Venous thromboembolism (VTE) represents a serious complication after oncologic surgery. Recent studies have shown that the risk of VTE persists several weeks after surgery. This study assesses the form of presentation and time course of VTE after abdominal and pelvic cancer surgery.
MethodsProspective, multicenter, observational study that analyzes data from an international registry (RIETE) that includes consecutive patients with symptomatic VTE. Our study assesses the form and time of presentation of postoperative VTE, as well as main outcomes, in patients operated for abdominopelvic cancer 8 weeks prior to VTE diagnosis. Variables related to the presentation of VTE after hospital discharge are identified.
ResultsOut of the 766 analyzed patients with VTE, 395 (52%) presented pulmonary embolism (PE). Most VTE cases (84%) were detected after the first postoperative week, and 38% after one month. Among patients with VTE in the first postoperative week, 70% presented PE. VTE presented after hospital discharge in 54% of cases. Colorectal, urologic, and gynecologic tumors, the use of radiotherapy, and blood hemoglobin levels were independently associated with VTE diagnosis after hospital discharge. Complications (thrombosis recurrence, bleeding, and death) occurred in 34% of patients with VTE detected before hospital discharge, compared to 24% in VTE after hospital discharge (P<0.01).
ConclusionsVTE occurs after hospital discharge in most patients, particularly in those operated for colorectal, urologic, and gynecologic cancer. Pulmonary embolism is more frequent in patients who develop early VTE, who also have worse prognosis.
La enfermedad tromboembólica venosa (ETV) representa una grave complicación tras la cirugía oncológica. Recientes estudios revelan que el riesgo de ETV postoperatoria se extiende durante varias semanas. Este estudio analiza la forma y momento de presentación de la ETV tras cirugía oncológica abdominal.
MétodosEstudio observacional, prospectivo y multicéntrico, que analiza los datos de un registro internacional (RIETE) que incluye pacientes consecutivos con ETV sintomática. Se evalúa la forma y momento de presentación de la ETV, así como su pronóstico, en pacientes operados por cáncer abdominopélvico en las 8 semanas previas a la ETV. Se identifican las variables que se asocian con la presentación de la ETV tras el alta.
ResultadosEntre los 766 pacientes analizados, 396 (52%) presentaron embolia pulmonar (EP). La mayoría (84%) de los casos de ETV se presentaron después de la primera semana de la intervención y un 38% pasado un mes. El 70% de los pacientes con ETV precoz presentaron EP. El 54% de los casos desarrollaron ETV tras el alta. Los tumores colorrectales y genitourinarios, el uso de radioterapia y los niveles de hemoglobina resultaron variables independientes de ETV tras el alta. El 34% de los pacientes con ETV antes del alta tuvieron complicaciones (recidiva, hemorragia y defunción), frente al 24% con ETV tras el alta (p<0.01).
ConclusionesLa ETV se presenta tras el alta en la mayoría de los pacientes, especialmente en aquellos con cáncer colorrectal y genitourinario. La EP es más frecuente en los pacientes con ETV precoz que, además, tienen peor pronóstico.
Postoperative venous thromboembolic (VTE) disease, which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), represents a frequent and potentially serious complication in oncologic surgery patients.1,2 In fact, PE was the first cause of mortality in the first month after cancer surgery in an Italian multicenter study.3 Subsequently, a review in the United States of more than 2.5 million patients treated surgically for cancer has shown that the probability of dying in the immediate postoperative period is 5 times greater in those with symptomatic VTE.4
Available evidence on the natural history and presentation of VTE in cancer surgery patients is available primarily from controlled clinical trials of selected patients, which do not necessarily reflect standard clinical practice. In order to estimate the actual impact of postoperative VTE, a series of unselected consecutive cases should be analyzed, and follow-up should be continued for at least 3 months.5 A better understanding of the natural history of this complication is essential, as there is controversy about the optimal duration of antithrombotic prophylaxis in cancer surgery. Thus, while some clinical practice guidelines recommend maintaining low-molecular-weight heparin (LMWH) prophylaxis for one month after abdominal and pelvic cancer surgery,6,7 others are more selective depending on patient characteristics and the presence of risk factors.8,9
The main purpose of this study was to evaluate the form and time of presentation of symptomatic VTE in patients operated on for abdominal and pelvic cancer, as well as the disease progression in the first 3 months of treatment. To do so, we analyzed data from the largest prospective observational VTE registry in the world.10 The information obtained allowed us to better understand the natural history and evolution of this preventable complication in our setting and to identify which patient variables or tumor location variables correlate with the moment and form of presentation of VTE after abdominal and pelvic cancer surgery, as well as its short-term prognosis.
