Sleeve gastrectomy (SG) has become a technique in its own right although a selective or global indication remains controversial. The weight loss data at 5 years are heterogeneous. The aim of the study is to identify possible prognostic factors of insufficient weight loss after SG.
MethodsA SG retrospective multicenter study of more than one year follow-up was performed. Failure is considered if EWL >50%. Univariate and multivariate study of Cox regression were performed to identify prognostic factors of failure of weight loss at 1, 2 and 3 years of follow up.
ResultsA total of 1565 patients treated in 29 hospitals are included. PSP per year: 70.58±24.7; 3 years 69.39±29.2; 5 years 68.46±23.1. Patients with EWL <50 (considered failure): 17.1% in the first year, 20.1% at 3 years, 20.8% at 5 years. Variables with influence on the weight loss failure in univariate analysis were: BMI >50kg/m2, age >50years, DM2, hypertension, OSA, heart disease, multiple comorbidities, distance to pylorus >4cm, bougie >40F, treatment with antiplatelet agents. The reinforcement of the suture improved results. In multivariate study DM2 and BMI are independent factors of failure.
ConclusionThe SG associates a satisfactory weight loss in 79% of patients in the first 5 years; however, some variables such as BMI >50, age >50, the presence of several comorbidities, more than 5cm section of the pylorus or bougie >40F can increase the risk of weight loss failure.
La gastrectomía vertical (GV) se ha convertido en una técnica con entidad propia cuya indicación selectiva o global sigue siendo objeto de controversia. Los resultados ponderales a 5 años son heterogéneos. El objetivo del estudio es identificar posibles factores pronósticos de pérdida de peso insuficiente tras GV.
MétodosEstudio multicéntrico retrospectivo de GV con seguimiento mayor de un año. Se considera fracaso si el PSP<50%. Se realiza estudio univariado y multivariado de regresión de Cox para determinar los factores que influyen en el fracaso ponderal a 1, 2 y 3 años de seguimiento.
ResultadosSe incluye a 1.565 pacientes intervenidos en 29 hospitales. PSP al año: 70,58±24,8; a los 3 años 69,39±29,2; a los 5 años 68,46±23,1. Pacientes con PSP<50 (considerado fracaso ponderal): 17,1% en el primer año, 20,1% a 3 años, 20,8% a 5 años. Las variables que mostraron relación con el fracaso ponderal en el estudio univariado fueron: IMC>50kg/m2, edad>50años, DM2, HTA, SAOS, cardiopatía, varias comorbilidades asociadas, distancia a píloro>5cm, bujía>40F, tratamiento con antiagregantes. La sobresutura mejora los resultados. Las variables que mostraron ser factores predictivos de fracaso en el seguimiento fueron la DM2 y el IMC.
ConclusiónLa GV asocia una pérdida de peso satisfactoria en el 79% de los pacientes en los primeros 5 años; sin embargo, algunas variables como el IMC>50, la DM2, la edad>50, la presencia de varias comorbilidades, la sección a más de 4cm del píloro o la bujía>40F pueden aumentar el riesgo de fracaso ponderal.
In recent years, sleeve gastrectomy (SG) is no longer the initial surgery for the duodenal switch proposed by Gagner,1 and it has become an alternative technique in its own right.2 The role of this technique in the therapeutic algorithm of morbid obesity has still not been well defined, and there continues to be controversy about its selective indication or application in all patients.3–5 Published data reporting long-term results (5 or more years of follow-up) are heterogeneous and range between 40 and 86% of the percentage of excess weight lost (%EWL) after 5 years in the different series.6,7 These differences may be due to technical variations, such as the distance to the pylorus of the first staple line or the size of the guide catheter (as suggested by some authors8–10), patient-related factors (eating habits, age, sex, initial body mass index [BMI], and comorbidities11–15) or factors that are still unknown.16 The identification of prognostic factors or weight loss, resolution of comorbidities or improved quality of life could be very useful when making decisions with the patient about which technique is best and whether SG is most appropriate.17,18 In this multicenter study, with the participation of 29 hospitals from Spain and Portugal, our objective is to determine which variables may help identify patients with the highest probability for presenting poorer weight loss results throughout the mid-term follow-up after SG.
