Aim of the present study is to report clinical characteristics and outcomes of patients treated in authors’ hospital for GI metastasis from primary lung cancer, and to report and analyze the same data concerning patients retrieved from a systematic literature review.
We performed a retrospective analysis of prospectively collected data, and a systematic review using the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines.
Ninety-one patients were included, 5 patients from the authors’ hospital and 86 through PubMed database using the keywords “intestinal metastasis” AND “lung cancer”. The median time between primary lung cancer diagnosis and GI metastasis diagnosis was 2 months and the median overall survival was 4 months.
This group of patients present a poor prognosis and the gold standard treatment is not defined. None of the reported treatments had a significant impact on survival.
El objetivo del presente estudio es describir las características clínicas y el manejo de los pacientes tratados en nuestra institución por metástasis GI de cáncer pulmonar primario; así como realizar una revisión sistemática de casos reportados en la literatura.
Se realizó un análisis retrospectivo de una base de datos prospectiva y revisión sistemática de la literatura utilizando las normas MOOSE (Meta-analysis Of Observational Studies in Epidemiology).
Se incluyeron 91 pacientes, 5 de la base de datos de nuestra institución y 86 de la base de datos PubMed usando las palabras claves «intestinal metastasis» y «lung cancer». La mediana de tiempo entre el diagnóstico de cáncer pulmonar y el diagnóstico de metástasis GI fue 2 meses, la mediana de supervivencia global fue 4 meses.
Este grupo de pacientes presentan mal pronóstico. El tratamiento estándar no se encuentra bien establecido. Ninguno de los tratamientos descritos ha mostrado tener impacto significativo sobre la supervivencia.
The lung cancer is the neoplasia with the highest known cancer-related death rate ranging from 18% to 23%.1,2 Despite advances in prevention, it has been reported that approximately 50% of cases present metastasis at the time of diagnosis.3 However, due to new technologies in diagnosis and treatment, patients’ survival is increased in the recent years, making it easier to develop metastasis in the long-term period.4
Several studies in literature, report that gastrointestinal (GI) metastasis from primary lung cancer are uncommon, with an incidence that ranges from 0.3% to 1.7%.5 On the other hand, post-mortem studies have estimated that the incidence of this type of metastatic lesions ranges from 4.6% to 14%.5,6 This discrepancy, between the estimated incidence in clinical case studies and autopsies, seems to indicate that, in most cases, there are asymptomatic undiagnosed metastasis.3
Despite the increasing of clinical publications in the literature, there is no still consensus regarding the management of these patients due to their high associated mortality.7 It has been estimated that GI metastasis represent a prognostic sign that occurs late in the lung cancer course.3 In addition, due to the difficulty in its clinical detection, it is complex to develop prospective studies and, due to the poor reported literature, principally case report and retrospective studies,8 until today an adequate diagnostic and therapeutic data to improve patients’ prognosis are missing.
The aim of the present study is to report clinical characteristics and outcomes of patients treated in authors’ hospital for GI metastasis from primary lung cancer, and to report and analyze the same data concerning patients retrieved from a systematic literature review.
MethodsThis study is a retrospective analysis of prospectively collected data of patients with GI metastasis from lung primary cancer treated in the authors’ center (Hospital de la Sant Creu i Sant Pau, Barcelona, Spain) between 2012 and 2017. All patients admitted to authors’ center sign an informed consent where they authorize us to use their clinical data for educational purposes. Institutional review board (IRB) approval was obtained.
At the same time, a systematic literature review was conducted according to the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines.9 It includes only papers about GI metastasis from primary lung cancer.
Search StrategyThe research was carried out on PubMed database using the keywords: “intestinal metastasis” AND “lung cancer”. The search revealed 414 published papers, dating from January 2000 to August 2017.
Studies were included in the review if present the following criteria: (1) studies that included the terms for GI metastasis from primary lung cancer; (2) they were in English or Spanish; and (3) they included adults patients only.
Studies were excluded if (1) involved animals and (2) they were systematic reviews, meta-analysis, image papers, comment papers or correspondence.
Data such as age, gender, smoking habit, lung neoplasia site, TNM, pathological histology, interval time between the lung cancer diagnosis and intestinal metastasis diagnosis, symptoms or diagnosis for hospital admission, diagnostic instrument employed, GI metastasis site, other metastasis site, GI treatment, and survival time were extracted and analyzed.
Search, data extraction and risk of bias analysis were independently performed by two authors to minimize error (A.B. and J.S.).
Statistical AnalysisQuantitative data are presented as mean±Standard Deviation (SD) and percentages. Survival analysis was performed by Kaplan–Meier method. Subgroups analysis was performed stratifying patients according to age, gender, smoking habit, tumor stage, lymph-node stage, pathological histology, synchronous vs diagnosis, clinical presentation, metastasis location and presence of extra-intestinal metastasis. A probability (P) value lower than .05 was considered statistically significant. All computations were carried out with SPSS software 22.0 (SPSS Inc., Chicago, IL, USA).
