This study was aimed to assess the main clinical, pathological and therapeutic characteristics of a cohort of gastrointestinal stromal tumors (GIST).
MethodsObservational study including 66 patients diagnosed with GIST admitted to our hospital between 2002 and 2015. Parameters related to medical history, clinical manifestations, medical and surgical treatment, histopathology, and morbi-mortality were studied. A review of the literature was included to correlate with the results.
ResultsThe most frequent location of GIST in our patients was the stomach (65.2%), in which the gastric fondo was the predominant region. The most common clinical manifestation was gastrointestinal hemorrhage (45.5%), followed by incidental finding after imaging or invasive procedures (33.3%). 58 patients underwent surgery (90.6%), 15.5% were urgent. A total of 69% of the GISTs had a size between 2 and 10cm. The one-year mortality was 7.9%, all cases related to local or remote extension, or surgical complications.
ConclusionThere is a large clinical variability among GIST cases. The first choice of treatment is surgery, which is feasible in most cases and should be as conservative as possible. The prognosis varies depending on the size and proliferation index, thus close follow-up should be performed. No tumor marker is clearly associated with a poor prognosis. New molecular biology studies are needed in order to find therapeutic targets.
Describir las principales características clínicas, anatomopatológicas, terapéuticas y evolutivas de una serie amplia de tumores estromales gastrointestinales (GIST).
MétodosEstudio observacional de una serie de 66 casos de GIST tratados en nuestro hospital de 2002 a 2015. Seleccionamos variables relacionadas con los antecedentes personales, las manifestaciones clínicas, el tratamiento médico y quirúrgico, la anatomía patológica y la morbimortalidad. Añadimos una revisión de la literatura para correlacionarla con nuestros resultados.
ResultadosLa localización más frecuente fue el estómago (65,2%), en el que destacó como región predominante el fondo. La manifestación clínica más habitual fue la hemorragia digestiva (45,5%), seguida del hallazgo casual tras la realización de alguna prueba de imagen o procedimiento invasivo (33,3%). Recibieron cirugía 58 pacientes (90,6%), el 15,5% de carácter urgente. El 69% de los GIST tenían un tamaño entre 2 y 10cm. La mortalidad al año debida al tumor fue de un 7,9% (5 casos), todos ellos relacionados con extensión local o a distancia, o complicación quirúrgica.
ConclusionesLa variabilidad clínica de los GIST es muy amplia. El tratamiento de primera elección es la cirugía, que es factible en la mayoría de los casos y debe ser lo más conservadora posible. El pronóstico es variable, dependiendo del tamaño y del índice de proliferación, por lo que debe realizarse un seguimiento estrecho. No existe un marcador tumoral claramente asociado a un peor pronóstico, por lo que se necesitan nuevos estudios de biología molecular con el objetivo de encontrar dianas terapéuticas.
Gastrointestinal stromal tumors (GIST) are relatively rare neoplasms, representing only 1%–3% of malignant stomach cancers and 15%–20% of cancers of the small intestine.1–3 Their incidence is around 0.72–0.85 cases per 100000 inhabitants.2 GIST originate in the interstitial cells of Cajal, whose mutation in the KIT gene (tyrosine kinase growth factor receptor) seems to be mainly responsible for the growth of these tumors.3 They present a wide range of behavior,4 from the incidental finding of small-sized GIST5 to large-sized tumors6 that are very aggressive and capable of dissemination. The classical treatment of GIST is surgical excision of the tumor.
The aim of this study is to report and contribute the experience of a regional hospital, providing epidemiological, clinical and pathological characteristics in the context of the treatment of a series of 66 GIST diagnosed in the last 13 years, together with a brief current review about this subject.
MethodsOurs is an observational study about the cases of GIST treated at our hospital from 2002 to 2015. To identify the cases, we have used two retrospective searches: one primary search in the general archives of our hospital, with the clinical diagnosis of “suspected GIST”; and later a secondary search using the Pathology Department database of all the submucosal gastrointestinal tumors from 2002 until 2015. Selected for study were all those cases with histologies (obtained by needle aspiration or biopsy of the tumor or surgical piece) identified as GIST, with a positive CD117 marker (c-KIT). We have excluded submucosal neoplasms diagnosed as leiomyosarcoma, hamartoma, ectopic pancreas, with c-KIT negativity (at our hospital, analyses of PDFGRA mutations are not done, nor are “wild-types” identified, so they were not able to be identified or included in the study). All the patients selected had undergone follow-up for one year.