MethodsThis observational study was designed to use data from the Computerized Registry of Patients with Venous Thromboembolism (RIETE), which is an active, prospective, multicenter, international registry that includes consecutive patients with symptomatic VTE confirmed by diagnostic tests with a minimum follow-up period of 3 months. Currently, 245 hospitals in 18 different countries collaborate with this registry.
We analyzed data corresponding with the study population: patients included in RIETE over a 12-year period.
The inclusion criteria were: patients with symptomatic VTE with presentation as DVT or PE, confirmed by objective studies, included in RIETE, who had been treated surgically for abdominal and pelvic cancer in the 8 weeks prior to the diagnosis of VTE. All patients were followed by researchers at the participating hospitals for a minimum of 3 months to register any complications or clinically relevant event.
Excluded from the study were those patients who participated in therapeutic clinical trials and those for whom a 3-month follow-up was not considered viable.
The variables analyzed included patient demographic characteristics at the time of diagnosis of VTE (sex, age height, weight) and presence of comorbidities and other risk factors; pharmacological prophylaxis received during the perioperative period (drug, dosage, duration); form of presentation of the VTE, either DVT (proximal or distal, unilateral or bilateral) or PE (isolated or associated with DVT); time of presentation of VTE after surgery; treatment received in the acute phase and during the first 3 months; and complications after the establishment of the initial treatment, mainly hemorrhages, thrombotic recurrence and mortality.
The data were collected when the patients gave their explicit consent to participate in the RIETE Registry, in accordance with the requisites of each hospital after having obtained approval for the study from the respective ethics committees.
Local researchers of the RIETE Project registered variables of the patients included consecutively using the online computer application. The identities of the patients remained anonymous, and each case was identified by a single numerical code assigned by the coordinating center.
Statistical AnalysisA descriptive analysis was conducted calculating measures of central tendency and dispersion for the numerical variables. Variables following normal distribution are expressed as mean and standard deviation. Non-normal variables are expressed as median and percentiles. Categorical variables are expressed as absolute and relative frequencies. The Kolmogorov–Smirnov test was used to test the normality of the variables.
For the categorical variables, the Pearson or Fisher's chi-squared test was used, depending on the conditions for applicability. For the numerical variables, the Student t test was used for independent samples or the Mann–Whitney test in cases of non-normality. Bivariate and multivariate analyses were performed to study the variables that were related with the time of onset of VTE as a dichotomized variable (detection before or after discharge). Data were analyzed using the IBM SPSS Statistics 19 software.