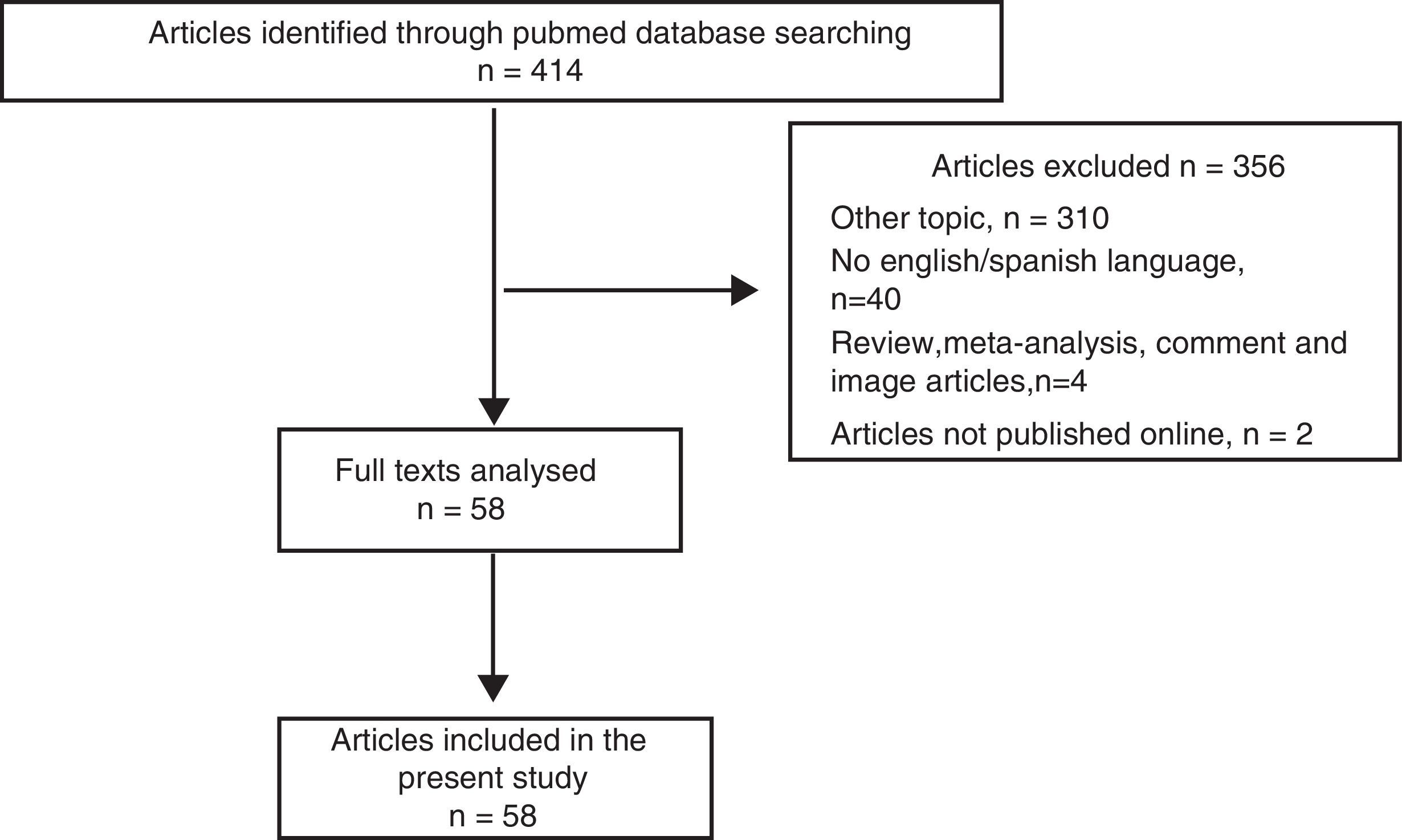
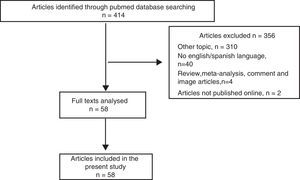
ResultsOf the 414 papers identified in the search, 356 were excluded because did not meet the inclusion criteria (Fig. 1). The remaining 58 studies, published between March 2001 and August 2016, were fully analyzed and all were included in the present review, as shown in the flow diagram (Fig. 1).10–67 All the papers were retrospective analysis, letters to the editor or case reports.10–67
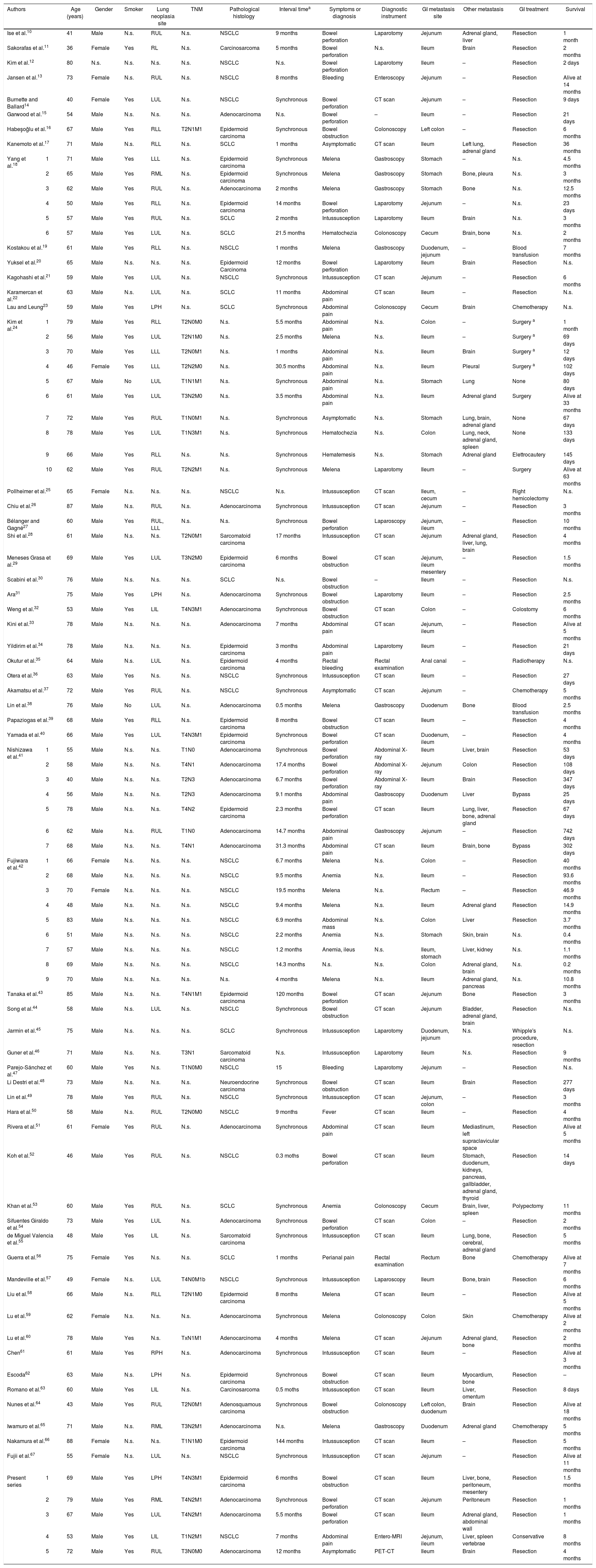
Patients’ characteristics are reported in Table 1. A total of 91 patients were recruited in the present review (86 patients from the literature and 5 form the authors’ series) 77 males (83.7%), 13 females (14.1%), 1 not specified gender patient. Mean age was 64.34±11.08 years, range 41–88.10–67
Patients’ Characteristics.