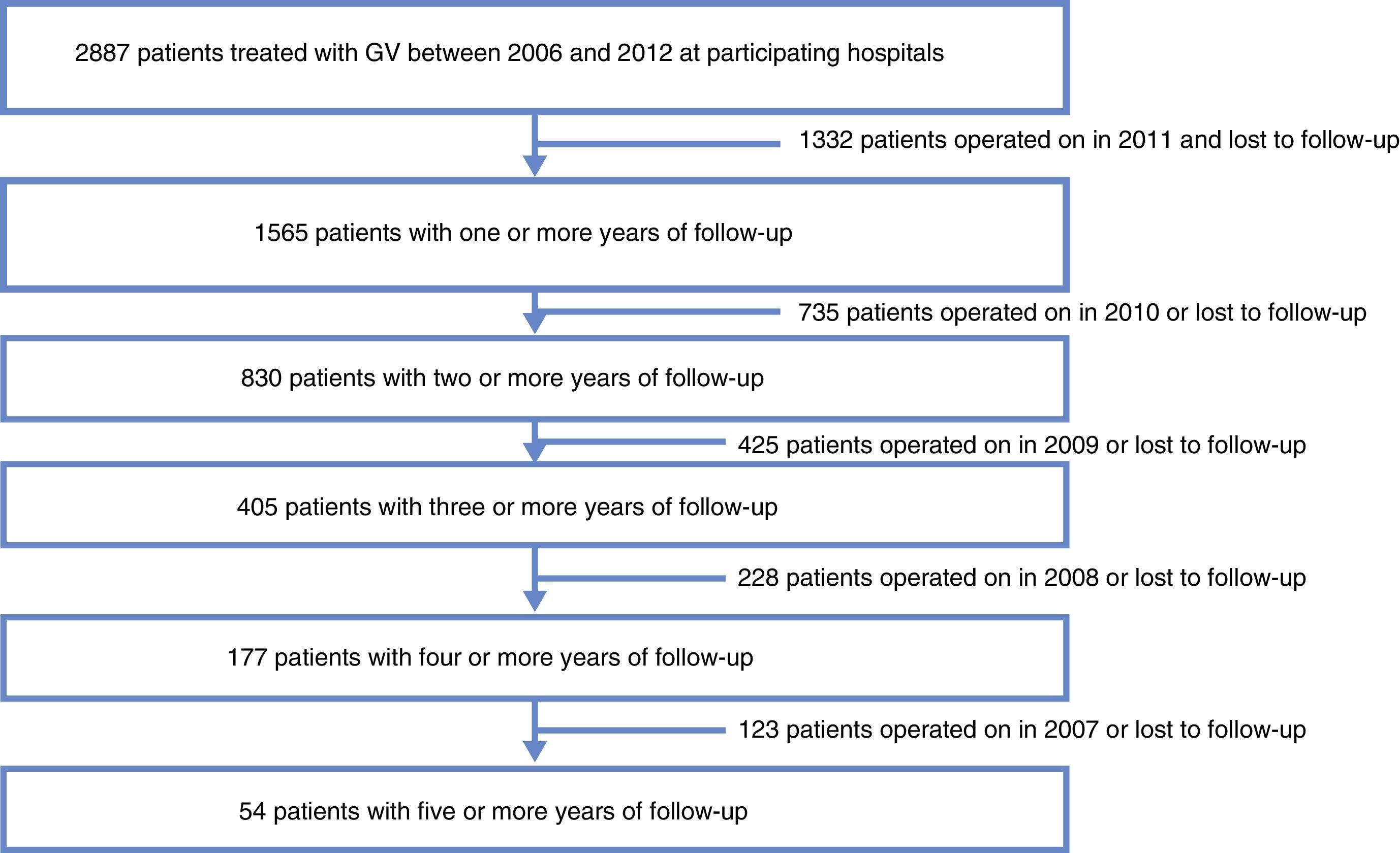
MethodsWe designed a retrospective multi-center cohort study including patients with the following selection criteria: BMI >40kg/m2 or BMI >35kg/m2 with 2 comorbidities; more than one year of progressive morbid obesity; no medical or psychiatric contraindications for bariatric surgery; capable of comprehending recommendations; and patients who had undergone SG between 2006 and 2012 at one of the participating hospitals in the study, with one or more years of follow-up.
Technique: the patient is placed in supine decubitus and the surgeon stands between the patient's legs or on the right side. Pneumoperitoneum is created and 4-5 trocars are placed in the upper hemiabdomen. The short vessels are divided from the region near the pylorus up past the angle of His. Usually, a bougie dilator is placed and gastric stapling or division is conducted, the greater curvature of the stomach is removed, and the remaining stomach is tubular in shape and sized according to the diameter of the bougie used.
Second surgeries include review surgeries conducted after the SG, in which the initial technique is modified and converted to another bariatric technique, such as gastric bypass, duodenal switch or single-anastomosis duodenal switch. Second surgeries also include excision of part of the excess stomach in cases of dilatation of the initial SG.
Baseline variables are considered potential predictive factors (factors prior to surgery or the immediate perioperative period). The variables studied were: (1) demographic variables and comorbidities: age, sex, weight, height, BMI, smoking, treatment with oral anticoagulants or antiplatelet drugs, heart disease, hypertension (HTN), type 2 diabetes mellitus (DM2), arthropathy, dyslipidemia, obstructive sleep apnea (OSA); (2) technical variations: surgeon, distance to the pylorus of the first staple, thickness of the bougie catheter, reinforcement and type; (3) complications: dehiscence/fistula, hemoperitoneum, staple-line bleeding, pneumonia, atelectasis, pulmonary thromboembolism, reoperation, death; (4) postoperative hospital stay; (5) follow-up: weight 1, 2, 3, 4 and 5 years after surgery; evolution of comorbidities at the end of follow-up (HTN, OSA, arthropathy, DM2).
A standardized Microsoft Excel spreadsheet was sent to all the participating hospitals, and each institution filled it out with the data from their prospective databases. Patients gave their consent for their anonymous data to be collected and utilized in scientific studies. The data were remitted to the coordinator of the study. With the data received, a global registry of the multicenter study was created, which calculated the additional intermediate variables necessary for the utilization of the data and statistical values. A specific variable was created to record the progressive experience gained by each of the surgeons specifically in SG procedures.