The following variables were analyzed: age, sex, clinical manifestations, tumor location, need and surgical indication (scheduled or emergency), surgical technique used, postoperative complications, tumor size, pathological markers (fusiform or epithelioid histology, mitosis of 50 high power fields, presence of necrosis, CD34, actin, desmin, protein S100 and Ki67 greater than 10%), Miettinen and Lasta classification grade,7,8 need for adjuvant treatment with imatinib or sunitinib, presence of metastases, tumor recurrence and one-year mortality. Absent or missing data have been marked blank and included within the analysis.
The database and its descriptive analysis have been created with the IBM SPSS© version 20 statistical package. For the composition of this article, we have followed the structure and checklist proposed by the STROBE declaration.9
ResultsWith the search performed based on the clinical diagnosis of “suspected GIST”, we have found 72 patients from 2002 to 2015. Six cases were excluded after the second review of the pathology database because they were CD117 negative tumors (hamartomas, inflammatory pseudotumor, submucosal lipoma), so the definitive series had 66 patients.
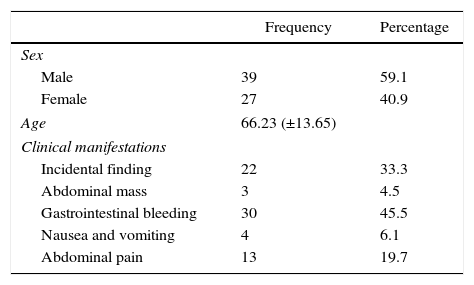
In the 66 cases, there was no clear predominance of one sex over another, although the frequency was slightly higher in males (59% vs 41%). The most frequent clinical manifestation was gastrointestinal bleeding (45.5%), followed by an incidental finding after an imaging study or invasive procedure (22 cases, 33.3%). Abdominal pain, nausea/vomiting and palpation of an abdominal mass completed the clinical spectrum of presentation (Table 1).
Patient Characteristics and Clinical Manifestations of GIST Treated at Our Hospital.
| Frequency | Percentage | |
|---|---|---|
| Sex | ||
| Male | 39 | 59.1 |
| Female | 27 | 40.9 |
| Age | 66.23 (±13.65) | |
| Clinical manifestations | ||
| Incidental finding | 22 | 33.3 |
| Abdominal mass | 3 | 4.5 |
| Gastrointestinal bleeding | 30 | 45.5 |
| Nausea and vomiting | 4 | 6.1 |
| Abdominal pain | 13 | 19.7 |
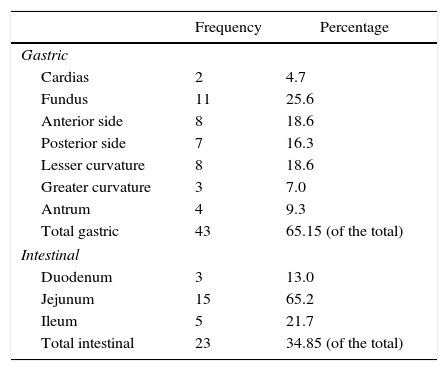
The tumor locations are summarized in Table 2. Approximately two-thirds of the tumors were located in the stomach, where the fundus (25% of the gastric GIST) was the predominant area. The remaining third were all located in some region of the small intestine, and the jejunum was the predominant site (65% of intestinal GIST).
Location of GIST in the Series Described.
| Frequency | Percentage | |
|---|---|---|
| Gastric | ||
| Cardias | 2 | 4.7 |
| Fundus | 11 | 25.6 |
| Anterior side | 8 | 18.6 |
| Posterior side | 7 | 16.3 |
| Lesser curvature | 8 | 18.6 |
| Greater curvature | 3 | 7.0 |
| Antrum | 4 | 9.3 |
| Total gastric | 43 | 65.15 (of the total) |
| Intestinal | ||
| Duodenum | 3 | 13.0 |
| Jejunum | 15 | 65.2 |
| Ileum | 5 | 21.7 |
| Total intestinal | 23 | 34.85 (of the total) |
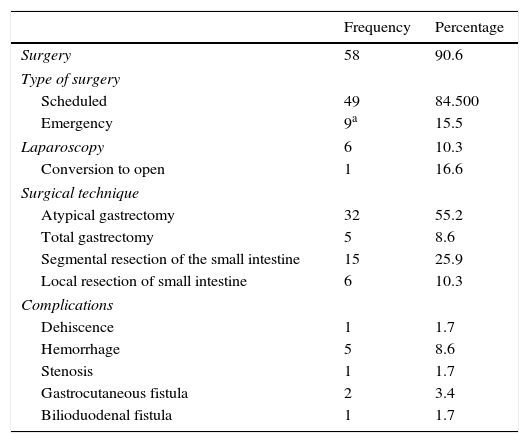
As for the treatment performed (Table 3), the vast majority underwent surgery (90.6%). Nine cases (15.5%) required urgent action, and 4 (6.1%) were incidental findings during emergency surgery for another reason (cholecystitis, adhesion-related bowel obstruction). The 5 most complicated patients (7.6%) presented with massive digestive bleeding (2 cases, 3.0%), gastric tumor perforation (2 cases, 3.0%) and bowel obstruction (one case, 1.5%).