| Authors | Age (years) | Gender | Smoker | Lung neoplasia site | TNM | Pathological histology | Interval timea | Symptoms or diagnosis | Diagnostic instrument | GI metastasis site | Other metastasis | GI treatment | Survival | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ise et al.10 | 41 | Male | N.s. | RUL | N.s. | NSCLC | 9 months | Bowel perforation | Laparotomy | Jejunum | Adrenal gland, liver | Resection | 1 month | |
| Sakorafas et al.11 | 36 | Female | Yes | RL | N.s. | Carcinosarcoma | 5 months | Bowel perforation | N.s. | Ileum | Brain | Resection | 2 months | |
| Kim et al.12 | 80 | N.s. | N.s. | N.s. | N.s. | NSCLC | N.s. | Bowel perforation | Laparotomy | Ileum | – | Resection | 2 days | |
| Jansen et al.13 | 73 | Female | N.s. | RUL | N.s. | NSCLC | 8 months | Bleeding | Enteroscopy | Jejunum | – | Resection | Alive at 14 months | |
| Burnette and Ballard14 | 40 | Female | Yes | LUL | N.s. | NSCLC | Synchronous | Bowel perforation | CT scan | Jejunum | – | Resection | 9 days | |
| Garwood et al.15 | 54 | Male | N.s. | N.s. | N.s. | Adenocarcinoma | N.s. | Bowel perforation | – | Ileum | – | Resection | 21 days | |
| Habeşoğlu et al.16 | 67 | Male | Yes | RLL | T2N1M1 | Epidermoid carcinoma | Synchronous | Bowel obstruction | Colonoscopy | Left colon | – | Resection | 6 months | |
| Kanemoto et al.17 | 71 | Male | N.s. | RLL | N.s. | SCLC | 1 months | Asymptomatic | CT scan | Ileum | Left lung, adrenal gland | Resection | 36 months | |
| Yang et al.18 | 1 | 71 | Male | Yes | LLL | N.s. | Epidermoid carcinoma | Synchronous | Melena | Gastroscopy | Stomach | – | N.s. | 4.5 months |
| 2 | 65 | Male | Yes | RML | N.s. | Epidermoid carcinoma | Synchronous | Melena | Gastroscopy | Stomach | Bone, pleura | N.s. | 3 months | |
| 3 | 62 | Male | Yes | RUL | N.s. | Adenocarcinoma | 2 months | Melena | Gastroscopy | Stomach | Bone | N.s. | 12.5 months | |
| 4 | 50 | Male | Yes | RLL | N.s. | Epidermoid carcinoma | 14 months | Bowel perforation | Laparotomy | Jejunum | – | N.s. | 23 days | |
| 5 | 57 | Male | Yes | RUL | N.s. | SCLC | 2 months | Intussusception | Laparotomy | Ileum | Brain | N.s. | 3 months | |
| 6 | 57 | Male | Yes | LUL | N.s. | SCLC | 21.5 months | Hematochezia | Colonoscopy | Cecum | Brain, bone | N.s. | 2 months | |
| Kostakou et al.19 | 61 | Male | Yes | RLL | N.s. | NSCLC | 1 months | Melena | Gastroscopy | Duodenum, jejunum | – | Blood transfusion | 7 months | |
| Yuksel et al.20 | 65 | Male | N.s. | N.s. | N.s. | Epidermoid Carcinoma | 12 months | Bowel perforation | Laparotomy | Ileum | Brain | Resection | N.s. | |
| Kagohashi et al.21 | 59 | Male | Yes | LUL | N.s. | NSCLC | Synchronous | Intussusception | CT scan | Jejunum | – | Resection | 6 months | |
| Karamercan et al.22 | 63 | Male | N.s. | LUL | N.s. | SCLC | 11 months | Abdominal pain | CT scan | Ileum | – | Resection | N.s. | |
| Lau and Leung23 | 59 | Male | Yes | LPH | N.s. | SCLC | Synchronous | Abdominal pain | Colonoscopy | Cecum | Brain | Chemotherapy | N.s. | |
| Kim et al.24 | 1 | 79 | Male | Yes | RLL | T2N0M0 | N.s. | 5.5 months | Abdominal pain | N.s. | Colon | – | Surgery a | 1 month |
| 2 | 56 | Male | Yes | LUL | T2N1M0 | N.s. | 2.5 months | Melena | N.s. | Ileum | – | Surgery a | 69 days | |
| 3 | 70 | Male | Yes | LLL | T2N0M1 | N.s. | 1 months | Abdominal pain | N.s. | Ileum | Brain | Surgery a | 12 days | |
| 4 | 46 | Female | Yes | LLL | T2N2M0 | N.s. | 30.5 months | Abdominal pain | N.s. | Ileum | Pleural | Surgery a | 102 days | |
| 5 | 67 | Male | No | LUL | T1N1M1 | N.s. | Synchronous | Abdominal pain | N.s. | Stomach | Lung | None | 80 days | |
| 6 | 61 | Male | Yes | LUL | T3N2M0 | N.s. | 3.5 months | Abdominal pain | N.s. | Ileum | Adrenal gland | Surgery | Alive at 33 months | |
| 7 | 72 | Male | Yes | RUL | T1N0M1 | N.s. | Synchronous | Asymptomatic | N.s. | Stomach | Lung, brain, adrenal gland | None | 67 days | |
| 8 | 78 | Male | Yes | LUL | T1N3M1 | N.s. | Synchronous | Hematochezia | N.s. | Colon | Lung, neck, adrenal gland, spleen | None | 133 days | |
| 9 | 66 | Male | Yes | RLL | N.s. | N.s. | Synchronous | Hematemesis | N.s. | Stomach | Adrenal gland | Elettrocautery | 145 days | |
| 10 | 62 | Male | Yes | RUL | T2N2M1 | N.s. | Synchronous | Melena | Laparotomy | Ileum | – | Surgery | Alive at 63 months | |
| Pollheimer et al.25 | 65 | Female | N.s. | N.s. | N.s. | NSCLC | N.s. | Intussusception | CT scan | Ileum, cecum | – | Right hemicolectomy | N.s. | |
| Chiu et al.26 | 87 | Male | N.s. | RUL | N.s. | Adenocarcinoma | Synchronous | Intussusception | CT scan | Jejunum | – | Resection | 3 months | |
| Bélanger and Gagné27 | 60 | Male | Yes | RUL, LLL | N.s. | N.s. | Synchronous | Bowel perforation | Laparoscopy | Jejunum, ileum | – | Resection | 10 months | |