The statistical analysis was performed with SPSS statistical software, version 21.0 (SPSS Inc., Chicago, IL, USA). Descriptive data have been expressed as mean±standard deviation or percentage. For the univariate study, the Chi-squared test or Fisher's test was used when necessary to compare the qualitative variables, and ANOVA or the Mann–Whitney test was used to compare the quantitative variables. The Cox regression model with the backward stepwise method was used to evaluate the risk factors for failure to lose weight after one year, 2 years and 3 years, while establishing as a dependent variable the time transpired until the %EWL was greater than 50% (%EWL >50) for the first time. Patients with a BMI >25kg/m2 were considered overweight. The percentage of patients who regained weight in successive years after having reached their goal was also evaluated.
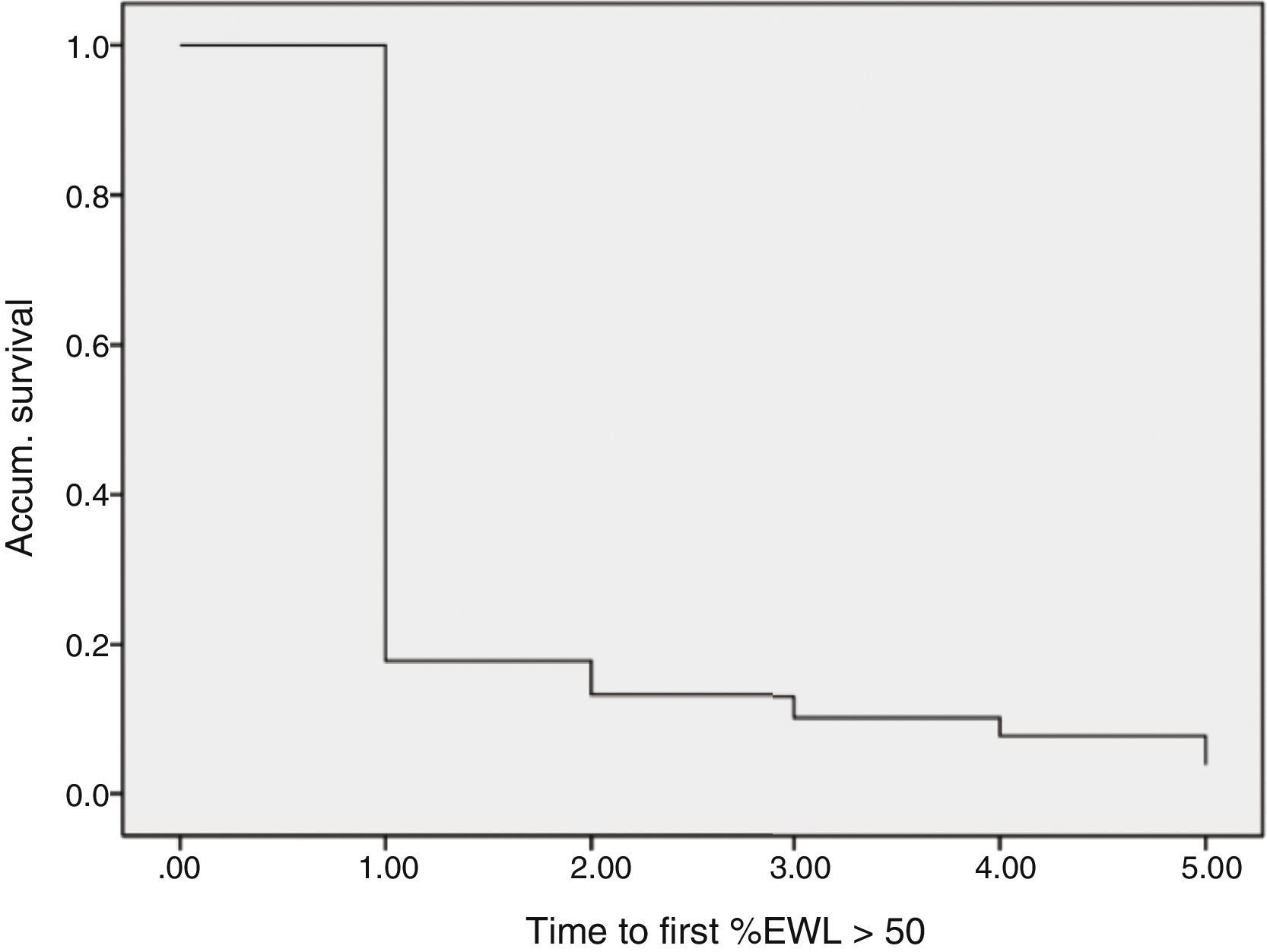
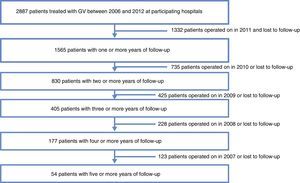
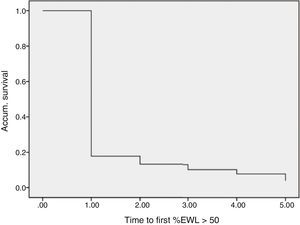
ResultsData were collected from 1565 patients who had undergone SG with a follow-up of more than one year from the 29 participating centers. Mortality follow-up rate (including immediate post-op up to 30 days) was 0.5%. Median follow-up was 2 years with an interquartile range of between 1 and 3 years (Fig. 1).
