Pilocytic astrocytoma is a rare tumour, usually occurring in paediatric ages, and mainly located in the posterior fossa. It can cause hydrocephalus and intracranial hypertension and, less frequently, seizures, or a focal neurological deficit. The main imaging study by magnetic resonance imaging, which shows a tumour with solid and cystic components without peri-lesional swelling. The election treatment is surgical, and the patient is considered cured if a total resection is accomplished.
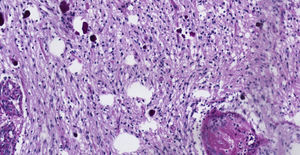
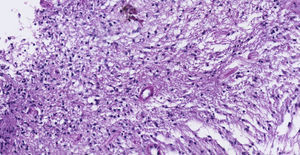
Clinical caseThe case is presented of 22-year-old female patient with a supratentorial pilocytic astrocytoma and epilepsy. Histopathology reported a low grade glial proliferation, with an extensive fibrillar matrix, small cells without atypia, extensive calcifications and piloid areas consisting of bipolar fusiform cells, and some Rosenthal fibres. There were also spongiotic areas consisting of multipolar cells and associated microcysts. The final report was a pilocytic astrocytoma.
ConclusionsPilocytic astrocytoma is more frequent in paediatric patients and in the posterior fossa. The case presented is of a young female adult with supratentorial location, making it a special case. The surgery achieved a total resection. The long-term prognosis is good, but it is necessary to perform a follow-up, particularly in adult patients because of a higher risk of recurrence.
El astrocitoma pilocítico es un tumor poco frecuente, con predilección por los pacientes pediátricos, localizado principalmente en la fosa posterior. Suele presentarse con síntomas de hidrocefalia y aumento de la presión intracraneana y menos frecuentemente con epilepsia o déficit neurológico focal. El estudio de elección es la resonancia magnética, en la cual se observa una lesión con componentes sólidos y quísticos, sin edema perilesional. El tratamiento de elección es la resección total y si se logra, se puede considerar curado al paciente.
Caso clínicoSe presenta el caso de una paciente femenina de 22 años de edad con un astrocitoma pilocítico supratentorial, quien comenzó con epilepsia. Patología: Se reportó proliferación glial de bajo grado, con matriz fibrilar extensa y células pequeñas sin atipia, con extensas calcificaciones, con áreas piloides conformadas por células fusiformes bipolares, con algunas fibras de Rosenthal. También se observaron áreas espongióticas conformadas por células multipolares asociadas a microquistes. El resultado fue un astrocitoma pilocítico.
ConclusionesEl astrocitoma pilocítico es más frecuente en pacientes pediátricos y en la fosa posterior. El caso presentado es de una adulta joven y con una localización supratentorial, lo cual lo hace un caso especial. La cirugía logró una resección completa. El pronóstico es muy bueno a largo plazo, aunque es necesario hacer un seguimiento especialmente en los pacientes adultos, ya que se han reportado casos con recurrencia.
Pilocytic astrocytoma is classified by the World Health Organisation as a grade I astrocytarian tumour, within the group of those derived from neuroepithelial tissue. It is a well-circumscribed, slow growing tumour. In general, a patient is considered cured when a complete tumour resection has been performed.1
The most common site is the posterior fossa in paediatric patients.
ObjectiveWe present the case of a young adult patient with a supratentorial pilocytic astrocytoma and epilepsy.
Clinical caseThis is the case of a 22 year-old female who presented with her current condition one week before her arrival to hospital, with generalised onset of a tonic-clonic seizure which caused mild head trauma. She subsequently had two further seizures. The patient denied having had any other symptoms. Neurological examination revealed that the patient's mental functions, cranial nerve functions, motor system, senses and cerebellum were all within normal limits.