| Shi et al.28 | 61 | Male | N.s. | N.s. | T2N0M1 | Sarcomatoid carcinoma | 17 months | Intussusception | CT scan | Jejunum | Adrenal gland, liver, lung, brain | Resection | 4 months | |
| Meneses Grasa et al.29 | 69 | Male | Yes | LUL | T3N2M0 | Epidermoid carcinoma | 6 months | Bowel obstruction | CT scan | Jejunum, ileum mesentery | – | Resection | 1.5 months | |
| Scabini et al.30 | 76 | Male | N.s. | N.s. | N.s. | SCLC | N.s. | Bowel obstruction | – | Ileum | – | Resection | N.s. | |
| Ara31 | 75 | Male | Yes | LPH | N.s. | Adenocarcinoma | Synchronous | Bowel obstruction | Laparotomy | Ileum | – | Resection | 2.5 months | |
| Weng et al.32 | 53 | Male | Yes | LIL | T4N3M1 | Adenocarcinoma | Synchronous | Bowel obstruction | CT scan | Colon | – | Colostomy | 6 months | |
| Kini et al.33 | 78 | Male | N.s. | N.s. | N.s. | Adenocarcinoma | 7 months | Abdominal pain | CT scan | Jejunum, ileum | – | Resection | Alive at 5 months | |
| Yildirim et al.34 | 78 | Male | N.s. | N.s. | N.s. | Epidermoid carcinoma | 3 months | Abdominal pain | Laparotomy | Ileum | – | Resection | 21 days | |
| Okutur et al.35 | 64 | Male | N.s. | LUL | N.s. | Epidermoid carcinoma | 4 months | Rectal bleeding | Rectal examination | Anal canal | – | Radiotherapy | N.s. | |
| Otera et al.36 | 63 | Male | Yes | N.s. | N.s. | NSCLC | Synchronous | Intussusception | CT scan | Ileum | – | Resection | 27 days | |
| Akamatsu et al.37 | 72 | Male | Yes | RUL | N.s. | NSCLC | Synchronous | Asymptomatic | CT scan | Jejunum | – | Chemotherapy | 5 months | |
| Lin et al.38 | 76 | Male | No | LUL | N.s. | Adenocarcinoma | 0.5 months | Melena | Gastroscopy | Duodenum | Bone | Blood transfusion | 2.5 months | |
| Papaziogas et al.39 | 68 | Male | Yes | RLL | N.s. | Epidermoid carcinoma | 8 months | Bowel obstruction | CT scan | Ileum | – | Resection | 4 months | |
| Yamada et al.40 | 66 | Male | Yes | LUL | T4N3M1 | Epidermoid carcinoma | Synchronous | Bowel perforation | CT scan | Duodenum, ileum | – | Resection | 4 months | |
| Nishizawa et al.41 | 1 | 55 | Male | N.s. | N.s. | T1N0 | Adenocarcinoma | Synchronous | Bowel perforation | Abdominal X-ray | Ileum | Liver, brain | Resection | 53 days |
| 2 | 58 | Male | N.s. | N.s. | T4N1 | Adenocarcinoma | 17.4 months | Bowel perforation | Abdominal X-ray | Jejunum | Colon | Resection | 108 days | |
| 3 | 40 | Male | N.s. | N.s. | T2N3 | Adenocarcinoma | 6.7 months | Bowel perforation | Abdominal X-ray | Ileum | Brain | Resection | 347 days | |
| 4 | 56 | Male | N.s. | N.s. | T2N3 | Adenocarcinoma | 9.1 months | Abdominal pain | Gastroscopy | Duodenum | Liver | Bypass | 25 days | |
| 5 | 78 | Male | N.s. | N.s. | T4N2 | Epidermoid carcinoma | 2.3 months | Bowel perforation | CT scan | Ileum | Lung, liver, bone, adrenal gland | Resection | 67 days | |
| 6 | 62 | Male | N.s. | RUL | T1N0 | Adenocarcinoma | 14.7 months | Abdominal pain | Gastroscopy | Jejunum | – | Resection | 742 days | |
| 7 | 68 | Male | N.s. | N.s. | T4N1 | Adenocarcinoma | 31.3 months | Abdominal pain | CT scan | Ileum | Brain, bone | Bypass | 302 days | |
| Fujiwara et al.42 | 1 | 66 | Female | N.s. | N.s. | N.s. | NSCLC | 6.7 months | Melena | N.s. | Colon | – | Resection | 40 months |
| 2 | 68 | Male | N.s. | N.s. | N.s. | NSCLC | 9.5 months | Anemia | N.s. | Ileum | – | Resection | 93.6 months | |
| 3 | 70 | Female | N.s. | N.s. | N.s. | NSCLC | 19.5 months | Melena | N.s. | Rectum | – | Resection | 46.9 months | |
| 4 | 48 | Male | N.s. | N.s. | N.s. | NSCLC | 9.4 months | Melena | N.s. | Ileum | Adrenal gland | Resection | 14.9 months | |
| 5 | 83 | Male | N.s. | N.s. | N.s. | NSCLC | 6.9 months | Abdominal mass | N.s. | Colon | Liver | Resection | 3.7 months | |
| 6 | 51 | Male | N.s. | N.s. | N.s. | NSCLC | 2.2 months | Anemia | N.s. | Stomach | Skin, brain | N.s. | 0.4 months | |
| 7 | 57 | Male | N.s. | N.s. | N.s. | NSCLC | 1.2 months | Anemia, ileus | N.s. | Ileum, stomach | Liver, kidney | N.s. | 1.1 months | |
| 8 | 69 | Male | N.s. | N.s. | N.s. | NSCLC | 14.3 months | N.s. | N.s. | Colon | Adrenal gland, brain | N.s. | 0.2 months | |
| 9 | 70 | Male | N.s. | N.s. | N.s. | N.s. | 4 months | Melena | N.s. | Ileum | Adrenal gland, pancreas | N.s. | 10.8 months | |
| Tanaka et al.43 | 85 | Male | N.s. | N.s. | T4N1M1 | Epidermoid carcinoma | 120 months | Bowel perforation | CT scan | Jejunum | Bone | Resection | 3 months | |
| Song et al.44 | 58 | Male | N.s. | LUL | N.s. | NSCLC | Synchronous | Bowel obstruction | CT scan | Jejunum | Bladder, adrenal gland, brain | Resection | N.s. | |
| Jarmin et al.45 | 75 | Male | N.s. | N.s. | N.s. | SCLC | Synchronous | Intussusception | Laparotomy | Duodenum, jejunum | N.s. | Whipple's procedure, resection | N.s. | |
| Guner et al.46 | 71 | Male | N.s. | N.s. | T3N1 | Sarcomatoid carcinoma | N.s. | Intussusception | Laparotomy | Ileum | N.s. | Resection | 9 months | |