Surgical Treatment and Postoperative Complications of the GIST in Our Series.
| Frequency | Percentage | |
|---|---|---|
| Surgery | 58 | 90.6 |
| Type of surgery | ||
| Scheduled | 49 | 84.500 |
| Emergency | 9a | 15.5 |
| Laparoscopy | 6 | 10.3 |
| Conversion to open | 1 | 16.6 |
| Surgical technique | ||
| Atypical gastrectomy | 32 | 55.2 |
| Total gastrectomy | 5 | 8.6 |
| Segmental resection of the small intestine | 15 | 25.9 |
| Local resection of small intestine | 6 | 10.3 |
| Complications | ||
| Dehiscence | 1 | 1.7 |
| Hemorrhage | 5 | 8.6 |
| Stenosis | 1 | 1.7 |
| Gastrocutaneous fistula | 2 | 3.4 |
| Bilioduodenal fistula | 1 | 1.7 |
Open surgery was the most commonly used technique. Laparoscopy was used in 6 selected cases (10.3% of patients who underwent surgery) and one required conversion to laparotomy (16.6% of laparoscopic surgeries). The usual surgical technique was atypical gastrectomy in the case of gastric GIST and segmental resection of the small intestine in more than two-thirds of intestinal GIST cases. Ten patients (17.2%) had major complications, half of which involved gastrointestinal bleeding originating at the anastomosis (one case required surgical re-operation), and three fistulas occurred (2 gastrocutaneous and one bilioduodenal, all with spontaneous resolution).
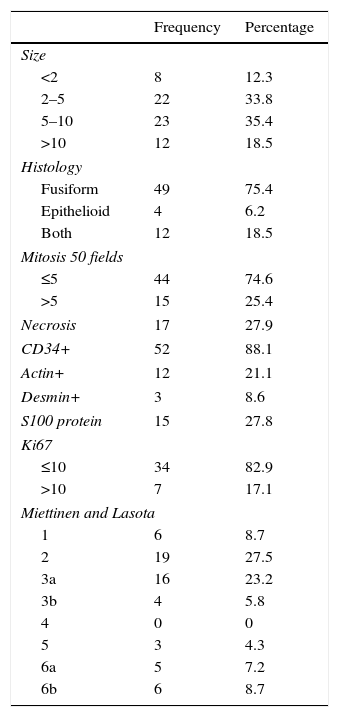
The pathological characteristics are summarized in Table 4. 69% of the GIST had a size between 2 and 10cm, and the predominant histology was fusiform (75% isolated fusiform, 18.5% with mixed epithelioid and fusiform physiological features). The Miettinen and Lasota classification (modified in 2006),7 which analyzes the size and presence of mitosis, was predominantly stages 2 and 3 (between 5% and 25% advanced disease after a 5-year follow up). None of the markers analyzed (CD34+, actin, desmin, S100 protein) was predominant in GIST with elevated Miettinen and Lasota.
Pathology Characteristics of the GIST Series.