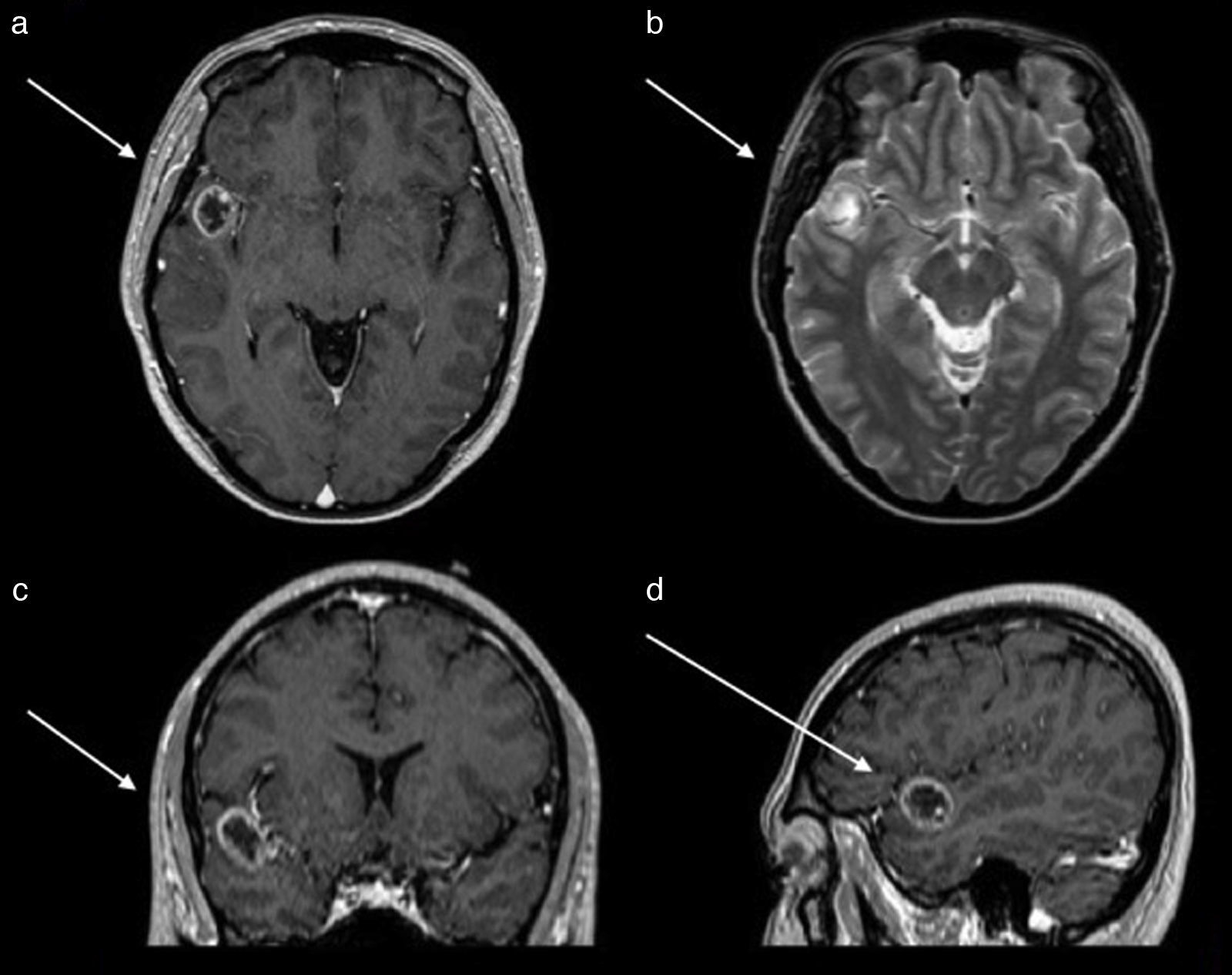
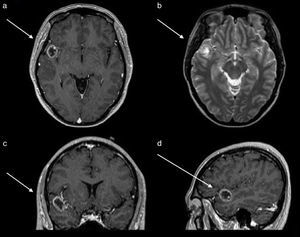
An EEG showed abnormal activity and treatment was initiated with 1g of levetiracetam every 12h. Simple and contrasted magnetic resonance of the skull was performed, where a lesion in the first gyrus of the right temporal lobe was observed. This was observed as a hypertense ring on simple T1 imaging, as a ring on contrast enhanced T1, with a hypointense centre and no perilesional oedema on T2 and FLAIR signals (Fig. 1). Computed tomography revealed calcification on the periphery, mainly towards the medial surface of the lesion. Preoperative studies were carried out in which thrombocytopenia was identified in 21,000 counts. It was evaluated by the haematology unit which diagnosed idiopathic thrombocytopenic purpura. The patient received treatment with platelet apheresis, corticosteroids and immunoglobulin. During her hospital stay and prior to surgery of the central nervous system, she presented with intense pain in the right hypochondrium and was therefore assessed by general surgery. She was diagnosed through questioning, physical examination and abdominal scan with an aseptic cholecystitis. She suffered from painful hepatomegaly and presented with a drop in haemoglobin. These symptoms were resolved by conservative treatment.