| Parejo-Sánchez et al.47 | 60 | Male | Yes | N.s. | T1N0M0 | NSCLC | 15 | Bleeding | Laparotomy | Jejunum | – | Resection | N.s. | |
| Li Destri et al.48 | 73 | Male | N.s. | N.s. | N.s. | Neuroendocrine carcinoma | Synchronous | Bowel obstruction | CT scan | Ileum | Brain | Resection | 277 days | |
| Lin et al.49 | 78 | Male | Yes | RUL | N.s. | NSCLC | Synchronous | Intussusception | CT scan | Jejunum, colon | – | Resection | 3 months | |
| Hara et al.50 | 58 | Male | N.s. | RUL | T2N0M0 | NSCLC | 9 months | Fever | CT scan | Ileum | – | Resection | 4 months | |
| Rivera et al.51 | 61 | Female | Yes | RUL | N.s. | Adenocarcinoma | Synchronous | Abdominal pain | CT scan | Ileum | Mediastinum, left supraclavicular space | Resection | Alive at 5 months | |
| Koh et al.52 | 46 | Male | Yes | RUL | N.s. | NSCLC | 0.3 moths | Bowel perforation | CT scan | Ileum | Stomach, duodenum, kidneys, pancreas, gallbladder, adrenal gland, thyroid | Resection | 14 days | |
| Khan et al.53 | 60 | Male | Yes | RUL | N.s. | SCLC | Synchronous | Anemia | Colonoscopy | Cecum | Brain, liver, spleen | Polypectomy | 11 months | |
| Sifuentes Giraldo et al.54 | 73 | Male | Yes | LUL | N.s. | Adenocarcinoma | Synchronous | Bowel perforation | CT scan | Colon | – | Resection | 2 months | |
| de Miguel Valencia et al.55 | 48 | Male | Yes | LIL | N.s. | Sarcomatoid carcinoma | Synchronous | Intussusception | CT scan | Ileum | Lung, bone, cerebral, adrenal gland | Resection | 5 months | |
| Guerra et al.56 | 75 | Female | Yes | N.s. | N.s. | SCLC | 1 months | Perianal pain | Rectal examination | Rectum | Bone | Chemotherapy | Alive at 7 months | |
| Mandeville et al.57 | 49 | Female | N.s. | LUL | T4N0M1b | NSCLC | Synchronous | Intussusception | Laparoscopy | Ileum | Bone, brain | Resection | 6 months | |
| Liu et al.58 | 66 | Male | N.s. | RLL | T2N1M0 | Epidermoid carcinoma | 8 months | Melena | CT scan | Ileum | – | Resection | Alive at 5 months | |
| Lu et al.59 | 62 | Female | N.s. | N.s. | N.s. | Adenocarcinoma | Synchronous | Melena | Colonoscopy | Colon | Skin | Chemotherapy | Alive at 2 months | |
| Lu et al.60 | 78 | Male | Yes | N.s. | TxN1M1 | Adenocarcinoma | 4 months | Melena | CT scan | Jejunum | Adrenal gland, bone | Resection | 2 months | |
| Chen61 | 61 | Male | Yes | RPH | N.s. | Adenocarcinoma | Synchronous | Intussusception | CT scan | Ileum | – | Resection | Alive at 3 months | |
| Escoda62 | 63 | Male | N.s. | LPH | N.s. | Epidermoid carcinoma | Synchronous | Bowel obstruction | CT scan | Ileum | Myocardium, bone | Resection | – | |
| Romano et al.63 | 60 | Male | Yes | LIL | N.s. | Carcinosarcoma | 0.5 moths | Intussusception | CT scan | Ileum | Liver, omentum | Resection | 8 days | |
| Nunes et al.64 | 43 | Male | Yes | RUL | T2N0M1 | Adenosquamous carcinoma | Synchronous | Bowel obstruction | Colonoscopy | Left colon, duodenum | Brain | Resection | Alive at 18 months | |
| Iwamuro et al.65 | 71 | Male | N.s. | RML | T3N2M1 | Adenocarcinoma | N.s. | Melena | Gastroscopy | Duodenum | Adrenal gland | Chemotherapy | 5 months | |
| Nakamura et al.66 | 88 | Female | N.s. | N.s. | T1N1M0 | Epidermoid carcinoma | 144 months | Intussusception | CT scan | Ileum | – | Resection | 5 months | |
| Fujii et al.67 | 55 | Female | N.s. | LUL | N.s. | NSCLC | Synchronous | Intussusception | CT scan | Jejunum | – | Resection | Alive at 11 months | |
| Present series | 1 | 69 | Male | Yes | LPH | T4N3M1 | Epidermoid carcinoma | 6 months | Bowel obstruction | CT scan | Ileum | Liver, bone, peritoneum, mesentery | Resection | 1.5 months |
| 2 | 79 | Male | Yes | RML | T4N2M1 | Adenocarcinoma | Synchronous | Bowel perforation | CT scan | Jejunum | Peritoneum | Resection | 1 months | |
| 3 | 67 | Male | Yes | LUL | T4N2M1 | Adenocarcinoma | 5.5 months | Bowel perforation | CT scan | Ileum | Adrenal gland, abdominal wall | Resection | 1 months | |
| 4 | 53 | Male | Yes | LIL | T1N2M1 | NSCLC | 7 months | Abdominal pain | Entero-MRI | Jejunum, ileum | Liver, spleen vertebrae | Conservative | 8 months | |
| 5 | 72 | Male | Yes | RUL | T3N0M0 | Adenocarcinoma | 12 months | Asymptomatic | PET-CT | Ileum | Brain | Resection | 4 months | |
Interval time between the lung cancer diagnosis and intestinal metastasis diagnosis. GI: Gastrointestinal. a: surgical procedure not specified. NSCLC: non-small cell lung carcinoma. SCLC: small cell lung carcinoma. N.s.: not specified. CT: computed tomography. MRI: magnetic resonance imaging. PET: positron emission tomography. RL: right lobe. RPH: right pulmonary hilum. RUL: right upper lobe. RML: right middle lobe. RLL: right lower lobe. LPH: left pulmonary hilum. LUL: left upper lobe. LIL: left inferior lobe. LLL: left lingual lobe.