| Frequency | Percentage | |
|---|---|---|
| Size | ||
| <2 | 8 | 12.3 |
| 2–5 | 22 | 33.8 |
| 5–10 | 23 | 35.4 |
| >10 | 12 | 18.5 |
| Histology | ||
| Fusiform | 49 | 75.4 |
| Epithelioid | 4 | 6.2 |
| Both | 12 | 18.5 |
| Mitosis 50 fields | ||
| ≤5 | 44 | 74.6 |
| >5 | 15 | 25.4 |
| Necrosis | 17 | 27.9 |
| CD34+ | 52 | 88.1 |
| Actin+ | 12 | 21.1 |
| Desmin+ | 3 | 8.6 |
| S100 protein | 15 | 27.8 |
| Ki67 | ||
| ≤10 | 34 | 82.9 |
| >10 | 7 | 17.1 |
| Miettinen and Lasota | ||
| 1 | 6 | 8.7 |
| 2 | 19 | 27.5 |
| 3a | 16 | 23.2 |
| 3b | 4 | 5.8 |
| 4 | 0 | 0 |
| 5 | 3 | 4.3 |
| 6a | 5 | 7.2 |
| 6b | 6 | 8.7 |
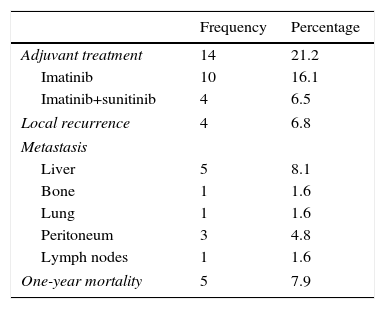
As for the progress and mortality of these patients (Table 5), 4 cases (6.8% of patients) had local recurrence, while 11 cases (17%) had a distant metastasis, the liver and peritoneum being the most frequent locations. A total of 14 patients (21.2%) required adjuvant therapy with tyrosine-kinase inhibitors, 10 of which (16.1%) only required imatinib and 4 (6.5%) required complementary treatment with sunitinib due to refractoriness to treatment with imatinib. Tumor-related mortality per year was 7.9% (5 cases), all of which was related with local or distant extension, or complications at the local level.
DiscussionThis paper provides descriptive data from an extensive series of GIST at a regional hospital, which in large part concur with those found in the latest global studies. The incidence of GIST ranges from 0.5 to 2 cases per 100000 inhabitants per year in different series.2,10 However, in the population covered by our hospital (about 260000 inhabitants), there is a greater incidence, ranging from 2 to 5 cases per 100000 inhabitants per year.11 Mean patient age is between 60 and 70. Although a slightly higher frequency is observed in males, the published series show no clear predominance toward either gender.2,10,12,13 All these data coincide with what we have found in our study.
In our series, the predominant location of GIST was the stomach, which is similar to the data provided by recent studies, which estimate gastric involvement at 55%–65%. The second most frequent location is the small intestine, preferably the jejunum (25%–30%); the remaining 5%–10% occur in atypical locations, such as the colon (5%–6%) and the esophagus (0.7%–1%).2,10,12
In terms of clinical presentation, a significant number of cases show no evident signs, and diagnosis is an incidental finding during an imaging test or surgery. In a review about the clinical manifestations of GIST that included 15 studies and more than 2400 patients,13 18.7% were incidental findings. In our study, the percentage was even higher: 33% were incidental findings, and 4 of these intraoperative. Among the cases with evident clinical manifestations, the most frequent finding is gastrointestinal bleeding (30%–40%), either as hematemesis/melena or anemia with no obvious signs of active bleeding. Other common findings are non-specific abdominal pain or palpable mass.10,13,14
Regarding treatment, in our series laparoscopic resection was performed in 7 patients, one of whom required conversion to open surgery for technical reasons. According to an observational study of 156 patients and a meta-analysis involving 1060 cases of GIST, laparoscopic surgery offers less bleeding, shorter time to oral tolerance, and shorter hospital stay, with no differences in surgical time, postoperative complications or mortality.15–17 This indicates that laparoscopic resection of gastric GIST could be considered standard procedure in small and medium-sized gastric GIST.18
On the other hand, in advanced tumors (larger than 10cm, Miettinen and Lasota greater than 4), the use of a tyrosine-kinase inhibitor should be considered, which can be either neoadjuvant to surgery or after its completion.12 The first line drug is imatinib and, if it produces little response, the second line drug is sunitinib.19 In our study, 4 patients (6.5%) required the use of this drug, although 2 of them died due to the tumor, which demonstrates that the drug has limited efficacy and other therapeutic pathways should be studied. The administration of imatinib at high doses (800mg) is another alternative, but there is less evidence. Likewise, regorafenib is suggested as a promising third-generation drug that still requires further study.20,21
The pathology of GIST is complex and significant due to its correlation with patient prognosis. The c-KIT mutation (CD 117) is the foremost mutation of these tumors and was essential for GIST diagnosis until not very long ago. Within the gene, the exon 11 mutation is most frequent and found in 50% of cases in some series.12 Another tumor subtype presents mutations in the platelet-derived growth factor receptor alpha (PDGFRA).22 However, in recent years, a small percentage of cases (less than 10%) have been found that do not have mutations in either the c-KIT or the PDGFRA. This new type of GIST has been called “wild-type”.23 They are usually seen in younger people and, up to 15% of the time, may be related with Carney's triad (GIST, paraganglioma and pulmonary chondroma), Carney-Stratakis syndrome (GIST and paraganglioma), type 1 neurofibromatosis and BRAF gene mutations. “Wild-type” GIST are unresponsive to treatment with imatinib, so it is therefore necessary to develop new therapies directed at the specific markers.23 Since the detection of wild-type markers is not available in our setting, we do not present any cases of this subtype, although there is probably more than one case categorized as another type of mesenchymal tumor.
