The aging population raises concerns about the adequacy of aggressive surgical procedures and their outcomes. The treatment of the elderly with hepatocellular carcinoma is one of the diseases that involve complicated management decisions. We set out to compare the results between an older and younger patient cohort with this disease.
Material and methodsA total of 36 hepatic resections were performed on patients with hepatocellular carcinoma between 2000 and 2011. The cohort was divided into 2 groups (14 patients <70 and 22 patients ≥70 years of age), and their results, disease free and overall survival were compared using Kaplan–Meir curves and log rank test. An attempt was also made at determining the predictive factors of a poor outcome among this patient cohort.
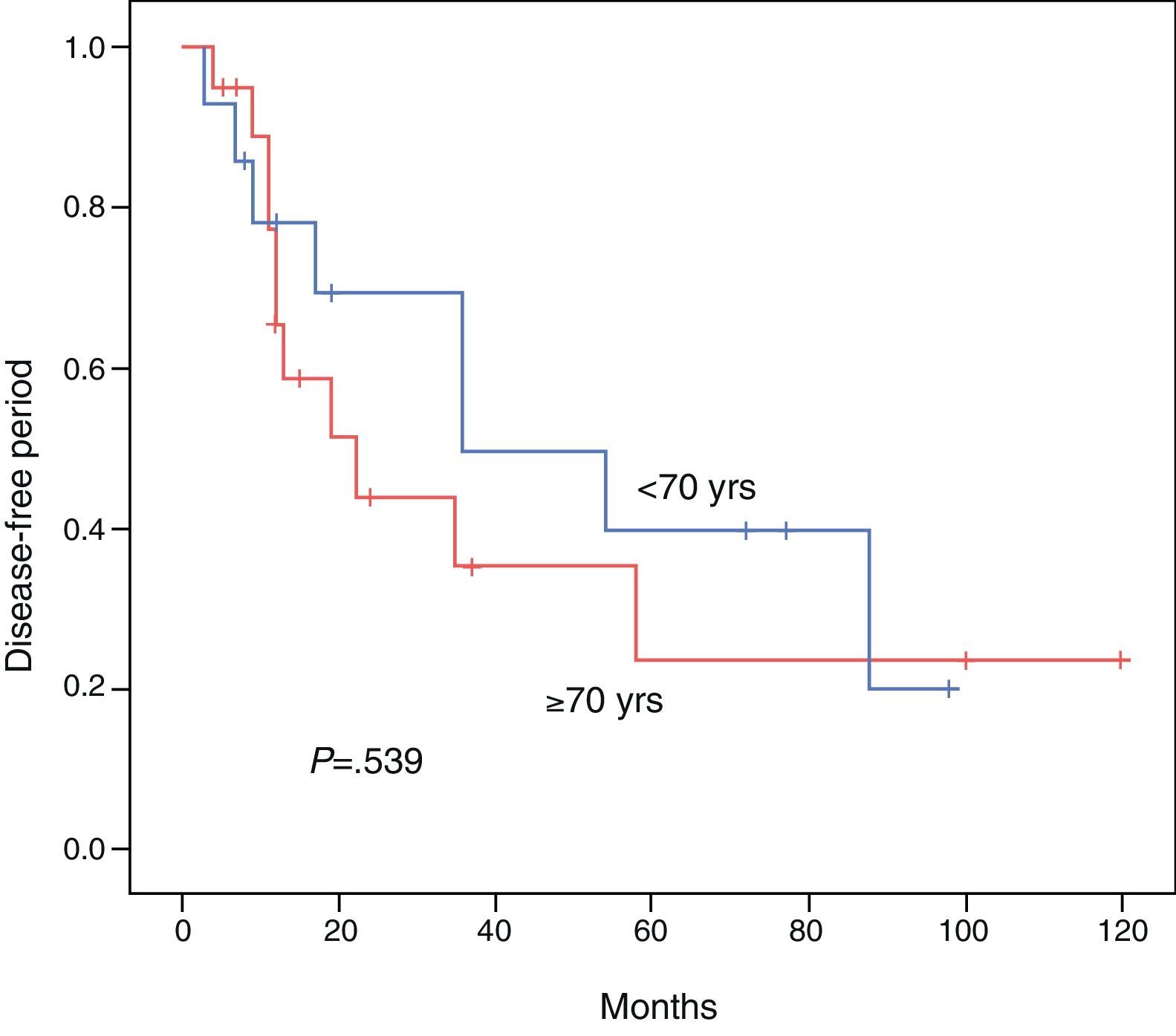
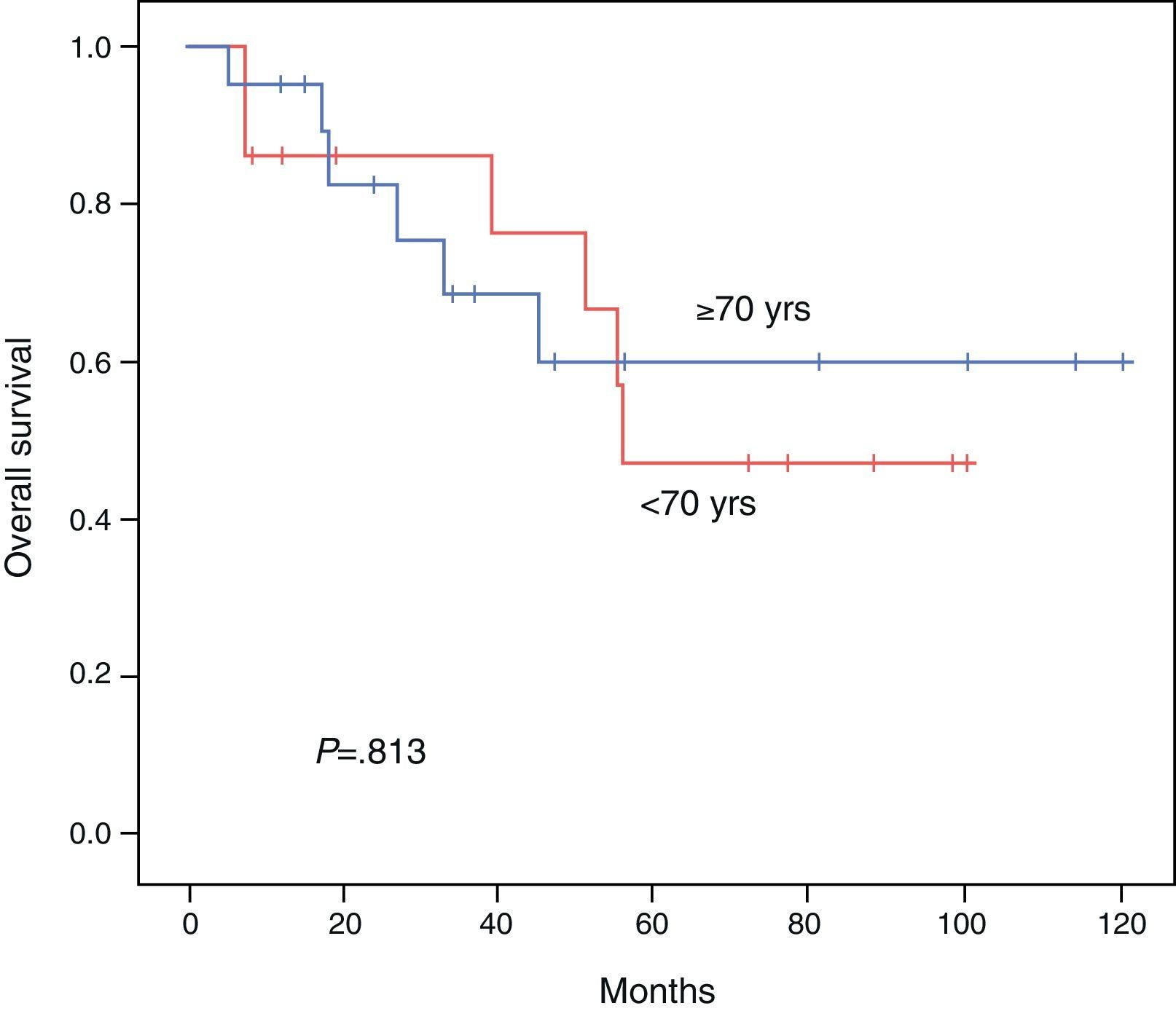
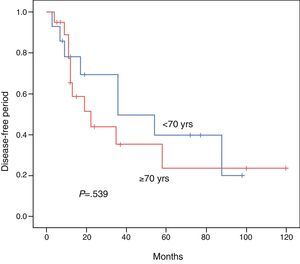
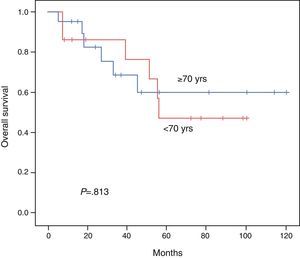
ResultsBoth groups were similar with regard to their pre-operative status. Operation time, procedure, hospital stay, and morbidity and mortality were similar. Overall survival at 3 and 5 years