The most common site of primary lung cancer was left upper lobe (17 patients, 18.7%), followed by right upper lobe in (16 patients, 17.6%) and right lower lobe (8 patients, 8.8%) (Table 1). Pathological histology has been reported in 78 patients (non-small cell lung cancer in 69 patients, 75.9%, small cell lung cancer in 9 patients, 9.9%). Non-small cell lung cancer was divided in: 23 adenocarcinomas (25.3%), 16 epidermoid carcinomas (17.6%), 3 sarcomatoid carcinomas (3.3%) and 2 carcinosarcomas (2.2%) (Table 1).
In 33 cases (38.8%), primary lung cancer and GI metastasis were diagnosed at the same time, and in 52 cases (61.2%) delayed. The median time between primary lung cancer diagnosis and GI metastasis diagnosis (reported in 85 cases, 93.4%) was 2 months (95% CI: 0.1–38) (Table 1).
Bowel obstruction was observed in 26 patients (28.6%; 15 intussusceptions, 10 neoplastic stenosis, 1 compression from mesenteric neoplastic infiltration), followed by GI bleeding in 25 patients (27.5%; 15 melena, 5 chronic anemia, 1 hematochezia, 1 hematemesis, 1 rectal bleeding and 2 anemia), bowel perforation in 17 patients (18.7%) and abdominal pain in 14 patients (15.4%). Five patients were asymptomatic (5.5%) and in 3 patients (3.3%) fever, perianal pain and abdominal mass were observed (Table 1).
Computed tomography (CT) scan was the most useful diagnostic test (35 patients, 38.5%) followed by endoscopy (gastroscopy, colonoscopy or enteroscopy in 15 patients, 16.5%) and laparotomy (11 patients, 12.1%) (Table 1).
The most common site of metastasis was the ileum (40 patients, 44%), followed by colon (18 patients, 19.8%) and jejunum (16 patients, 17.6%). Extra-intestinal metastasis was observed in 50 patients (54.9%) (Table 1).
Regarding treatment options, bowel resection was performed in 67 patients (73.6%), chemotherapy or radiotherapy and conservative treatment in 6 patients (6.6%), respectively, and endoscopic treatment in 2 patients (2.2%) (Table 1).
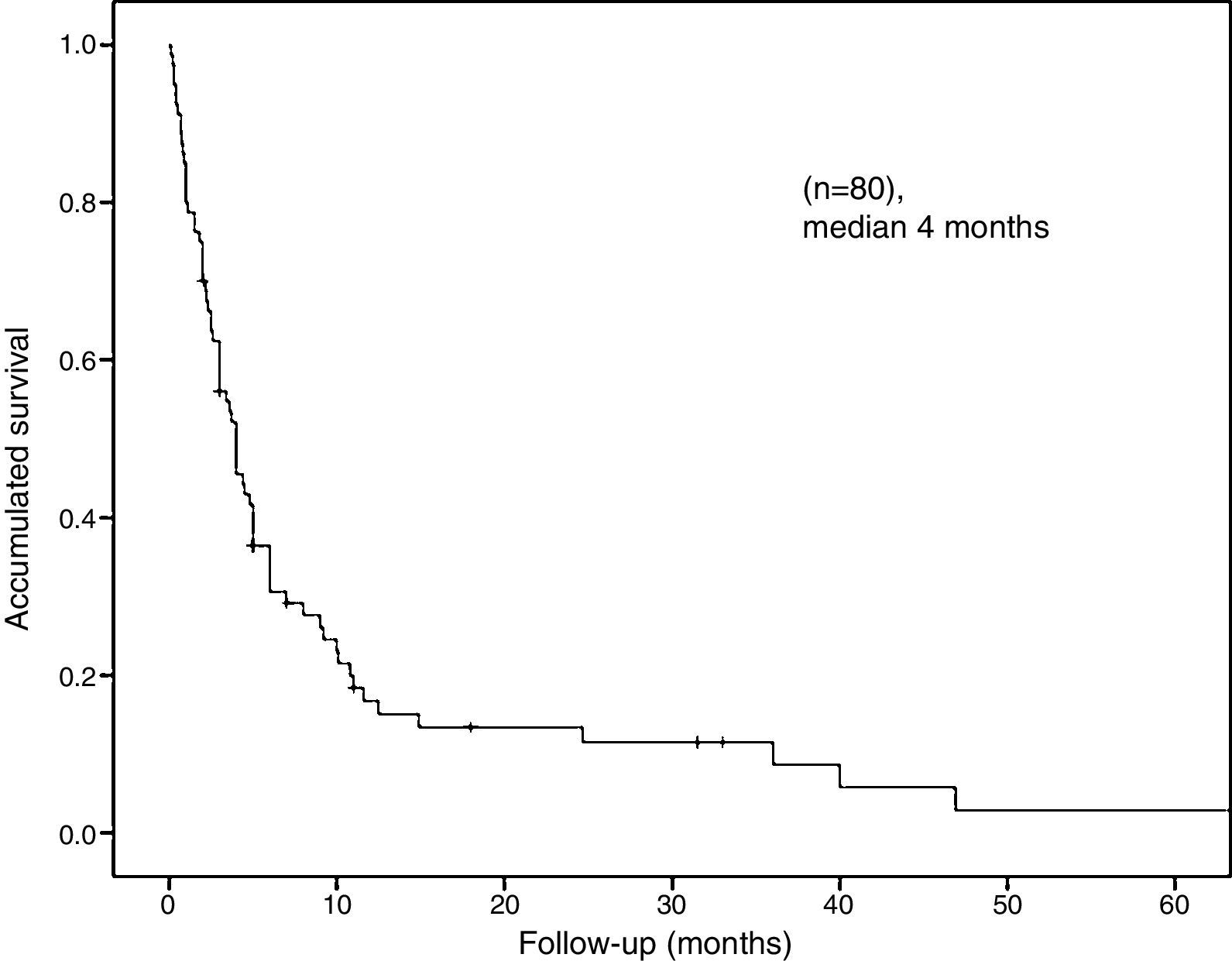
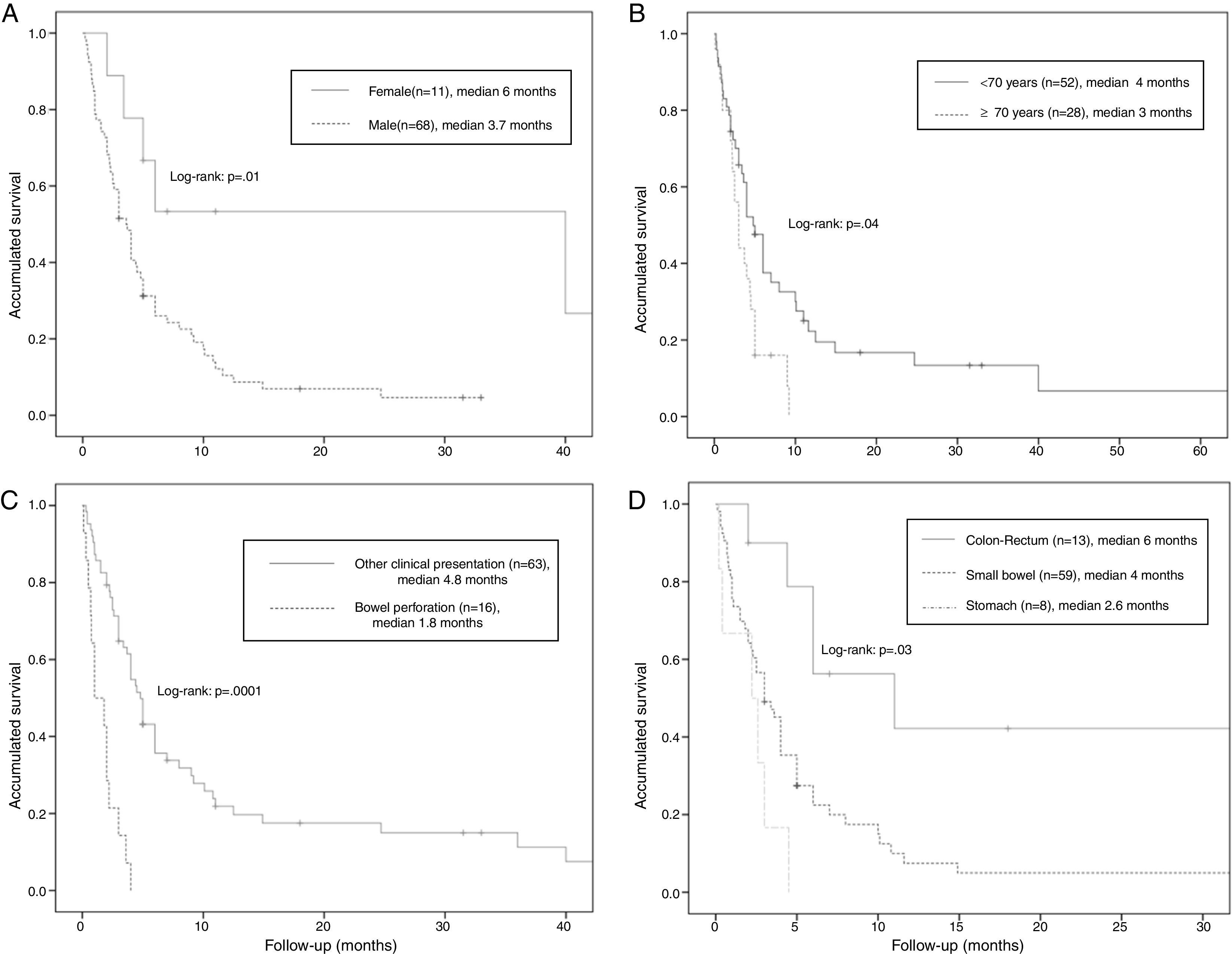
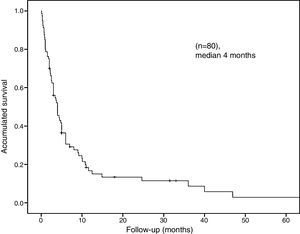
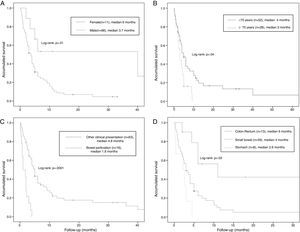