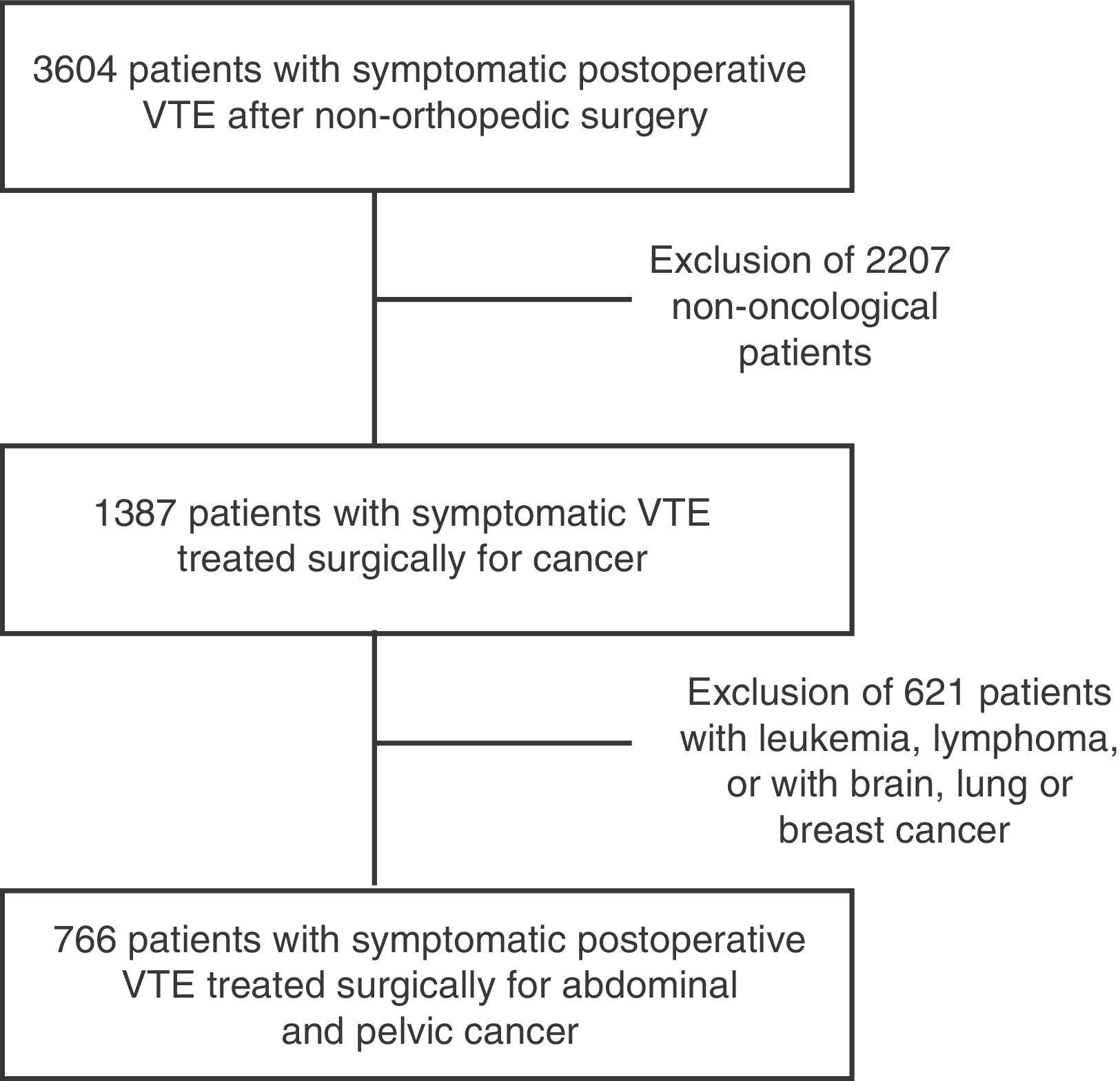
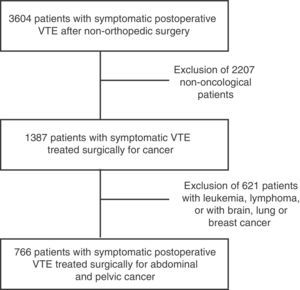
ResultsWith regard to the patient characteristics over the course of 12 years, the RIETE study included 3604 patients with VTE after non-orthopedic or trauma surgery, out of which 766 presented this complication after abdominal and pelvic oncologic surgery (Fig. 1).
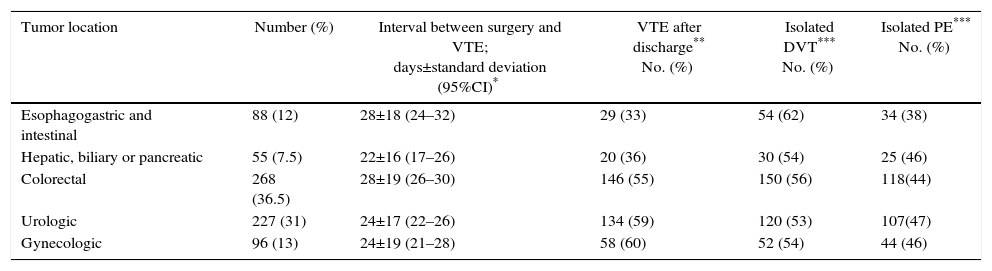
Mean patient age (±standard deviation) was 66±11 years (range 22–94). Table 1 provides the grouped location of the tumors for which patients were treated. Gastrointestinal tumors accounted for 54% of the total, followed by urological tumors (30%) and gynecological tumors (12.5%). In terms of tumor stage, 68% of patients had metastases and 47 and 8% had received chemotherapy and radiotherapy, respectively.