comparing the younger vs the elderly group was 85.7% vs 68.7% and 47.6% vs 60%, respectively (P=.813). Disease free survival at 3 and 5 years comparing the younger vs the elderly group was 69.3% vs 35.2% and 39.6% vs 23.4%, respectively (P=.539). Multivariate analysis of the whole cohort revealed multicentric diseases and elevated alpha-fetoprotein as independent factors of poor disease free survival and overall survival, respectively.
ConclusionsElderly patients with hepatocellular carcinoma should be managed in a similar fashion to younger patients. Surgeons should expect similar post-operative complications, disease free and overall survival.
El manejo quirúrgico agresivo de ciertos tipos de tumores es cuestionado en el paciente anciano debido a la posibilidad de tener un aumento en la morbimortalidad. Este es el caso del paciente anciano con carcinoma hepatocelular. Comparamos los resultados obtenidos con la resección hepática entre pacientes mayores y menores de 70 años de edad con hepatocarcinoma.
Material y métodosSe realizaron 36 resecciones hepáticas curativas para tratar cáncer hepatocelular. Dividimos nuestra población en 2 grupos (14 pacientes < 70 años y 22 pacientes ≥ 70 años de edad) y comparamos su morbimortalidad, periodo libre de enfermedad y sobrevida global utilizando curvas de Kaplan–Meir y prueba de log rank. Además buscamos factores de mal pronóstico en la población.
ResultadosAmbos grupos son similares en cuanto al estado pre-operatorio. El tiempo quirúrgico, tipo de procedimiento, estancia hospitalaria y morbimortalidad fueron similares. La sobrevida global para los pacientes jóvenes y seniles a 3 y 5 años fue de 85,7 vs 68,7% y 47,6 vs 60% respectivamente (p = 0,813). El periodo libre de enfermedad a 3 y 5 años fue de 69,3 vs 35.2% y 39,6 vs 23.4% respectivamente (p = 0,539). El análisis multivariado reveló la enfermedad multicéntrica y la alfa-feto-proteína elevada como factores pronósticos independientes de una sobrevida libre de enfermedad y global más cortas.
ConclusiónLos pacientes mayores de 70 años con carcinoma hepatocelular deben ser manejados en una manera similar a pacientes más jóvenes. Hay que tomar en cuenta factores de mal pronóstico como la multicentricidad y la alfa-feto-proteína elevadas.