The median overall survival was 4 months (95% CI: 2.68–5.31) (Fig. 2). Survival stratified analysis revealed statistical significant differences when the patients were compared according to gender (male vs female: 3.7 months, 95% CI: 2.7–4.6 vs 6 months, 95% CI: 0–36.5, Log-rank: P=.01), age (<70 vs ≥70: 4 months, 95% CI: 2.4–5.5 vs 3 months, 95% CI: 2.1–3.8, Log-rank: P=.04), clinical presentation (bowel perforation vs others: 1.8 months. 95% CI: 0.4–3.1 vs 4.8 months, 95% CI: 3.9–5.6, Log-rank: P=.0001) and metastasis location (colon-rectum vs small bowel vs stomach: 6 months, 95% CI: 3.3–86 vs 4 months, 95% CI: 3–4.9 vs 2.6 months, 95% CI: 1.6–3.5, Log-rank: P=.03) (Fig. 3). No statistically significant differences were observed when patients were stratified according smoking habit, pathological histology, tumor stage, lymph-node stage, presence of extra-intestinal metastasis and synchronous vs delay diagnosis.
DiscussionFrom March 2001 to August 2017, 86 cases of GI metastasis from primary lung cancer are reported in literature10–67 and between 2012 and 2017, in authors’ hospital, 5 patients were treated for the same reason, for a total of 91 patients included in the present study. The treatment of choice of this type of lesions, is still debated in literature, due to the lack of reported large series and the difficulty to perform Randomized Control Trials (RCTs) due to the patients’ heterogeneous characteristics who experience GI metastasis. Often, the treatment is affected by patients’ urgency conditions, such as bowel perforation or obstruction and bleeding, that do not allow to choose a different treatment than surgery. For these reasons and due to the patients’ poor condition at the time of GI metastasis diagnosis (advanced stage cancer, old age, severe symptoms) the median survival rate is very low.7
We have investigated with the aim to analyze our data and those reporting in literature regarding GI metastasis from primary lung cancer. Anyway, the present study has several biases, such as the relative small number of patients and several missing data from the literature.