Number of Patients With VTE, Time of Presentation and Presentation Type According to the Tumor Location.
| Tumor location | Number (%) | Interval between surgery and VTE; days±standard deviation (95%CI)* | VTE after discharge** No. (%) | Isolated DVT*** No. (%) | Isolated PE*** No. (%) |
|---|---|---|---|---|---|
| Esophagogastric and intestinal | 88 (12) | 28±18 (24–32) | 29 (33) | 54 (62) | 34 (38) |
| Hepatic, biliary or pancreatic | 55 (7.5) | 22±16 (17–26) | 20 (36) | 30 (54) | 25 (46) |
| Colorectal | 268 (36.5) | 28±19 (26–30) | 146 (55) | 150 (56) | 118(44) |
| Urologic | 227 (31) | 24±17 (22–26) | 134 (59) | 120 (53) | 107(47) |
| Gynecologic | 96 (13) | 24±19 (21–28) | 58 (60) | 52 (54) | 44 (46) |
Antithrombotic prophylaxis with anticoagulants was administered to 575 patients (75%). The most frequently used method was LMWH, used in 96% of cases at a mean dose of 4.000±2.300IU. The mean duration of the prophylaxis was 13±9 days.
As for the form of presentation of postoperative VTE, 370 patients developed isolated DVT (48%), 288 patients (38%) isolated PE and 108 PE associated with DVT (14%). In short, 48% presented DVT without embolism and 52% PE with or without associated DVT. Thrombosis affected the right lower limb in 203 patients (41%), the left in 237 (49%) and both limbs in 49 (10%). Meanwhile, 272 (78%) DVT of the lower limbs were located in venous sectors proximal to the knee. Out of the 61 cases with thrombosis in the upper extremities, in 48 (80%) this was associated with the presence of a central venous catheter.
Although the differences were not statistically significant (P=.07), the percentage of patients with VTE who presented isolated PE was greater in the 119/283 (42%) who were overweight or obese than in the 57/146 (39%) patients who had normal weights or the 8/36 (22%) who were underweight.