Magnetic resonance of the skull. (a) Axial section contrast enhanced T1 sequences which shows tumour with a hypointense centre and enhancement of the contrast in the shape of a ring. (b) Axial section in T2 sequence which shows a hypointense ring and with a hyperintense centre, with no perilesional swelling. (c) Coronal section contrast enhanced in T1 sequence which shows the location in the first right temporal gyrus.
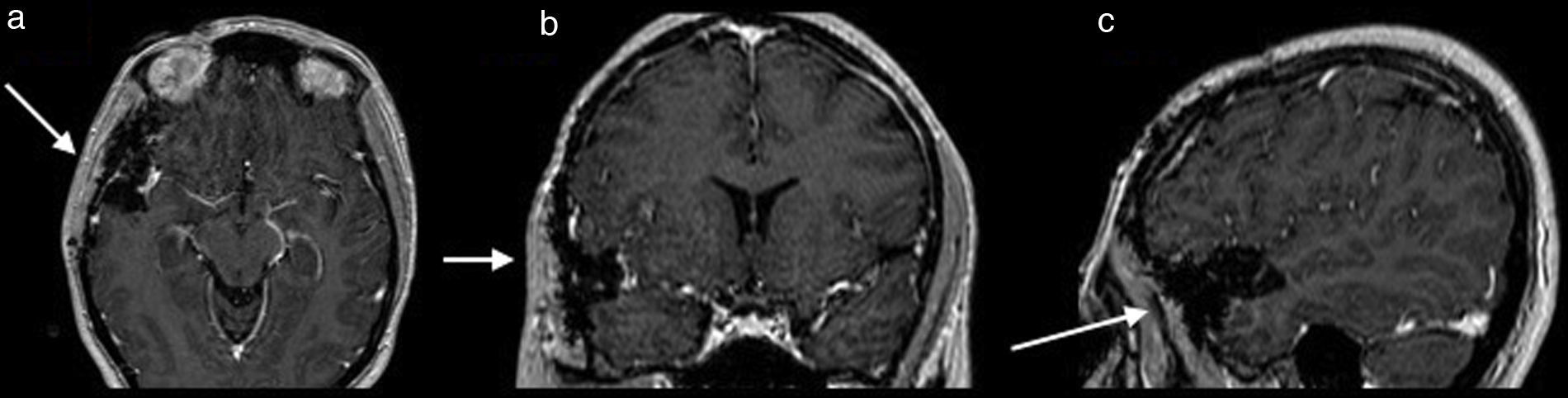
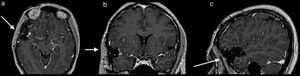
Once surgery had been authorised it was performed with a Falconer type right incision, a temporal craniotomy and complete resection of the lesion by microsurgery with the use of a neuronavegator with ultrasound and ultrasonic aspirator (Fig. 2). There were no events or complications during the procedures. The patient was discharged neurologically intact. She evolved satisfactorily, with no seizures, and is still taking 1g of oral levetiracetam every 12h. She has been followed up now for a little over 5 years.