Regarding the descriptive data, mean patient age was 43.52±11.38 years, with a range from 16 to 74 years; 33.2% were older than 50 years of age, and 32.2% were males. Mean BMI was 48.39±9.18kg/m2, and 39.2% were superobese (BMI ≥50kg/m2); 15.7% were smokers. As for comorbidities, 29.1% had DM2, 45.7% HTN, 39.5% OSA, 28% arthropathy, 29.1% dyslipidemia, and 8.7% heart disease. Treatment for these included oral anticoagulants in 11.4% and antiplatelet agents in 7.4%; 26.6% had previous abdominal surgery.
With regard to variations in the technique, the surgeries were performed by 68 surgeons, and the following variations in the technique were recorded: suture reinforcement was used in 84.6% of cases (polyglycolic acid sutures in 17%, oversewn in 61.2%, and the combination of the two in 3.1%). The bougie size was <34F in 50%, between 35 and 39F in 30.1%, and ≥40F in 19.9%. The distance to the pylorus of the first staple was ≤4cm in 24%, 5cm in 52.5% and ≥6cm in 23.5%.
As for complications and hospital stay, the rate of complications was 12.5% and there was 0.3% deaths. Recorded complications included: staple line bleeding (2.1%), hemoperitoneum (2.2%), upper digestive hemorrhage (0.6%), dehiscence/fistula (3.4%), pulmonary thromboembolism (2%), pneumonia (0.7%), and atelectasis (1.5%). Reoperations were necessary in 5%, and the length of hospital stay was 5.92±9.44 days.
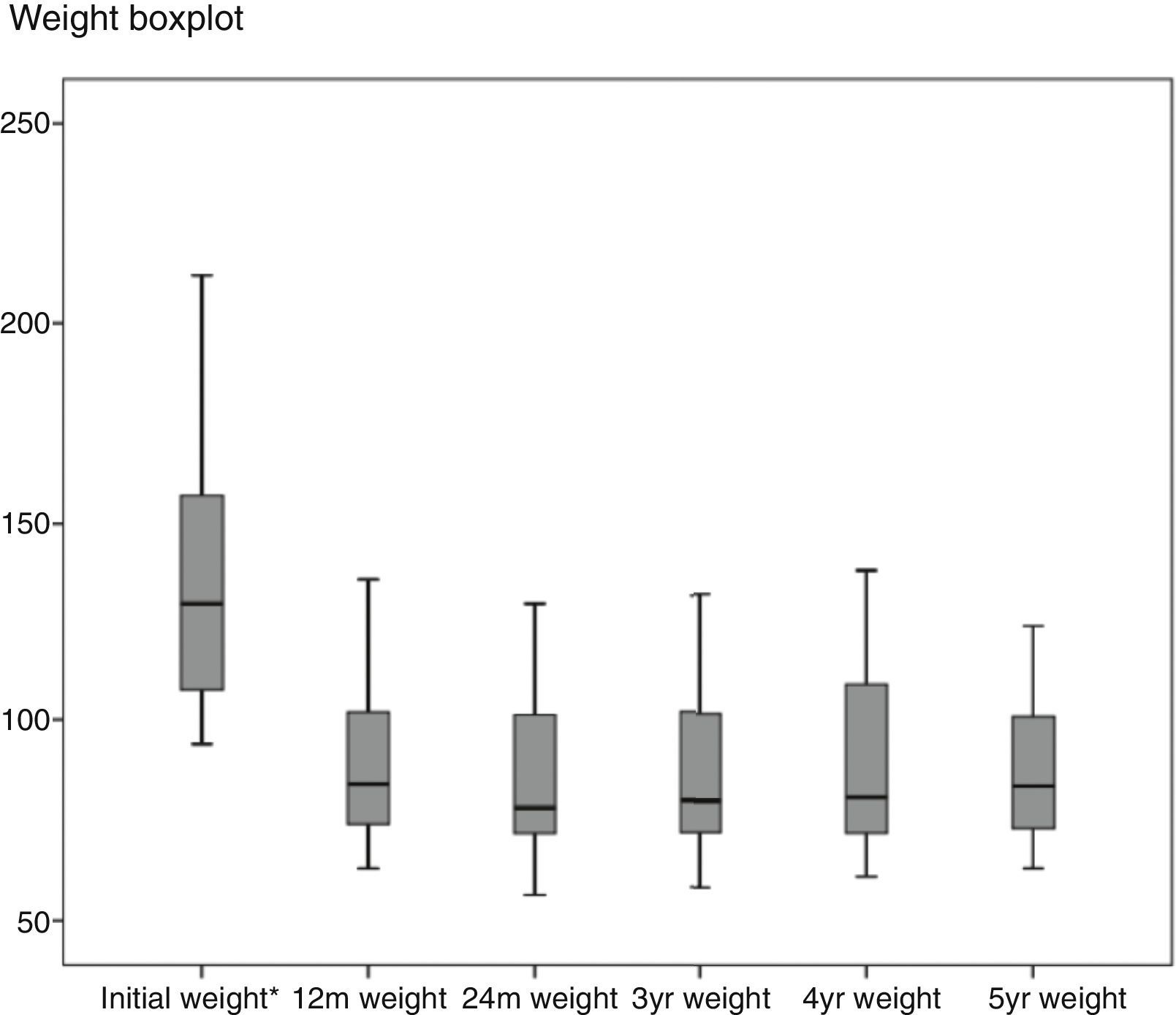
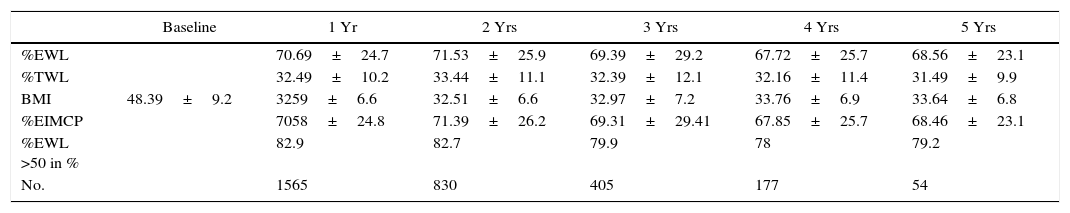
Regarding weight results, more than 80% of the patients lost 50% of their excess weight in the first year (Figs. 2 and 3). The %EWL after the first year was 70.58±24.8; after 3 years, 69.39±29.2; and after 5 years, 68.46±23.1. Other weight loss measurements are shown in Table 1. The percentage of failures (%EWL <50) in the first year was 17.1%; in the third year, 20.1%; and in the fifth year, 20.8%. At the end of the follow-up (considering the last recorded follow-up), 79% of DM2, 70% of HTN, 76% of OSA and 65% of arthropathies had abated.
Weight Loss Measurements.
| Baseline | 1 Yr | 2 Yrs | 3 Yrs | 4 Yrs | 5 Yrs | |
|---|---|---|---|---|---|---|