Among the patients who received pharmacological prophylaxis and developed VTE, 43% presented isolated PE versus 46% of those who had not received this treatment, although these differences were not statistically significant (P=.38).
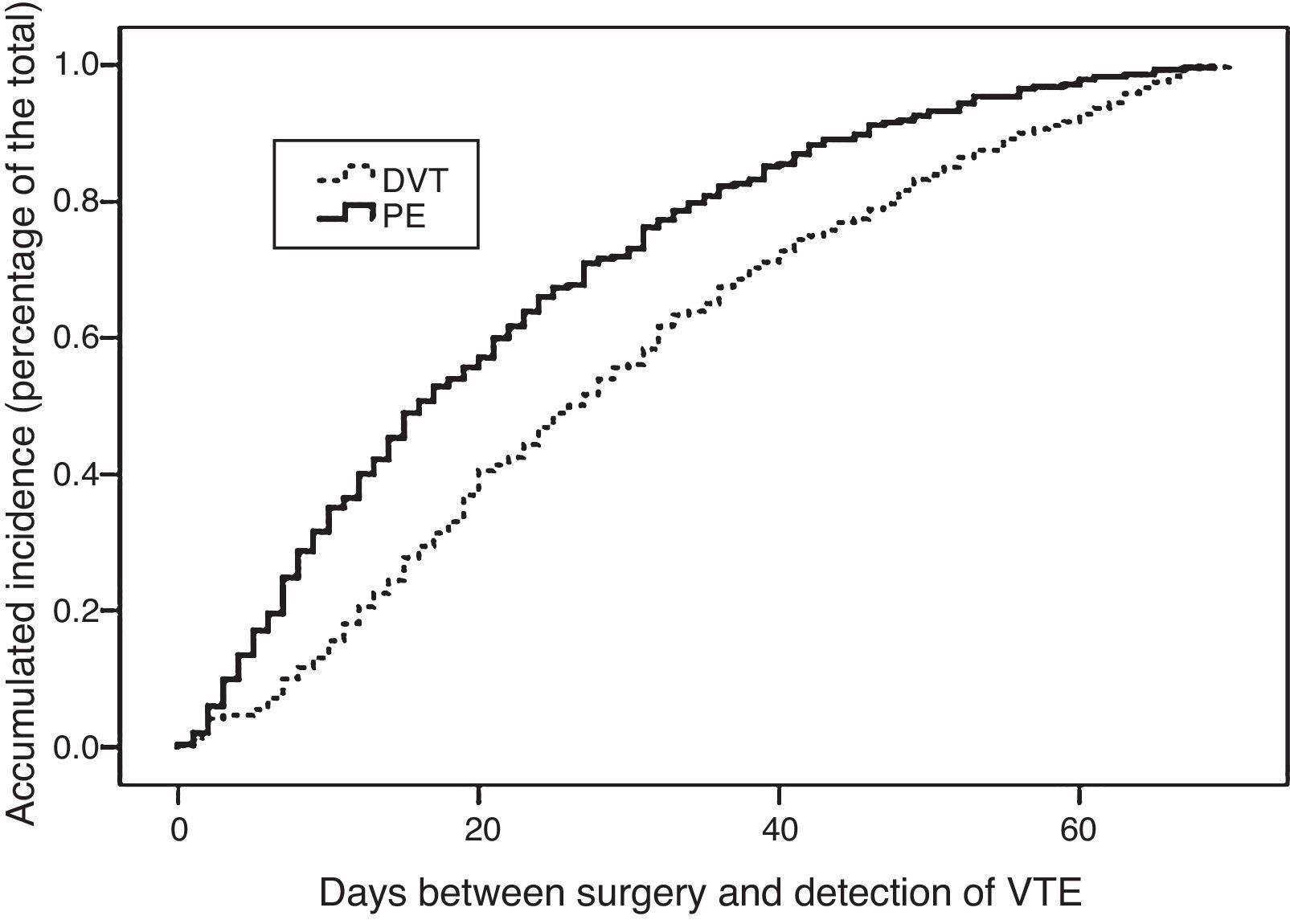
With regards to the time of appearance, 123 patients (16%) presented VTE in the first 7 days after surgery, 353 (46%) between the 8th and 30th day post-op, and 290 (38%) patients more than 30 days post-op. In terms of the presentation of VTE as DVT, PE or PE±DVT (PE with or without associated DVT) and the moment of presentation, as seen in Table 2, the majority of patients with early VTE (<1 week) developed PE±DVT (70%), while almost 60% of the patients with late-onset VTE (>30 days) developed isolated DVT (P<.001).
Time Transpired Between Surgery and VTE Diagnosis According to the Type of Presentation.
| Type of presentation | Interval between surgery and VTE diagnosis* | ||
|---|---|---|---|
| ≤7 days | 7–30 days | ≥30 days | |
| DVT n (%) | 36 (30) | 168 (48) | 166 (57) |
| PE±DVT n (%) | 87 (70) | 185 (52) | 124 (43) |
The mean interval (±standard deviation) expressed in days between the intervention and the diagnosis of VTE was 26.4±18 days. In addition, depending on the form of presentation, a mean of 29.8±18 days transpired in the cases that presented DVT and 21.4±17 days for PE (P<.05). There were no significant differences in the interval between surgery and diagnosis of VTE, which was 25.8±2 days in patients who had received prophylaxis and 26.2±2 days in those who had not (P=.9).
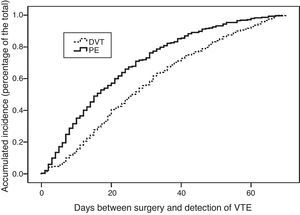
The analysis of these intervals (Table 1) according to the location of the cancer shows that VTE occurred earlier after hepatobiliary pancreatic surgery compared to colorectal or esophagogastric and intestinal cancers, with statistically significant differences (P<.05). Fig. 2 shows the actuarial curve of the percentage of presentation at different intervals after surgery in cases with DVT and PE.