The worldwide population is getting older,1 and as a consequence there has been a simultaneous increase in the diseases that are most prevalent among elderly patients. Approximately 60% of all tumors are diagnosed in the population over the age of 65.2,3 The age-adjusted incidence of cancer varies according to age groups: in the population under the age of 65, it is 208/100000 and in the population over the age of 65 it is 2151/100000.2,3
Hepatocellular carcinoma (HCC) is one of the most frequent tumors worldwide4 and generally presents in patients with liver cirrhosis. Its management is multidisciplinary and is focused on the treatment of the tumor as well as management of the liver disease.
Treatment of HCC is primordially surgical and the best results are obtained with liver transplantation, followed by resection.5,6 Patients who are considered for resection are those who present tumors without extrahepatic extension, Child–Pugh A, with no involvement of the main vascular or biliary structures and with adequate residual liver function.
Careful selection of patients for resection of hepatocarcinoma is extremely important as it provides good results and low mortality even in patients with cirrhotic livers.7 There is a certain controversy regarding the resection of multicentric tumors, because their presence is considered to be an intrahepatic dissemination of the lesion with possible micrometastatic foci. Even so, the resection of these lesions is recommended as long as there is adequate remaining liver function.8–10
Patients with Child–Pugh classification B or C who meet the Milan criteria are considered for transplantation, limited by the availability of healthy organs.1 The overall 4-year survival (OS) of patients who receive transplants is 75%, with a 4-year disease-free interval (DFI) of 83%.11 In patients who undergo liver resection, 5-year OS is 33%–44%,12 and recurrence of the disease in these patients is almost the “norm” at about 40%–100% within 5 years.13,14
Although surgery is the treatment of choice in HCC, it is used less frequently in elderly patients than in younger ones. This is in contrast with other tumors, such as colon and breast carcinomas, where surgery is used with similar frequency in both young and elderly patients.15
Because of this, we believe that it is important to establish the safety of liver resection in elderly patients. Most of the experiences published on liver resection for HCC in seniors are based on Asian populations; nevertheless, it is not known if these results are specific to the Asian context and, therefore, if they are reproducible in western populations. A search of the medical literature about liver resection for hepatocarcinoma in patients >70 identified only 3 non-Asian studies that evaluated these results. Only 1 analyzed patient OS and long term survival.
Three studies only analyzed the postoperative results, with no survival analysis. Fortner et al. published a study in 1990 that reported a high operative mortality in elderly patients (11.1%).16 In contrast, Riffat et al. and Aldrighetti et al. more recently established the safety of hepatic surgery in the context of HCC in patients over the age of 70.17,18
The Italian study by Ferrero et al. evaluated the results of hepatic resection in patients with HCC in this population segment and also made an analysis of OS and DFI. It included 64 patients over the age of 70, and they observed DFI and OS similar to the cohort of patients under the age of 70.19
Our aim was to review and report our experience over the last 10 years as a contribution to the limited amount of western literature published on liver resection exclusively for the treatment of HCC in patients over the age of 70.
Material and MethodsWe performed a retrospective cohort study comparing the results obtained in liver resections for HCC in patients both younger and older than 70 years of age at Hospital de la Santa Cruz y San Pablo, which is a tertiary hospital. We chose this age as a cut-off point because it has been used consistently in similar studies to define the senior population.
Our study included 36 patients who were treated surgically between January 2000 and January 2011 and had undergone liver resection as primary treatment for HCC. The patients were studied preoperatively with computed tomography (CT) and/or magnetic resonance imaging (MRI), in addition to laboratory tests including liver function tests and alpha-fetoprotein (AFP) serum values.
All patients were evaluated prior to surgery by a multidisciplinary team consisting of hepatobiliary surgeons, gastroenterologists, oncologists, radiotherapists and interventional radiologists.
The patients considered for surgery were Child–Pugh A with no significant portal hypertension (<10mmHg), no invasion of main vascular or biliary structures and with acceptable remaining liver function.