| %EWL | 70.69±24.7 | 71.53±25.9 | 69.39±29.2 | 67.72±25.7 | 68.56±23.1 | |
| %TWL | 32.49±10.2 | 33.44±11.1 | 32.39±12.1 | 32.16±11.4 | 31.49±9.9 | |
| BMI | 48.39±9.2 | 3259±6.6 | 32.51±6.6 | 32.97±7.2 | 33.76±6.9 | 33.64±6.8 |
| %EIMCP | 7058±24.8 | 71.39±26.2 | 69.31±29.41 | 67.85±25.7 | 68.46±23.1 | |
| %EWL >50 in % | 82.9 | 82.7 | 79.9 | 78 | 79.2 | |
| No. | 1565 | 830 | 405 | 177 | 54 |
%EIMCP >50: percentage of patients who lose more than 50% of excess BMI (greater than BMI >25); BMI: body mass index; %TWL: percentage of total weight loss; %EWL: percentage of excess weight lost; %EWL >50: percentage of patients who lose more than 50% of excess weight (determined by weight equivalent to BMI >25).
A second surgery was performed on 109 patients (6.9%) up until the time of data collection. The mean initial BMI of this group was 54.58±9.1kg/m2 before SG. Before the second procedure, the BMI was 41.70±6.9kg/m2. The indication for the second surgery was regained weight in 35%, insufficient weight loss in 49% and gastroesophageal reflux in 13.7%. As for the techniques, duodenal switch was performed in 49.5%, gastric bypass in 35.7%, duodenal switch of an anastomosis in 11.9% and another SG (regastrectomy) in 9.1%.
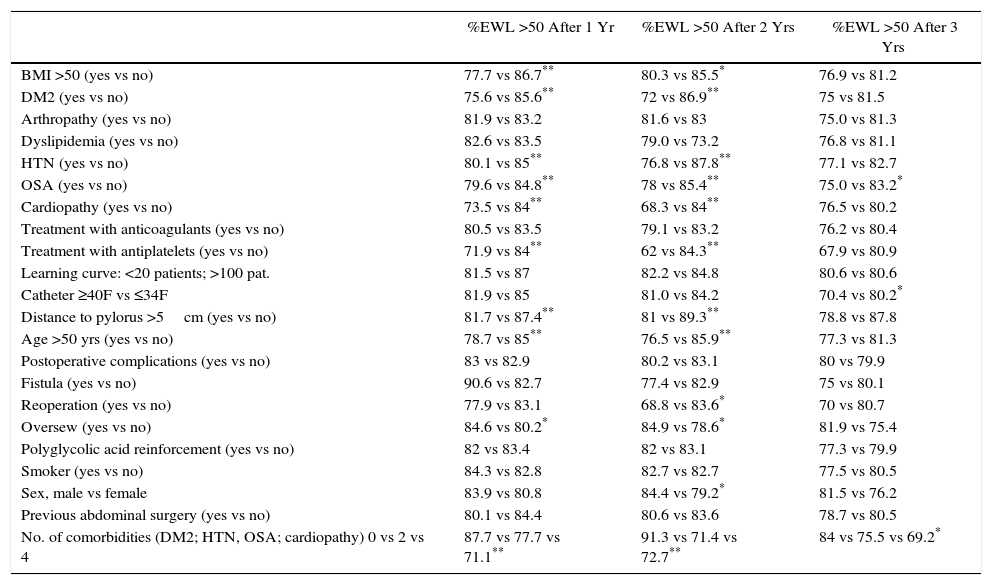
In the univariate study of predictive factors for weight loss failure, the following variables were associated with a significantly greater percentage of weight loss failure (%EWL <50) in the first year: BMI ≥50kg/m2, DM2, HTN, OSA, cardiopathy, antiplatelet treatment, distance from the pylorus of the first stapling >5cm, age >50 years and the sum of several comorbidities. Having oversewn the staple line favored a significantly greater percentage of success. In the second year, the following were associated with worse outcomes: BMI ≥50kg/m2, DM2, HTN, OSA, heart disease, age ≥50 years, postoperative reoperation, female sex, and the association of several comorbidities. Reinforcement sutures also favored a significantly greater percentage of success in the second year. In the third year, the only variable associated with poorer weight loss results was the use of a >40F catheter (Table 2).