In the total series, VTE occurred after discharge in 408 patients (54%). As for the form of presentation among the patients who experienced VTE after discharge, 194 (47.5%) developed PE±DVT and 214 (52.5%) presented isolated DVT, which were significant (P<.05). Table 1 shows the percentage of VTE presentation before or after discharge, and most colorectal and genitourinary cancers were diagnosed after discharge. This is in contrast with those in esophagogastric-intestinal or hepatobiliary-pancreatic locations, in which almost two-thirds occurred before (P<.001).
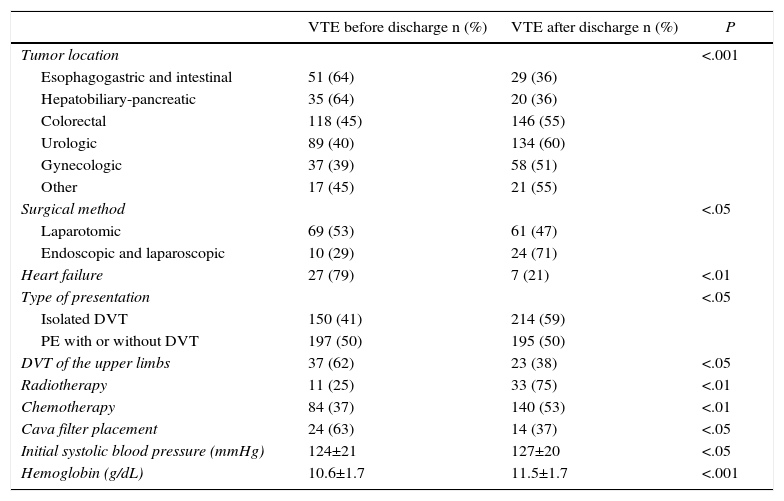
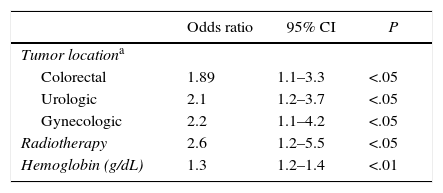
Table 3 shows the variables that were significantly associated with the clinical presentation of VTE after hospital discharge in a bivariate analysis. Table 4 shows the predictive variables of presentation after discharge, by multivariate analysis. The area under the curve of the receiver operator characteristics (ROC) model was 0.672.
Bivariate Analysis of the Variables That Significantly Correlated With the Detection of VTE After Hospital Discharge.
| VTE before discharge n (%) | VTE after discharge n (%) | P | |
|---|---|---|---|
| Tumor location | <.001 | ||
| Esophagogastric and intestinal | 51 (64) | 29 (36) | |
| Hepatobiliary-pancreatic | 35 (64) | 20 (36) | |
| Colorectal | 118 (45) | 146 (55) | |
| Urologic | 89 (40) | 134 (60) | |
| Gynecologic | 37 (39) | 58 (51) | |
| Other | 17 (45) | 21 (55) | |
| Surgical method | <.05 | ||
| Laparotomic | 69 (53) | 61 (47) | |
| Endoscopic and laparoscopic | 10 (29) | 24 (71) | |
| Heart failure | 27 (79) | 7 (21) | <.01 |
| Type of presentation | <.05 | ||
| Isolated DVT | 150 (41) | 214 (59) | |
| PE with or without DVT | 197 (50) | 195 (50) | |
| DVT of the upper limbs | 37 (62) | 23 (38) | <.05 |
| Radiotherapy | 11 (25) | 33 (75) | <.01 |
| Chemotherapy | 84 (37) | 140 (53) | <.01 |
| Cava filter placement | 24 (63) | 14 (37) | <.05 |
| Initial systolic blood pressure (mmHg) | 124±21 | 127±20 | <.05 |
| Hemoglobin (g/dL) | 10.6±1.7 | 11.5±1.7 | <.001 |
Multivariate Analysis of the Predictive Variables for the Detection of VTE After Discharge.