Resection of multicentric tumors was considered in patients with unilobular lesions as long as the oncological criteria for resection were respected and there was no contraindication due to unacceptable residual liver function volume.
For the analysis, the resection type was classified according to the Brisbane classification from 200020 and for comparison these were later grouped as major (≥3 segments) and minor (<3 segments) resections.
The follow-up in our center was done regularly at 6-month intervals. AFP was documented at each subsequent visit and a CT was performed every 6 months-1 year unless there was a suspicion of recurrence, in which case CT was ordered immediately. When a recurrence was documented, the case was reevaluated by the multidisciplinary team and treatment options were discussed.
In cases that were lost to follow-up, we tried to contact the patients at the telephone number listed in their patient files.
Based on their age at the time of surgery, we divided the patients into 2 groups: control group (<70) and experimental group (>70). We collected the preoperative variables related to patient status, surgical procedure variables and postoperative data.
The post-surgical complications were classified according to the Clavien classification proposed by Dindo et al.21 In this classification, events that deviate from normal post-op and require no intervention are classified as grade I. Grade II are those complications that can be resolved with pharmacological intervention alone. Complications classified as grade III require surgical, endoscopic or radiologic intervention. Grade IV complications put the patient's life at risk and grade V complications lead to death.
In addition, we analyzed the population as a whole, in an attempt to identify preoperative variables associated with poorer results (related with DFI and OS).