Univariate Study of Prognostic Factors for Weight Loss of More Than 50% of Excess Weight (%EWL >50) After 1, 2 and 3 Years.
| %EWL >50 After 1 Yr | %EWL >50 After 2 Yrs | %EWL >50 After 3 Yrs | |
|---|---|---|---|
| BMI >50 (yes vs no) | 77.7 vs 86.7** | 80.3 vs 85.5* | 76.9 vs 81.2 |
| DM2 (yes vs no) | 75.6 vs 85.6** | 72 vs 86.9** | 75 vs 81.5 |
| Arthropathy (yes vs no) | 81.9 vs 83.2 | 81.6 vs 83 | 75.0 vs 81.3 |
| Dyslipidemia (yes vs no) | 82.6 vs 83.5 | 79.0 vs 73.2 | 76.8 vs 81.1 |
| HTN (yes vs no) | 80.1 vs 85** | 76.8 vs 87.8** | 77.1 vs 82.7 |
| OSA (yes vs no) | 79.6 vs 84.8** | 78 vs 85.4** | 75.0 vs 83.2* |
| Cardiopathy (yes vs no) | 73.5 vs 84** | 68.3 vs 84** | 76.5 vs 80.2 |
| Treatment with anticoagulants (yes vs no) | 80.5 vs 83.5 | 79.1 vs 83.2 | 76.2 vs 80.4 |
| Treatment with antiplatelets (yes vs no) | 71.9 vs 84** | 62 vs 84.3** | 67.9 vs 80.9 |
| Learning curve: <20 patients; >100 pat. | 81.5 vs 87 | 82.2 vs 84.8 | 80.6 vs 80.6 |
| Catheter ≥40F vs ≤34F | 81.9 vs 85 | 81.0 vs 84.2 | 70.4 vs 80.2* |
| Distance to pylorus >5cm (yes vs no) | 81.7 vs 87.4** | 81 vs 89.3** | 78.8 vs 87.8 |
| Age >50 yrs (yes vs no) | 78.7 vs 85** | 76.5 vs 85.9** | 77.3 vs 81.3 |
| Postoperative complications (yes vs no) | 83 vs 82.9 | 80.2 vs 83.1 | 80 vs 79.9 |
| Fistula (yes vs no) | 90.6 vs 82.7 | 77.4 vs 82.9 | 75 vs 80.1 |
| Reoperation (yes vs no) | 77.9 vs 83.1 | 68.8 vs 83.6* | 70 vs 80.7 |
| Oversew (yes vs no) | 84.6 vs 80.2* | 84.9 vs 78.6* | 81.9 vs 75.4 |
| Polyglycolic acid reinforcement (yes vs no) | 82 vs 83.4 | 82 vs 83.1 | 77.3 vs 79.9 |
| Smoker (yes vs no) | 84.3 vs 82.8 | 82.7 vs 82.7 | 77.5 vs 80.5 |
| Sex, male vs female | 83.9 vs 80.8 | 84.4 vs 79.2* | 81.5 vs 76.2 |
| Previous abdominal surgery (yes vs no) | 80.1 vs 84.4 | 80.6 vs 83.6 | 78.7 vs 80.5 |
| No. of comorbidities (DM2; HTN, OSA; cardiopathy) 0 vs 2 vs 4 | 87.7 vs 77.7 vs 71.1** | 91.3 vs 71.4 vs 72.7** | 84 vs 75.5 vs 69.2* |
Data in percentage.
Regarding the multivariate study of factors predictive of weight loss (Cox regression), 80% of the patients reached the goal of %EWL >50 in the first year. Some 5.3% of patients with %EWL >50 in the first year regained weight and did not meet the goal in the second year. 4% of the patients who did not reach the goal in the first year exceeded the %EWL of 50 during follow-up. Among patients who reached the target for the first time in the second year, 20% regained weight and did not meet the goal in the third year.
Variables that have shown a statistically significant relationship with failure to lose weight in the Cox regression (%EWL <50) were the initial BMI and DM2. Thus, the probability to not reach %EWL >50 is multiplied by 1.01 (1.001–1.016; P=.03) for each unit increase in BMI; in the case of diabetics, the probability of not reaching %EWL >50 increases by 1.17 (1.02–1.34; P=.023).
