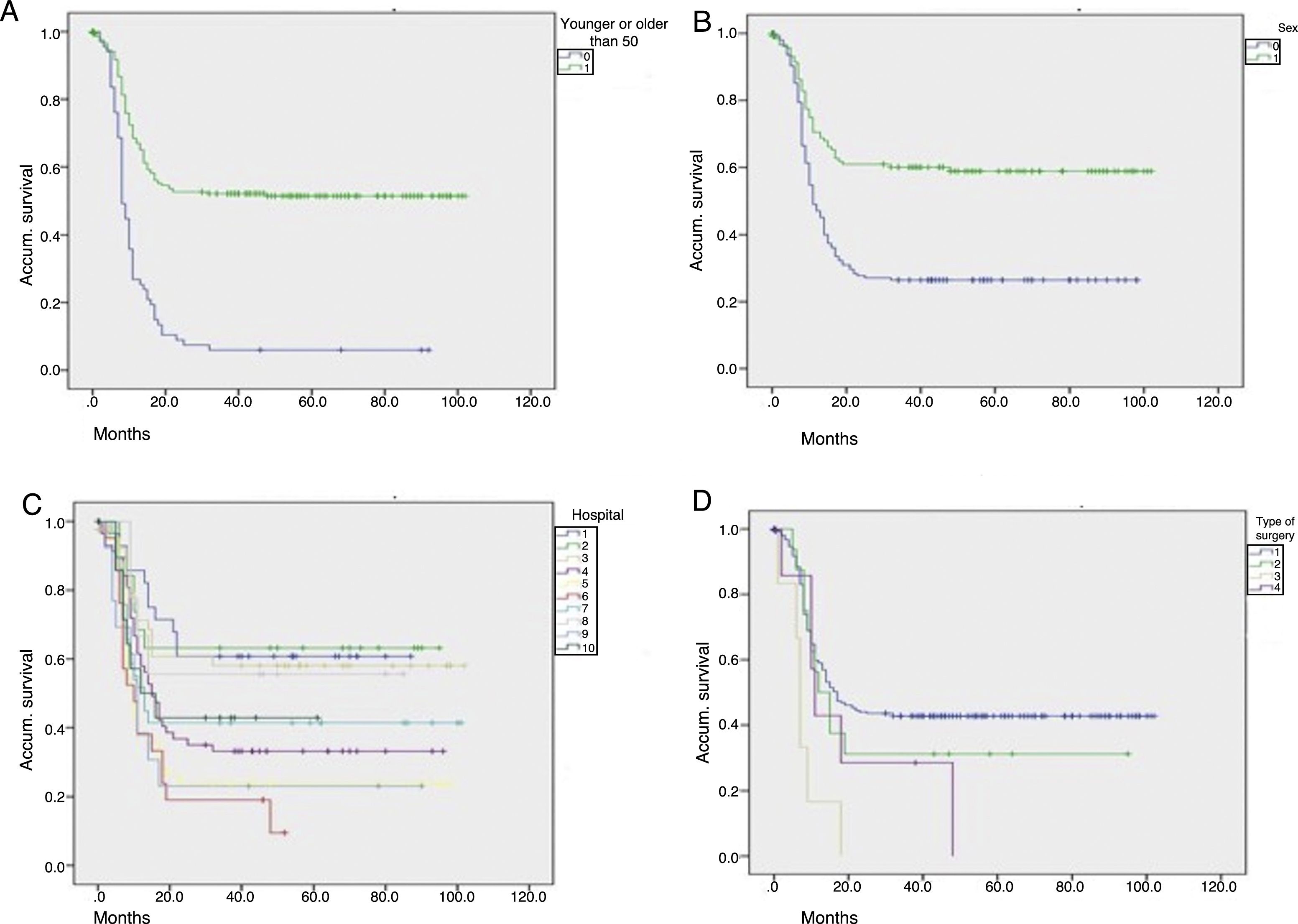
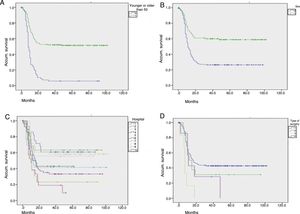
Fig. 2 shows graphs of actuarial maintenance of the stoma in relation to statistically significant variables. There were differences due to ASA surgical risk, immunosuppression or intraoperative haemodynamic instability.
Actuarial maintenance of the stomata according to several parameters (No.=312 patients): (A) age: 0=50 or younger; 1=51 or older; log-rank (Mantel–Cox)=61.591; P<.0001; (B) sex: 0=males; 1=females; log-rank (Mantel–Cox)=26.514; P<.0001; (C) hospital: 1,2,3…; log-rank (Mantel–Cox)=30.622; P<.0001; (D) initial surgerya: 1=Hartmann; 2=resection and primary anastomosis; 3=peritoneal lavage; 4=colostomy and drainage; log-rank (Mantel–Cox)=13.723; P=.003. aPatients who received a primary stoma or rather after reoperation during hospitalization for complicated acute diverticulitis (CAD).