We used Student's t test to compare the means of continuous variables and Fisher's test for categorical variables. For survival analysis, Kaplan–Meier curves and the log rank test were used. For the multivariate analysis, we used the Cox regression. P<.05 was considered statistically significant. The results were analyzed with SPSS software.
ResultsThirty-six patients were included in our study with an average age of 67.5, 30 (83.3%) were men. All the patients were classified as Child–Pugh A; 14 patients <70 years of age and 22 patients ≥70.
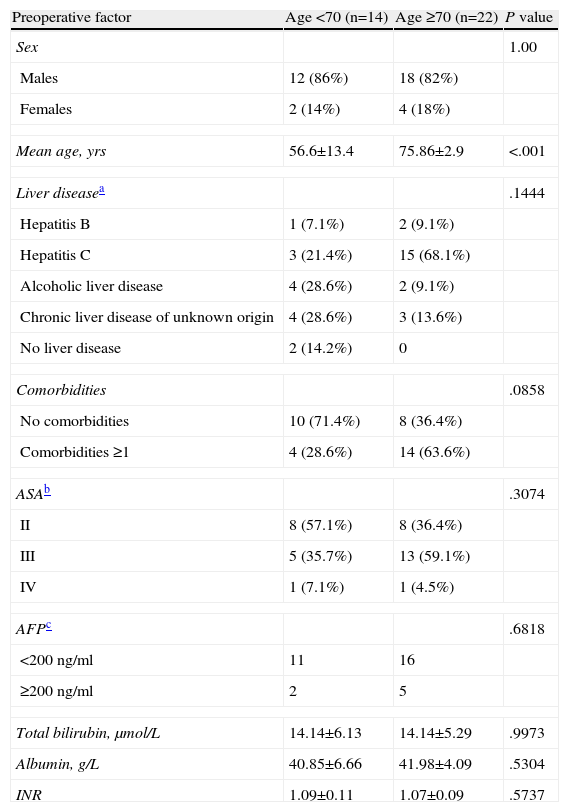
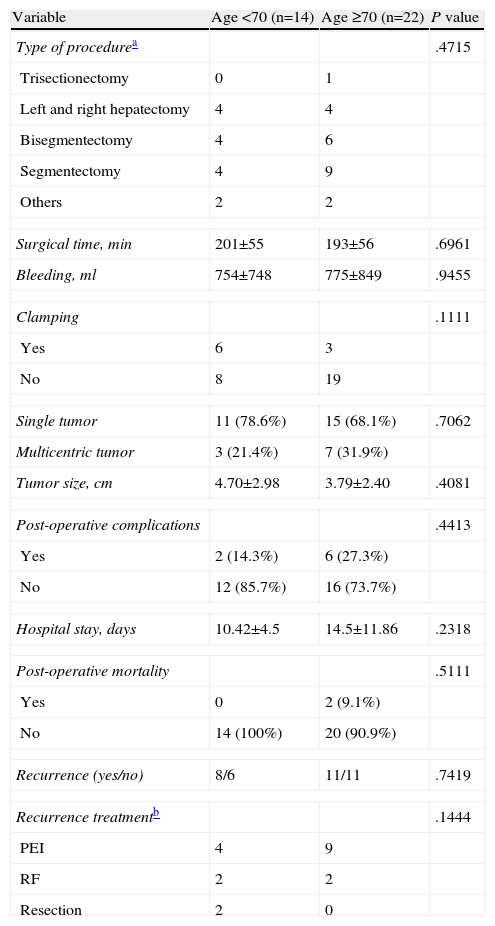
In our cohort, 18 patients (50%) had chronic HCV infection, 3 (8.3%) chronic HBV, 6 (16.7%) alcoholic hepatopathy, 7 (19.4%) cirrhosis with no clear etiology and 2 (5.5%) healthy livers. The comparison of the preoperative variables between both groups is summarized in Table 1. Only one patient in the entire cohort (<70 yrs) received adjuvant treatment with transarterial chemoembolization. None of the others received neoadjuvant or adjuvant therapy. Perioperative variables are compared in Table 2.
Preoperative Variables by Group.
| Preoperative factor | Age <70 (n=14) | Age ≥70 (n=22) | P value |
| Sex | 1.00 | ||
| Males | 12 (86%) | 18 (82%) | |
| Females | 2 (14%) | 4 (18%) | |
| Mean age, yrs | 56.6±13.4 | 75.86±2.9 | <.001 |
| Liver diseasea | .1444 | ||
| Hepatitis B | 1 (7.1%) | 2 (9.1%) | |
| Hepatitis C | 3 (21.4%) | 15 (68.1%) | |
| Alcoholic liver disease | 4 (28.6%) | 2 (9.1%) | |
| Chronic liver disease of unknown origin | 4 (28.6%) | 3 (13.6%) | |
| No liver disease | 2 (14.2%) | 0 | |
| Comorbidities | .0858 | ||
| No comorbidities | 10 (71.4%) | 8 (36.4%) | |
| Comorbidities ≥1 | 4 (28.6%) | 14 (63.6%) | |
| ASAb | .3074 | ||
| II | 8 (57.1%) | 8 (36.4%) | |
| III | 5 (35.7%) | 13 (59.1%) | |
| IV | 1 (7.1%) | 1 (4.5%) | |
| AFPc | .6818 | ||
| <200ng/ml | 11 | 16 | |
| ≥200ng/ml | 2 | 5 | |
| Total bilirubin, μmol/L | 14.14±6.13 | 14.14±5.29 | .9973 |
| Albumin, g/L | 40.85±6.66 | 41.98±4.09 | .5304 |
| INR | 1.09±0.11 | 1.07±0.09 | .5737 |
Surgical Procedure and Postoperative Variables.