| Odds ratio | 95% CI | P | |
|---|---|---|---|
| Tumor locationa | |||
| Colorectal | 1.89 | 1.1–3.3 | <.05 |
| Urologic | 2.1 | 1.2–3.7 | <.05 |
| Gynecologic | 2.2 | 1.1–4.2 | <.05 |
| Radiotherapy | 2.6 | 1.2–5.5 | <.05 |
| Hemoglobin (g/dL) | 1.3 | 1.2–1.4 | <.01 |
As for the treatment received and patient progress in the first 3 months, in the initial phase of treatment, 680 (90%) patients received full-dose therapeutic LMWH (12.524±3.288IU daily) and 75 (10%) received unfractionated heparin at a mean daily dose of 25.402±9.637IU. Meanwhile, in the extended phase of treatment, 409 (56%) received LMWH and 305 (42%) received oral antivitamin K drugs. The mean duration of initial treatment in the acute phase was 13±5 days, whereas in the chronic phase it was 180±130 days.
Bed rest was recommended in 335 (46%) patients and mobility was not restricted in 117 (17%). Among the 351 patients with isolated DVT of the lower limbs, bed rest was recommended in 116 (33%), compared with 157 (60%) of the 260 with PE (P<.01). The use of compression stockings was recommended in 191 (58%) patients with isolated DVT and 45 (18%) with PE without thrombosis (P<.001).
In the first 3 months of follow-up, 37 patients (4.8%) relapsed. This recurrence was as DVT in 20 cases (54%) and as PE in 17 (46%). Moreover, 68 patients (9%) had hemorrhagic complications, 53% of which were considered severe. The most frequent locations were the gastrointestinal (34%) and urinary tracts (25%). In total, 157 patients (20%) died due to the following causes: attributed to neoplasms in 86 cases (59%), bleeding in 7 (4.8%) and pulmonary embolism in 6 cases (4%). Death was considered due to respiratory failure in 6 patients (4%), to heart failure in 3 (2%), and in 19 (12%) the cause was unknown. Early VTE had a worse prognosis, as 117 (34%) of the 347 patients with VTE before discharge experienced a poor outcome (bleeding, relapse or death) compared to 98 (24%) of the 408 who had VTE after discharge (P=.004).
After discharge, 268 patients had to be re-hospitalized, representing 77% of the 348 discharged patients for whom this information was available. The percentage of re-admittance was greater in patients with PE (127 cases; 93%) than with isolated DVT (141; 66%) (P<.05).
DiscussionThis study demonstrates that, in patients with symptomatic VTE after abdominopelvic cancer surgery, it appears after one week in almost 80% of cases and after one month in more than one-third. VTE appears after discharge in the majority of patients, especially those treated surgically for colorectal and genitourinary cancer, while most hepatobiliary pancreatic tumors occur beforehand. In turn, PE is more frequent in patients with early VTE, who, in addition, have a poorer prognosis.
Our results demonstrate the importance of following these cases for at least 3 months because if we had restricted the follow-up to one month, we would not have detected 38% of the cases. Some studies on the natural history of postoperative VTE in other groups of surgical patients have restricted follow-up to one month3,11–13 and detected that 18–28% of VTE occur after discharge; however, those who increased this follow-up to at least 3 months detected 40–50% of VTE after discharge,14–18 which coincides with our findings.
Although most recent clinical practice guidelines recommend prolonging prophylaxis for 4 weeks after abdominopelvic surgery,6,7,19–22 others restrict this recommendation to patients with certain risk factors.8,9 In our series, the mean duration of prophylaxis, which was 13 days, seems insufficient when one considers that VTE was detected on average 26 days after the intervention. The fact that most VTE occur after discharge may explain why many surgeons underestimate the incidence and actual impact of postoperative VTE and, therefore, do not extend prophylaxis after discharge. Prospective registries such as RIETE that have a 3-month follow-up are able to detect these late-onset cases.23
While the high mortality in the first 3 months (around 20%) was related to cancer in half the cases, it is noteworthy that bleeding, which is logical in association with anticoagulant treatment, and PE caused about 10% of deaths. In general, VTE had a worse prognosis when it occurred before discharge and complicated the initial postoperative progression.