There are several tumor markers that appear to be related with increased tumor aggressiveness. In our study, 6 patients had a Ki-67 index greater than 10%: 3 with recurrences and one who died due to the tumor. This indicates that this marker may be a good candidate to identify tumor cell proliferation, but prospective studies with larger patient numbers are needed for confirmation.24 Another possible marker is the DOG 1 gene, which is expressed up to 50% more in advanced or metastatic GIST and also seems to be a predictor of a poorer prognosis in wild-type c-KIT tumors.
As well as GISTS with a high potential for malignancy, we should contemplate those tumors that have a minimal possibility for local and distant invasion due to their small size. Tumors smaller than 1cm are called “micro-GIST”, although tumors smaller than 2cm could also have very little capacity for dissemination.25 In our series, 8 micro-GIST were detected, representing 12.3% of the series. All of them were resected, although there is controversy about whether these GISTs should be removed and, if done, whether minimally invasive endoscopic resection would be sufficient.26
The prognosis of GIST is generally better than that of other digestive tract cancers and greatly exceeds the survival of gastric adenocarcinoma. The 5-year tumor-specific survival ranges from 70% to 80%, while the overall 5-year survival of these patients is slightly lower, between 65% and 70%.2 Our study has only studied one-year global mortality since the latest collected cases are recent (years 2014–2015). This mortality rate was 7.9%, which is similar to the mentioned figures. In terms of the degree of dissemination, our series includes 11 cases with distant metastasis and 2 of these presented one-year mortality (18.2%), which is more than double the global mortality rate of GIST. In other series studying longer-term mortalities, tumors with distant metastases are attributed a 5-year mortality rate of between 34% and 60%.2,25
Risk factors associated with poorer overall survival coincide in most studies: advanced age, male sex, African descent, tumor size, intestinal location, p53 positivity, high mitotic index and distant metastasis. The latter 2 produce the most independent risk. No clear evidence has been found to show that any of the tumor markers studied in GIST (CD34, actin, desmin, S100 protein, vimentin) are related to prognosis, and our study also concurs with this fact.2,13,14
In conclusion, GISTs are “young” tumors, as they were described relatively few years ago. Although they are considered not very prevalent in the population, their incidence is not negligible and should therefore always be included in the differential diagnosis of submucosal intestinal or gastric masses. Surgical treatment is paramount in all cases. It should be laparoscopic and as conservative as possible in well located tumors with no local or distant invasion. As for pathological studies, although new markers and genetic mutations have been discovered in recent years, there is still much to investigate, with the ultimate goal of finding possible therapeutic targets to improve survival in advanced GIST. The prognosis is very variable and depends on location, size and proliferation index. Therefore, the management of these tumors should always be cautious and accompanied by close monitoring.
Authorship/collaborationsFlores-Funes D: study design, data collection, analysis and interpretation of the results, composition of the article
Lirón-Ruiz R.J.: study design, analysis and interpretation of the results, critical review
Pérez-Guarinos C.V.: data collection, analysis and interpretation of the results
Martín-Lorenzo J.G.: analysis and interpretation of the results, critical review
Torralba-Martínez J.A.: analysis and interpretation of the results, critical review
Giménez-Bascuñana A.: data collection, analysis and interpretation of the results, critical review
Chaves-Benito M.A.: data collection, critical review, approval of the final version
Aguayo-Albasini J.L.: critical review, approval of the final version
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Flores-Funes D, Lirón-Ruiz RJ, Pérez-Guarinos CV, Martín-Lorenzo JG, Torralba-Martínez JA, Giménez-Bascuñana A, et al. Perfil clínico y anatomopatológico de los tumores estromales gastrointestinales de un hospital de área: Estudio descriptivo y revisión de la literatura. Cir Esp. 2017;95:391–396.