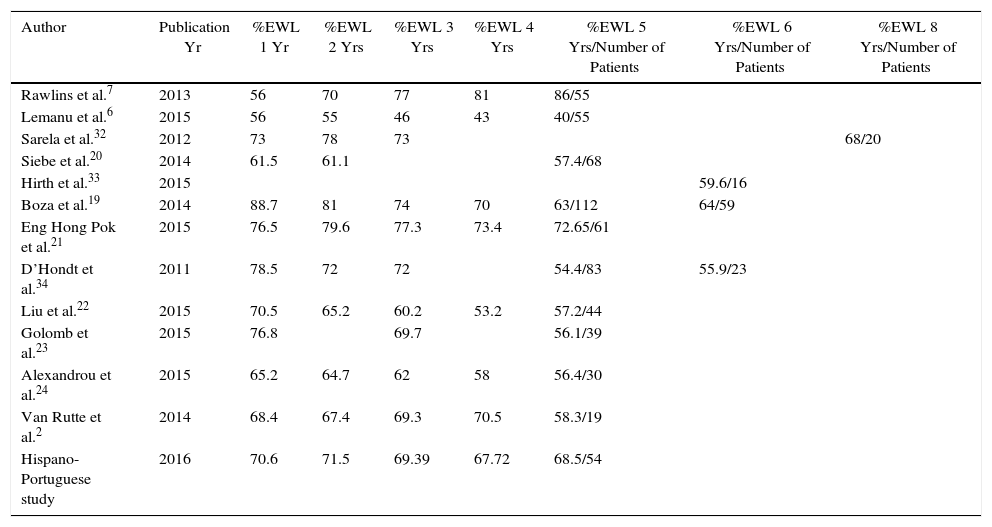
DiscussionIn the present multicenter Hispano-Portuguese study that collected data from 1565 patients who underwent SG at 29 hospitals with more than one year of follow-up, weight loss was satisfactory in a high percentage of patients throughout follow-up. Weight loss remained fairly stable after the first year in 90% of patients. In patients with long-term follow-up, the overall 5-year mean %EWL was 68.46±23.1, and 79.2% achieved the goal of more than 50% excess weight lost. These results support the use of SG as a solitary technique and are consistent with other series published in the literature6,19–25 (Table 3).
Comparison of Weight Follow-up With Other Series.
| Author | Publication Yr | %EWL 1 Yr | %EWL 2 Yrs | %EWL 3 Yrs | %EWL 4 Yrs | %EWL 5 Yrs/Number of Patients | %EWL 6 Yrs/Number of Patients | %EWL 8 Yrs/Number of Patients |
|---|---|---|---|---|---|---|---|---|
| Rawlins et al.7 | 2013 | 56 | 70 | 77 | 81 | 86/55 | ||
| Lemanu et al.6 | 2015 | 56 | 55 | 46 | 43 | 40/55 | ||
| Sarela et al.32 | 2012 | 73 | 78 | 73 | 68/20 | |||
| Siebe et al.20 | 2014 | 61.5 | 61.1 | 57.4/68 | ||||
| Hirth et al.33 | 2015 | 59.6/16 | ||||||
| Boza et al.19 | 2014 | 88.7 | 81 | 74 | 70 | 63/112 | 64/59 | |
| Eng Hong Pok et al.21 | 2015 | 76.5 | 79.6 | 77.3 | 73.4 | 72.65/61 | ||
| D’Hondt et al.34 | 2011 | 78.5 | 72 | 72 | 54.4/83 | 55.9/23 | ||
| Liu et al.22 | 2015 | 70.5 | 65.2 | 60.2 | 53.2 | 57.2/44 | ||
| Golomb et al.23 | 2015 | 76.8 | 69.7 | 56.1/39 | ||||
| Alexandrou et al.24 | 2015 | 65.2 | 64.7 | 62 | 58 | 56.4/30 | ||
| Van Rutte et al.2 | 2014 | 68.4 | 67.4 | 69.3 | 70.5 | 58.3/19 | ||
| Hispano-Portuguese study | 2016 | 70.6 | 71.5 | 69.39 | 67.72 | 68.5/54 |
Data in percentage.
%EWL: percentage of excess weight loss after 1 yr, 2 yrs, 3 yrs, 4 yrs, 5 yrs.