The most frequent lung histological subtype cancer related to GI metastasis is still debated in literature3,5,7,8,24 but in the present series, Large cell carcinoma is the most common observed (75.9%).
The majority of patients with GI metastasis from a primary lung cancer are asymptomatic,5 as evidenced by the discrepancy between the estimated incidence in clinical studies and post-mortem studies.24 This clinical under-diagnosis may be due to the fact that the GI discomfort, referred by these patients, is often confused with chemotherapy side effects68 and for this reason requires a greater degree of suspicion by the clinician for early identification. The most frequent uncomplicated symptoms observed are the abdominal pain occurred in 50% of patients and weight loss.7,69 Among complicated symptoms, small bowel perforation is described,7,70 such as the obstruction that can occur as a consequence of an occlusive mass24 or by intussusception of the affected ileal tract.7,33 Finally, acute digestive hemorrhage may be observed as melena in case of stomach, duodenum or small bowel lesions71 and rectal bleeding in case of lower GI tract involvement.68 In the present series, small bowel obstruction is the most frequent complicated symptom observed (28.6%) as well as reported by Di et al. (35%).7
Lung metastasis can be observed in all GI tract such as esophagus (6.3%), small bowel (2.6%), stomach (1.2%) and colon (0.7%).3 Based on the literature, the most frequent small bowel site are the jejunum and ileum,7,24,72 while the duodenum is usually affected in a smaller number of cases.73 In the present series, the most frequent metastasis site is in the ileum (44%).
CT scan is the most used diagnostic test, both in authors’ experience and in the literature.5 This test has been widely described5 due to its versatility and easy access in the context of urgency, with an estimated sensitivity rate of 72%,74 however, it has been shown difficulty in detecting small lesions.74 Positron emission tomography-CT (PET-CT) scan shown to be useful in the diagnosis of metastasis from primary lung cancer also in case of asymptomatic GI metastasis.75,76 Although, in the present study is employed only in one patient, and, at now, no sufficient data about sensitivity and specificity of this test are available.77 The use of endoscopic approach has been described in several series as a diagnostic test in patients who present digestive bleeding or anemia.68,69,76,77 A recent study reports the use of endoscopic capsule such as feasible diagnostic test for small bowel lesions and as a non-invasive diagnostic method that could reduce the metastasis under-diagnosis.78 Magnetic resonance imaging (MRI) is a less common diagnostic test proposed in the literature,47 and has been employed in only one patient of the present series.
The treatment of choice of the lung GI metastasis is still debated in literature, between authors that support conservative approach8,79 and authors in favor of surgical approach.3,24 Anyway, in case of bowel perforation or obstruction or in case of massive bleeding, the surgical approach is often the only possible treatment.3 A perioperative mortality rate between 20% and 100%, is reported in literature, in patients who underwent surgery8,79 but it should be noted that most of these deaths were not due to immediate surgical complications.7 Goh et al. suggest that an adequate selection of patients who underwent surgery could increase the survival rate,3 considering multiple extra-intestinal metastasis and bowel perforation the most important factors that affected the postoperative outcomes.3 In the present series perioperative mortality was observed in one case. The most common surgical technique performed, in the literature such as in the present series, is the bowel resection with primary anastomosis by laparotomy,3,8 but a recent study shows the feasibility of laparoscopic approach.80
The median survival ranges between 1 and 6 months.3,7,8,24 The largest series published in literature, that reports a retrospective analysis of 100 previously published cases (including autopsies), shows a median survival of 2.3 months7 and reports that the risk factors mostly associated with mortality are: age greater than 70 years, bowel perforation and presence of extra-intestinal metastasis,7 as well as confirmed in this series. In the present study, the survival rate is similar to that published in literature (4 months, 95% CI: 2.68–5.31), and stratifying the patients, the statistical significant differences were observed only for gender, age, clinical presentations and metastasis site.
The gold standard treatment is not still available based on the published data, and none of the reported treatments had a significant impact on survival. Anyway, surgical treatment cannot defer in case of bowel perforation but also in some case of massive bleeding or bowel obstruction. For this reason, in patients with acute abdomen and history of lung cancer, GI metastasis should be considered such as differential diagnosis. At the time of primary lung cancer diagnosis, after CT scan, PET-CT scan could be helpful to diagnose eventual synchronous metastasis.
To the best of the authors’ knowledge, this is the first systematic review, concerning GI metastasis from primary lung cancer, reported in literature.
Randomized clinical trials are difficult to perform due to the rarity and the heterogeneity of these lesions, however a wider sample size of patients, and further studies are required to confirm these data.
Authors’ ContributionAndrea Balla: study designs, acquisition of data, analysis and interpretation of results, manuscript preparation, critical revision and approval of the final version of the manuscript.
José D. Subiela: study designs, acquisition of data, analysis and interpretation of results, manuscript preparation, critical revision and approval of the final version of the manuscript.
Jesús Bollo: study designs, acquisition of data, analysis and interpretation of results, manuscript preparation, critical revision and approval of the final version of the manuscript.
Carmen Martinez: analysis and interpretation of results, manuscript preparation, critical revision and approval of the final version of the manuscript.
Carlos Rodriguez Luppi: analysis and interpretation of results, manuscript preparation, critical revision and approval of the final version of the manuscript.
Pilar Hernández: analysis and interpretation of results, manuscript preparation, critical revision and approval of the final version of the manuscript.
Yuliana Pascual: analysis and interpretation of results, manuscript preparation, critical revision and approval of the final version of the manuscript.
Silvia Quaresima: analysis and interpretation of results, manuscript preparation, critical revision and approval of the final version of the manuscript.
Eduard M. Targarona: study designs, acquisition of data, analysis and interpretation of results, manuscript preparation, critical revision and approval of the final version of the manuscript.
Conflict of InterestDr. Andrea Balla, Dr. José D. Subiela, Dr. Jesús Bollo, Dr. Carmen Martinez, Dr. Carlos Rodriguez Luppi, Dr. Pilar Hernández, Dr. Yuliana Pascual, Dr. Silvia Quaresima and Professor Eduard M. Targarona have no conflicts of interest or financial ties to disclose.
Please cite this article as: Balla A, Subiela JD, Bollo J, Martinez C, Rodriguez Luppi C, Hernández P, et al. Metástasis gastrointestinales de carcinoma pulmonar primario. Serie de casos y revisión sistemática de la literatura. Cir Esp. 2018;96:184–197.