A century after its description, the Hartmann procedure continues to be used frequently.11 This means that patients usually require a complex surgery to restore intestinal continuity with risk of complications, and some 20%–50% are never reconstructed.4–7
Our ECT rate has been similar to that of other publications focused on diverticulitis, ranging from 45% to 68.5%.12–16 A British multicentre review of 3950 Hartmann interventions for any reason (2853 of them urgent) showed a reconstruction rate of only 22.3% (4%–34%),5 and in two Spanish series, patients with benign pathology had a rate almost twice as much as those who had a malignant process.6,7
The most common causes of not performing the intervention in our series were comorbidity and death of the patient, some 33% each. ECT occurred significantly more in men, which was possibly influenced by the older age of the women. In fact, younger age was associated with a higher rate of ECT, as was a lower surgical risk prior to the initial surgery, factors that are also significant in other series.6 Reconstruction was also associated with several variables, although the multivariate analysis showed only younger age as a predictive factor. The same occurred when comparing in the actuarial analysis the temporal relationship of stoma maintenance with various variables, the most adverse being age over 50 years, female sex, hospital, ASA, immunosuppression, intraoperative hypotension, faecal peritonitis or Hartmann's intervention. In this context, Riansuwan et al. defined a risk/benefit score for stoma closure in patients treated surgically for CAD.17
Another point of discussion would be the minimum time to reconstruct the stoma, in order to wait for the reduction of the peritoneal adhesions while the patient recovers from the initial surgery, which is generally about 3 months.18 However, this period tends to increase in practice, and the waiting lists in our setting can mean an additional important amount of time; in fact, this was the most common cause in our series.
We observed a very low rate of laparoscopic surgery for colostomy reversal. However, good results have been published with its use,19,20 although there are no prospective randomized studies that stratify patients due to operative difficulties and risks. In a Scottish multicentre study with 252 patients, the reconstruction approach was laparoscopic in only 15%, with a conversion rate of 64%.21
Mechanical preparation of the colon was the norm in our series, although its use decreased as the study progressed due to evidence of its problems.22 Most of the anastomoses were performed with a circular stapler generally at the level of the sacral promontory, although in 7 cases it was done in the sigmoid colon, which increases the recurrence rate of diverticular disease.8,23 Only in one patient was ECT possible.
Our rate of postoperative complications is similar to that of other studies5–7,12,21 and, although the risk factors are similar to those of other digestive anastomoses,7,23,24 we have only identified immunosuppression and the cause of the stoma being a reoperation after peritoneal lavage as related factors. It is important to note that 7.9% of patients continued with a stoma after surgery to reverse it or for its complications. The review by Aquina et al.15 including 10487 patients found that surgeons with the highest volume of resections were associated with better results after reconstruction. In our study, there were significant differences between hospitals, but not necessarily related to their level or volume.
It has been demonstrated that a primary anastomosis can be safe in the presence of bowel obstruction or even diffuse peritonitis,25 although it is also true that experience is required to construct anastomoses under adverse conditions. Thus, on-call surgeons often evade anastomoses, which does not avoid the possible complications associated with the creation of a stoma. A second operation is required for stoma reversal, which puts the patient at risk of further complications, generates another hospital stay, more costs and socioeconomic repercussions.7,26
Given the low percentage of reconstructions, their delay and morbidity, the indications of a Hartmann procedure for CAD should be questioned as it means two surgeries that together must be compared with a resection and primary anastomosis, at least when faced with localized or diffuse purulent peritonitis in patients with acceptable general conditions.8,27–29 We should also not forget that another option is to perform an anastomosis protected by a stoma, which is later reconstructed with greater frequency.13
Many publications support the idea that primary resection and anastomosis does not lead to more morbidity and mortality, but that the opposite is true.3,27,30–35 In a randomized study by Oberkofler et al.36 in patients with diffuse peritonitis due to CAD, which included the reconstruction of the stoma if done, the differences in favour of the primary anastomosis were significant in terms of reconstruction rate, morbidity, hospital stay and costs. Something similar is seen in the recent multivariate randomized DIVERTI study, with a significantly higher rate of stoma reconstruction after protected primary anastomosis.37 In practice, this should be weighed against the surgical risk and risk factors for anastomotic failure, particularly hypotension and hypoxia.
The daily practice of our emergency services is not always ideal. Many times patients are operated on by surgeons dedicated to other areas of general surgery, who only occasionally perform colon resections and, when faced with a truly difficult context, tend to conduct more end colostomies. This trend could be improved by providing up-to-date information on the management of these highly prevalent problems in surgical departments.
Our study has the limitations of retrospective data collection from a multicentre group, involving many surgeons who are not necessarily specialized in colorectal surgery. Other weaknesses are the low number of patients with diverting stomata and the definition of comorbidities, which may have been interpreted differently at the participating hospitals. This implies possible errors despite having provided detailed instructions to the coordinators at each medical centre. However, the value of our study is that it demonstrates what happens in a large sample of patients treated surgically for a prevalent and benign process and how its management can affect a long period of their lives.
In conclusion, the possibility of maintaining a permanent stoma after surgery for CAD is high in our setting, and the intention to reverse the stoma becomes delayed and involves significant morbidity. Therefore, although the Hartmann procedure saves lives, its indications must be carefully considered.
Conflict of InterestThe authors have no conflict of interests to declare.
Marta Aguado, Javier Aguiló, Zutoia Balciscueta, Sylvia Barros, Juan Carlos Bernal, Miriam Cantos, Javier Espinosa, Matteo Frasson, Juan García Armengol, Rafael García-Calvo, Eduardo García-Granero, Lucas García-Mayor, Juan Hernandis, Francisco Landete, Félix Lluís, David Martínez-Ramos, Emilio Meroño, Isabel Rivadulla, Rodolfo Rodríguez, María Dolores Ruiz, José Vicente Roig, Vicente Roselló, Antonio Salvador-Martínez, Natalia Uribe, Celia Villodre.
The names of the members of the Cooperative Group of the Valencian Society of Surgery can be consulted in Addendum.
Please cite this article as: Roig JV, Salvador A, Frasson M, García-Mayor L, Espinosa J, Roselló V, et al. Reconstrucción de la continuidad digestiva tras cirugía de la diverticulitis aguda complicada. Estudio retrospectivo multicéntrico. Cir Esp. 2018;96:283–291.