Among the limitations of our study, it should be noted that only patients with confirmed symptomatic VTE were analyzed, and that the evolution of patients without this complication is unknown. Another of the limitations found stems from the fact that the postoperative complications of these patients are not collected in the RIETE. In addition, some selection bias related to the hospitals participating in RIETE cannot be ruled out, although the inclusion of a large number of hospitals that include non-selected consecutive patients could reduce that possibility. Among the advantages, we can highlight the prospective nature of the registry, the inclusion of unselected consecutive cases, the completeness of the data collected, and especially, that there is a 3-month clinical follow-up.
In conclusion, VTE presents after discharge inpatients treated surgically for abdominopelvic oncologic surgery, especially in those with colorectal and genitourinary cancer and those who were administered radiotherapy. However, PE appears earlier than DVT.
Conflict of InterestsThe authors have no conflict of interests to declare.
The authors would like to thank Sanofi Spain for the support of this registry with an unrestricted educational grant. Likewise, we extend our thanks to Bayer Pharma AG for supporting this registry. The support of Bayer Pharma AG was limited to the international section of the RIETE, representing 24.26% of the total of patients included. We would also like to thank the Coordination Center of the RIETE Registry, S&H Medical Science Services, for their data quality control and administrative/logistical support. Lastly, thanks go to Manuela Expósito for her invaluable help with the statistical analysis and data interpretation.
Coordinator of the RIETE project: Dr. Manuel Monreal (Spain)
Members of the RIETE Directive Committee: Dr. Hervè Decousus (France), Dr. Paolo Prandoni (Italy), Dr. Benjamin Brenner (Israel)
National Coordinator of RIETE Spain: Dr. Raquel Barba (Spain)
Coordinating Center of the RIETE Registry: S & H Medical Science Services
Members of the RIETE Group in Spain:
Adarraga M.D., Aibar M.A., Alfonso M., Arcelus J.I., Ballaz A., Baños P., Barba R., Barrón M., Barrón-Andrés B., Bascuñana J., Blanco-Molina A., Camón A.M., Cruz AJ., de Miguel J., del Pozo R., del Toro J., Díaz-Pedroche M.C., Díaz-Peromingo J.A., Falgá C., Fernández-Capitán C., Fernández-Muixi J., Fidalgo M.A., Font C., Font L., Furest I., García M.A., García-Bragado F., García-Morillo M., García-Raso A., Garnés C.M., Gavín O., Gómez C., Gómez V., González J., Grau E., Guijarro R., Gutiérrez J., Hernández-Blasco L., Hernando E., Isern V., Jara-Palomares L., Jaras M.J., Jiménez D., Jiménez R., Jiménez S., Joya M.D., Lecumberri R., Lima J., Llamas P., Lobo J.L., López-Jiménez L., López-Reyes R., López-Sáez J.B., Lorente M.A., Lorenzo A., Loring M., Lumbierres M., Madridano O., Maestre A., Manrique-Abos I., Marchena P.J., Martín M., Martín-Martos F., Monreal M., Morales M.V., Nieto J.A., Nieto S., Núñez M.J., Odriozola M., Olivares M.C., Otalora S., Otero R., Pedrajas J.M., Pellejero G., Pérez-Ductor C., Peris M.L., Pons I., Porras J.A., Ramírez L., Reyes R., Riera-Mestre A., Rivas A., Rodríguez-Dávila M.A., Rosa V., Rubio C.M., Ruiz-Artacho P., Sáez M.T., Sahuquillo J.C., Sala-Sainz M.C., Sampériz A., Sánchez-Martínez R., Sancho T., Soler S., Soto M.J., Suriñach J.M., Tolosa C., Torres M.I., Troya J., Trujillo-Santos J., Uresandi F., Usandizaga E., Valero B., Valle R., Vela J., Vicente M.P., Vidal G., Villalobos A. and Xifre B.
The list of RIETE researchers is included in the Appendix.
Please cite this article as: Bustos Merlo AB, Arcelus Martínez JI, Turiño Luque JD, Valero B, Villalobos A, Aibar MÁ, et al. Forma de presentación, historia natural y evolución de la enfermedad tromboembólica venosa postoperatoria en pacientes operados por cáncer abdominal y pélvico. Análisis del registro RIETE. Cir Esp. 2017;95:328–334.