| Variable | Age <70 (n=14) | Age ≥70 (n=22) | P value |
| Type of procedurea | .4715 | ||
| Trisectionectomy | 0 | 1 | |
| Left and right hepatectomy | 4 | 4 | |
| Bisegmentectomy | 4 | 6 | |
| Segmentectomy | 4 | 9 | |
| Others | 2 | 2 | |
| Surgical time, min | 201±55 | 193±56 | .6961 |
| Bleeding, ml | 754±748 | 775±849 | .9455 |
| Clamping | .1111 | ||
| Yes | 6 | 3 | |
| No | 8 | 19 | |
| Single tumor | 11 (78.6%) | 15 (68.1%) | .7062 |
| Multicentric tumor | 3 (21.4%) | 7 (31.9%) | |
| Tumor size, cm | 4.70±2.98 | 3.79±2.40 | .4081 |
| Post-operative complications | .4413 | ||
| Yes | 2 (14.3%) | 6 (27.3%) | |
| No | 12 (85.7%) | 16 (73.7%) | |
| Hospital stay, days | 10.42±4.5 | 14.5±11.86 | .2318 |
| Post-operative mortality | .5111 | ||
| Yes | 0 | 2 (9.1%) | |
| No | 14 (100%) | 20 (90.9%) | |
| Recurrence (yes/no) | 8/6 | 11/11 | .7419 |
| Recurrence treatmentb | .1444 | ||
| PEI | 4 | 9 | |
| RF | 2 | 2 | |
| Resection | 2 | 0 | |
PEI: percutaneous ethanol injection; RF: radiofrequency ablation.
Mean follow-up for the group of patients <70 yrs was 49.2 months, and the follow-up for the group ≥70 was 45.9 months.
In the group of patients <70, there were 5 complications: one intraabdominal collection classified as Clavien grade I; 4 abscesses classified as Clavien grade II. In the older patient group, there were 2 bile collections classified as Clavien grade I. Likewise, there were 6 complications (3 abscesses, one wound infection, one ascitic decompensation and one patient with fever and positive blood cultures for gram-negative bacilli) classified as Clavien grade II.
As for major complications, in the cohort <70 yrs there was one Clavien grade IIIb complication that consisted of a biliary fistula requiring endoscopic retrograde cholangiopancreatography with the placement of endoprosthesis. We had a Clavien grade IVa complication in a patient with a right pneumothorax that required the placement of a pleural drain and transfer to the ICU. In the older group, there was a Clavien IIIb complication in a patient with respiratory difficulties related to hemoperitoneum and bleeding that required surgical reoperation. Two patients presented Clavien grade V complications: one patient presented postoperative necrosis in two liver segments that required reoperation; another patient presented anastomotic leak of an ileal repair and needed reoperation. The 2 patients who died in this group were the patient with the ileal leak repair who developed sepsis during the postoperative period and the patient with liver segment necrosis; both patients were excluded from the survival analysis because this analysis is focused on the assessment of those patients whose resection led to no post-op mortality.
The DFI for patients aged <70 vs ≥70 was 52.1 vs 45.3 months, respectively (P=.539). The 3- and 5-year survival rates after successful resection were 85.7 vs 68.7% and 47.6% vs 60%, respectively (P=.813). The Kaplan–Meier curves for DFI and OS are shown in Figs. 1 and 2.
We carried out univariate and multivariate analyses of the cohort as a whole to detect any variables that would function as predictors of a poor prognosis in terms of DFI and OS. In the univariate analysis, none of the preoperative liver function tests (AST, ALT, albumin, GGT, total bilirubin, alkaline phosphatase and platelets) were associated with a poorer prognosis. In the univariate analysis, the patients with AFP<200ng/ml vs>200ng/ml had a mean DFI of 10.5 vs 60.8 months (P=.001) and an OS of 91.0 vs 20.6 months (P<.001), respectively.
In the comparison of single vs multicentric tumors, mean DFI was 11.7 vs 64.4 months (P<.001) and mean OS was 31.2 vs 100.3 months, respectively (P<.001). Patients with tumor size ≤3 vs >3cm had a DFI of 64.8 vs 60.4 months (P=.806) and an OS of 112 vs 76.2 months (P=.200).
Our multivariate analysis showed that the presence of a single vs multicentric tumor independently affected DFI (P=.001, relative risk 8.1). Likewise, an AFP>200ng/ml independently affected OS (P<.001, relative risk 15.0).