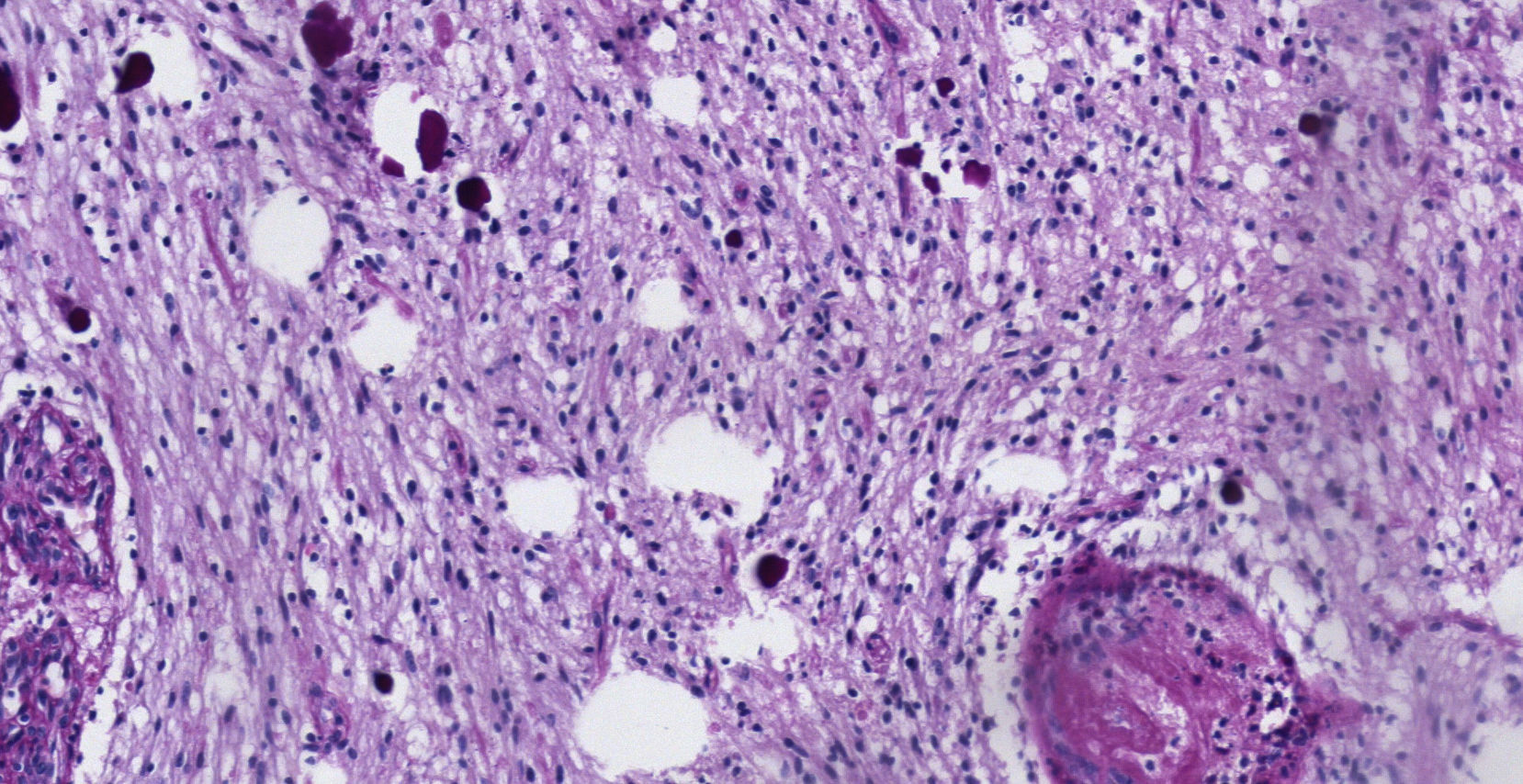
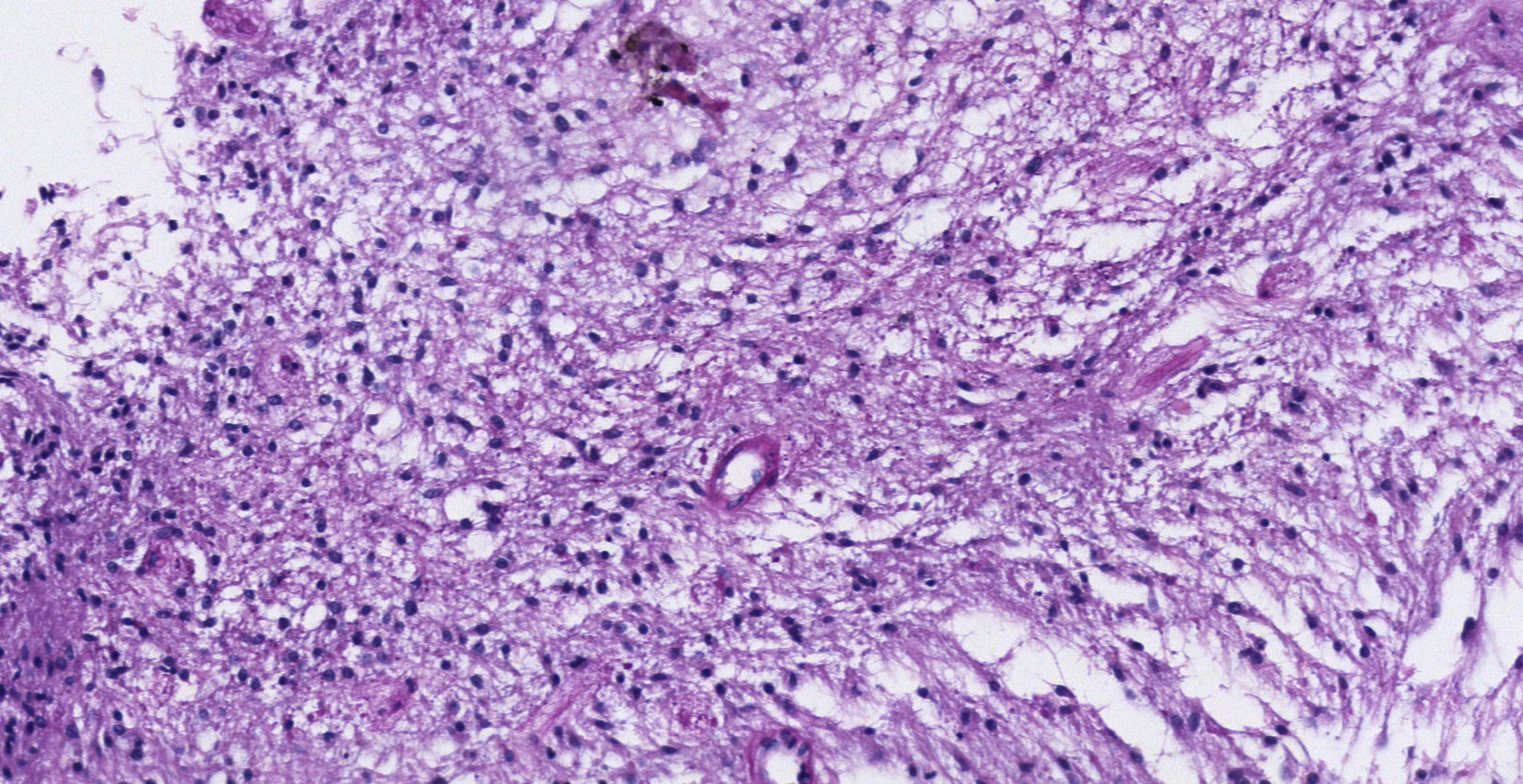
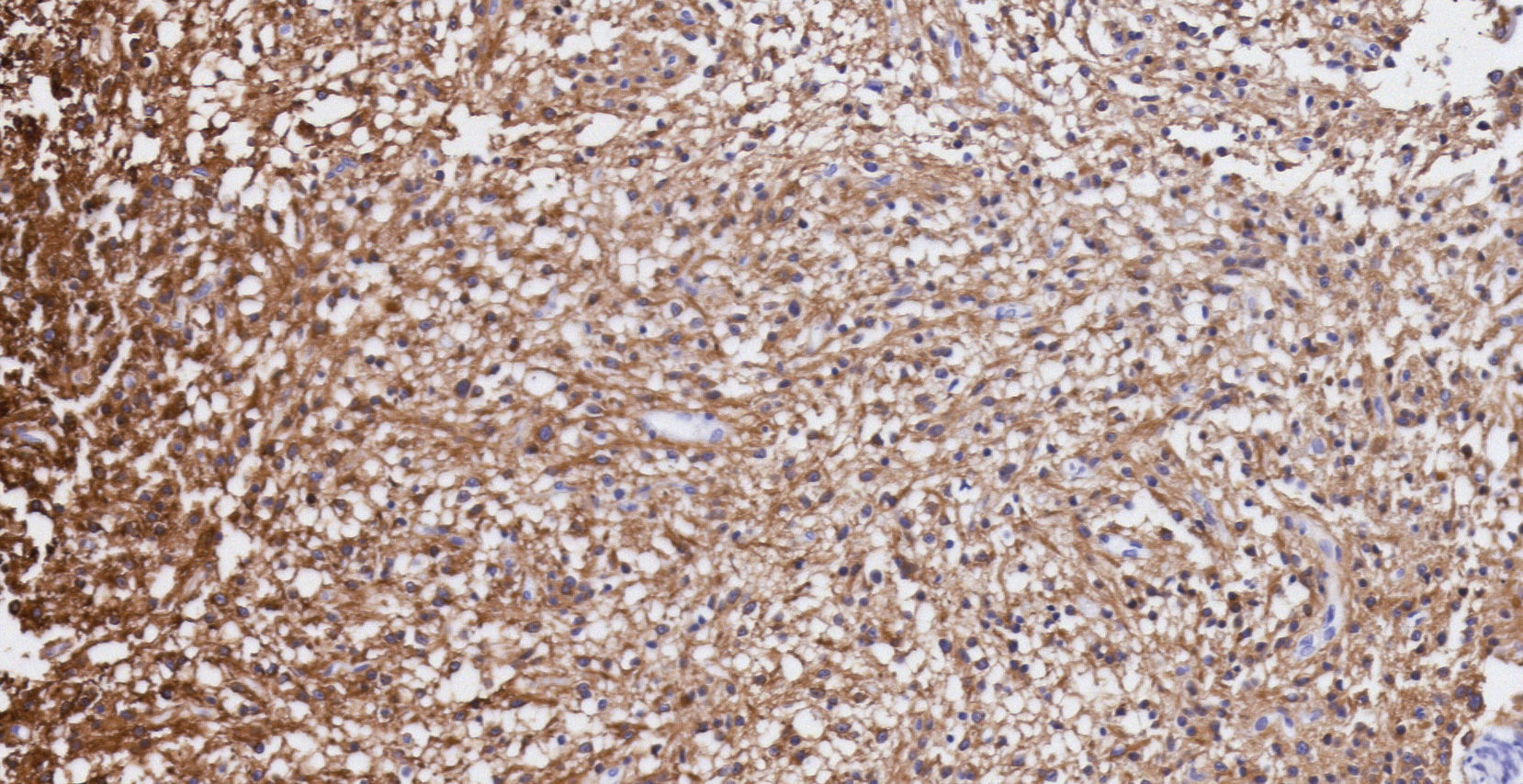
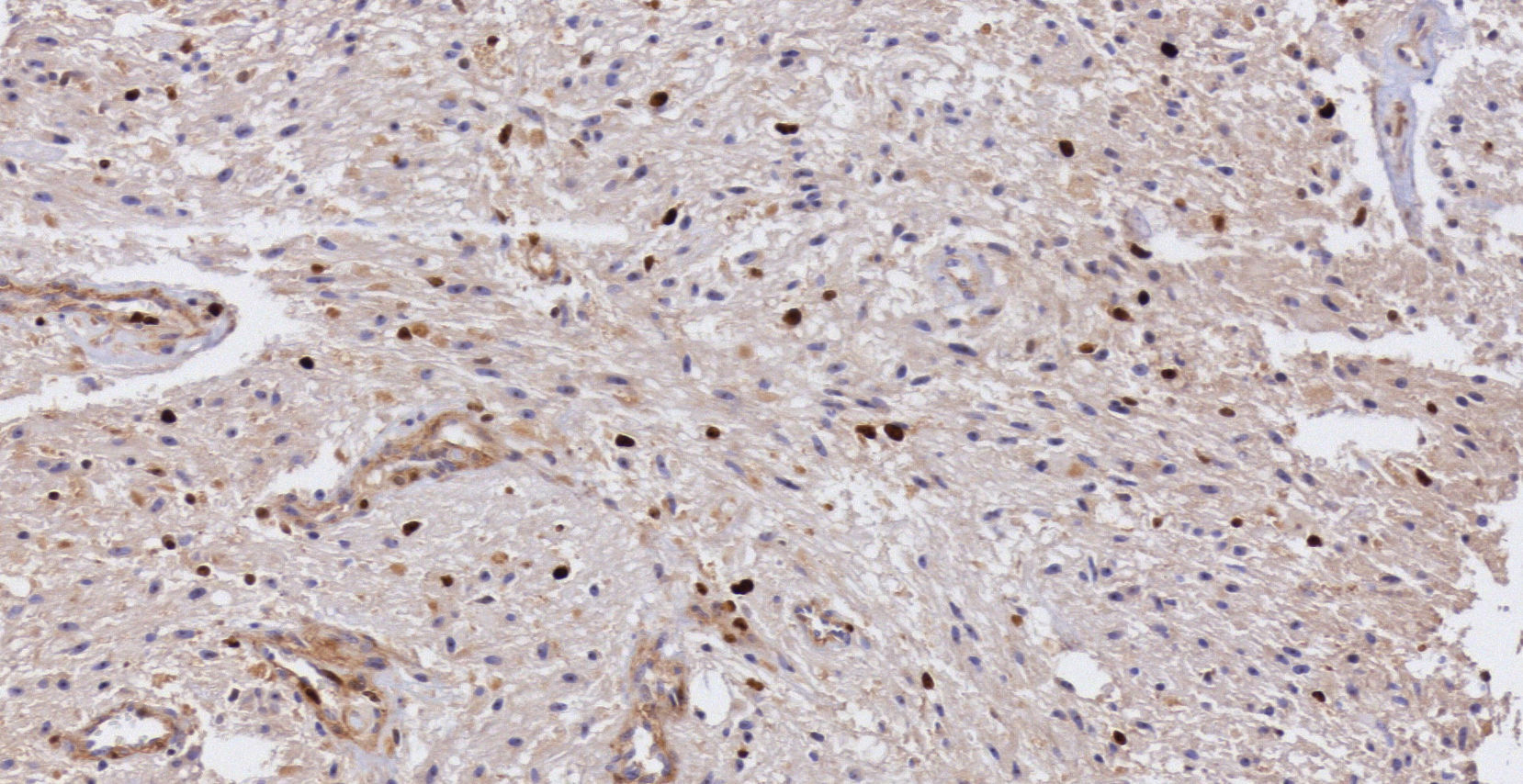
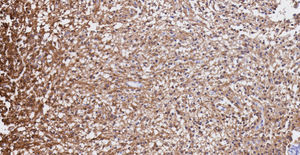
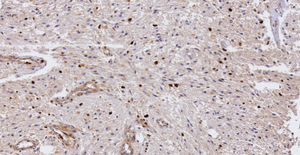
Pathology. Histopathology reported a low grade glial proliferation (Fig. 3), with an extensive fibrillar matrix, small cells without atypia, extensive calcifications and pyloid areas consisting of bipolar fusiform cells, and some Rosenthal fibres and calcifications (Fig. 4). There were also spongiotic areas consisting of multipolar cells and associated microcysts (Fig. 5). Immunohistochemistry analysis was performed in which a glial fibrillary acidic protein of the positive glia in the fibrillar matrix and neoplastic cells was found. Synaptophysin and