However, almost 20% of patients do not reach the recommended results. Our study aims to determine variables that may help identify patients who are more likely to have worse weight loss results. In this group of patients, another initial bariatric surgery technique could be considered, or a second surgery could be scheduled shortly after SG. In our study, patients who presented insufficient weight loss after the first year hardly improved afterwards: only 4% achieved the goal of %EWL >50 in the following years, and 20% of these regained weight. In patients with insufficient loss after the first year, a second surgery could be considered after having ruled out a possible defect of the gastroplasty. Some variables seem to influence the poor weight loss results of the patients studied, including the comorbidities comprising metabolic syndrome (DM, HTN, dyslipidemia), especially in diabetics, and BMI ≥50kg/m2. In this regard, it seems logical to argue that, in patients with higher BMI or with DM, a second surgery would be more frequently needed, as initially proposed by Gagner.26,27
Another variable that shows a relationship with a higher percentage of failures in our univariate study is age over 50 years. This could call into question the indication of SG in patients over 50, but other techniques have also shown poorer results in older patients. Gender, however, has not influenced the results of our study, unlike other published series.15,28 Technical variations also seem to have an influence on weight loss, as the distance from the first stapling of more than 5cm from the pylorus is associated with a higher percentage of weight loss failures in our univariate study. Nonetheless, the division made less than 4cm from the pylorus can increase complications, so the ideal distance for the first stapling seems to be 4–5cm.29 The size of the bougie also seems to influence weight loss: if a bougie larger than 40F is used, weight loss results worsen; on the other hand, the use of a narrow bougie can increase the percentage of fistulas. Therefore, a mean size between 38 and 40F seems most recommendable.8,9 Oversewing the staple line also seems to favor weight loss, at least in the early years.
As it is a multicenter study, our study has limitations associated with the inherent heterogeneity of the participation of multiple surgeons and several hospitals. It is a retrospective study, with a greater number of records in the first 2 years of follow-up and in which variables were prospectively collected from the databases of the participating hospitals, although some data may be missing that could potentially influence weight loss such as exercise, eating habits or psychosocial factors.16,30,31 However, given the limited amount of reports in the literature on prognostic factors for weight loss following SG, our results can serve as a guide to inform the patient and adjust their weight loss expectations after the intervention, which will help make an informed decision on the most appropriate technique in their situation. Studies with an elevated number of long-term follow-up patients will be necessary to better understand the variables determining possible weight loss failure after SG. Furthermore, these future studies should contemplate other possible variables, such as exercise, eating habits, psychosocial aspects, etc.
In conclusion, SG is associated with satisfactory weight loss that persists for 5 years in almost 80% of patients. Some variables, such as high BMI or DM, may increase the risk of weight loss failure and the need for a second surgery. Information provided to patients about the possible risks, weight loss expectations with GS and the possible need for a second procedure are essential in order for patients to be able to participate in the decision-making process regarding the choice of the most appropriate bariatric surgery technique for their situation.
Authorship/CollaboratorsAll the authors have contributed to the study design, data collection, and critical review/final review. They are listed in order according to the amount of data contributed and their overall contribution to the manuscript. The analysis and interpretation of the results and the composition of the article were carried out by the first author.
- 1.
Raquel Sanchez Santos: design, data, review, analysis, composition;
- 2.
Ricard Corcelles: design, data, review;
- 3.
Ramón Vilallonga Puy: design, data, review;
- 4.
Salvadora Delgado Rivilla: design, data, review;
- 5.
José Vicente Ferrer: design, data, review;
- 6.
Javier Foncillas Corvinos: design, data, review;
- 7.
Carlos Masdevall Noguer: design, data, review;
- 8.
Maria Socas Macias: design, data, review;
- 9.
Pedro Gomes: design, data, review;
- 10.
Carmen Balague Ponz: design, data, review;
- 11.
Jorge de Tomas Palacio: design, data, review;
- 12.
Sergio Ortiz Sebastian: design, data, review;
- 13.
Andrés Sanchez Pernaute: design, data, review;
- 14.
José Julián Puche Pla: design, data, review;
- 15.
Fátima Sabench Pereferrer: design, data, review;
- 16.
Julen Abasolo Vega: design, data, review;
- 17.
Xavier Suñol Sala: design, data, review;
- 18.
Ana Garcia Navarro: design, data, review;
- 19.
Carlos Duran Escribano: design, data, review;
- 20.
Norberto Cassinello Fernandez: design, data, review;
- 21.
Nieves Perez: design, data, review;
- 22.
José Antonio Gracia Solanas: design, data, review;
- 23.
Francisca Garcia-Moreno Nisa: design, data, review;
- 24.
Alberto Hernández Matias: design, data, review;
- 25.
Víctor Valentí Azcarate: design, data, review;
- 26.
José Eduardo Perez Folques: design, data, review;
- 27.
Inmaculada Navarro Garcia: design, data, review;
- 28.
Eduardo Dominguez-Adame Lanuza: design, data, review;
- 29.
Sagrario Martinez Cortijo: design, data, review;
- 30.
Jesús González Fernández: design, data, review.
The authors have no conflict of interests to declare. The study has not received funding from any entity, either public or private.
The authors would like to thank the Spanish Association of Surgeons (Asociación Española de Cirujanos), the Spanish Society of Obesity Surgery And Metabolic Diseases (Sociedad Española de Cirugía de la Obesidad y de las Enfermedades Metabólicas) and the Portuguese Society of Obesity Surgery and Metabolic Diseases (Sociedade Portuguesa de Cirurgia da Obesidade e Doenças Metabólicas) for their support of this multicenter study. The authors would also like to acknowledge the multidisciplinary teams at the participating hospitals in the study for their impartial collaboration.
Please cite this article as: Sanchez Santos R, Corcelles R, Vilallonga Puy R, Delgado Rivilla S, Ferrer JV, Foncillas Corvinos J, et al. Factores predictivos de pérdida ponderal tras la gastrectomía vertical. Estudio multicéntrico hispano-portugués. Cir Esp. 2017;95:135–142.