DiscussionPatients over the age of 70 should be considered for liver resection with similar expected morbi-mortality results, DFI and OS as in patients under the age of 70. When we compared the preoperative variables in our population, these were all similar with the exception of a tendency in the older patients to present more comorbidities (28.6% vs 63.6%) and a higher proportion in the older group of HCV (68.1%). The high proportion of comorbidities is expected as a natural result of the aging process, which has been reported by other authors.22 Regarding HCV, it is not clear whether patients with HCV infection have a poorer prognosis, as there are reports with varying results.23–25
Blood loss, procedure and operative time were similar between both groups. This is important since the type of resection should not be minimized in the elderly group, and the surgeon should try to perform a curative resection in accordance with the principles that guide oncologic resections in patients with HCC (segmental resections). The use of less aggressive procedures in elderly patients is common due to their high rate of comorbidities.22,26 Other treatment options (radiofrequency ablation or injection of ethanol) can seem attractive to surgeons, but the results with these techniques are inferior to resection, even in small lesions,27 and their use in elderly patients may adversely affect DFI and OS.
Preoperative AFP, tumor size and the presence/absence of multicentric disease were similar in both groups. This is important because it shows that the patients who were treated surgically in the two groups had similar characteristics and, therefore, the possibility of patient selection bias was limited. Having taken this into account, we found that the DFI and OS were similar between both populations. Likewise, postoperative complications and mortality were similar in the two groups. These results are similar to reports from larger Asian studies.28–30
Tumor recurrence was mainly treated with ablative techniques and 2 patients in the <70 yr age group were treated with re-resection. Although this difference was not statistically significant, it is possible that there is a certain amount of caution when considering patients >70 yrs for re-resection. Nonetheless, other studies have shown that re-resection is the most effective treatment for recurrent HCC,31 and that this procedure is an acceptable alternative in the elderly population.29
Due to the small number of patients in our series, univariate and multivariate analyses were used in the cohort as a whole in order to try to identify prognostic factors related with OS and DFI. Our univariate analysis revealed that multicentric disease and AFP levels >200ng/ml are associated with shorter DFI and OS. In our univariate analysis, we did not find tumor size or preoperative liver function test variables to have an impact on DFI or OS.
In the multivariate analysis, the only independent prognostic factor for a shorter DFI was the presence of multicentric disease. The only independent factor associated with shorter OS was AFP>200ng/ml. It has been proposed that high AFP may be related with vascular invasion and progression of HCC,32 and also could have immunosuppressant effects in patients.33,34
Our study did not show statistically significant differences in DFI or OS in patients with tumors >3 vs <3cm, although there was a tendency toward lower OS in patients with tumors >3cm. With regard to this aspect, there is conflicting evidence about tumor size and its correlation with prognosis. Shah reports that DFI was significantly shorter in patients with very large tumors, while OS had no significant differences in patients with tumors <10 vs >10cm.35 Ng did a multi-institutional analysis and compared patients with <5cm tumors, single vs multicentric tumors or those >5cm, and he found that the group with large or multicentric tumors had a poorer prognosis after resection36; furthermore, the largest tumors had a more aggressive tumor biology.36 We believe that the prognosis of the patients with large adenocarcinomas are related with a more aggressive tumor biology as well as with a greater possibility for them to be associated with greater vascular invasion,37 resulting in poorer prognosis and OS.
A limitation of our study is the small number of patients. Nonetheless, we believe that it is a valuable study that establishes the safety of liver resection in a non-Asian population.
In conclusion, we believe that elderly patients should be treated following the same criteria as their younger counterparts since the results and prognoses are similar. Appropriate patient selection and assessment by a multidisciplinary team are essential and should be performed by a specialized liver surgery unit.
Conflict of InterestsThe authors declare having no conflict of interests.
Please cite this article as: Murillo A, Artigas V, González JA, Gallego A, Montserrat E, Rodriguez M, et al. Resección hepática por hepatocarcinoma: estudio comparativo entre pacientes menores y mayores de 70 años. Cir Esp. 2013;91:224–230.