chromogranin tested negative and the rate of cellular proliferation measured with Ki67 was 5%. The final report was a pilocytic astrocytoma (Fig. 6).
We present the case of a young adult patient with a right temporal pilocytic astrocytoma, who began with epilepsy.
The most common pilocytic astrocytomas in paediatric patients compared to adults, as demonstrated in several reported series,2–6 corresponds to 2.3% of brain tumours in the latter.7
Several series report a slightly higher incidence in men, than in women, as was reported by Ohgaki et al.8 with a male/female rate of 1.26. However, other series have reported a slightly higher frequency in women with a female/male rate of 1.22 in adult patients.9 In general it is accepted that the pilocytic astrocytoma has no predilection for either gender.2,4,8
There is a clear correlation between the patient's age and tumour site. Infratentorial is more common in paediatric age patients,6,8 and indistinct in adults, but with a tendency to be supratentorial,4,10 and often in the temporal lobes.11 A right temporal cystic lesion in a young adult patient should lead to suspicion of possible pilocytic astrocytoma, as occurred in our case.
The posterior fossa is the most common site (61.7%) and in descending order locations include: hemispheric (10.8%), optic nerve (6.7%), brain stem (5.8%), sellar or suprasellar area (5.8%), ventricles (5%) and spinal cord (4.2%).3,4,12
The presentation of symptoms is generally insidious, due to slow tumour growth.6
Clinical presentation in hemispheric site include: headache, epilepsy, hemiparesis, nausea and vomiting.13 Our patient began with epilepsy.
Intraparenchymal bleeding has also been described as a form of presentation, but it is rare.11
Grade I reflects the absence of malignant morphological characteristics.
Most pilocytic astrocytomas astrocitomas have normal karyotypes but approximately 32% show chromosomic abnormalities, especially in adults,14 such as gain in chromosomes 5, 7 and possibly 8, although a great many other abnormalities have been reported.5 It was not possible to carry out these tests in our patient.
The first genetic association with the pilocytic astrocytoma was the mutation of gene NF1 and the type I (NF-1) neurofibromatosis. This alteration is associated with a more indolent behaviour.10
One tool which may help with differential diagnosis of in pilocytic astrocytoma and other tumours is spectroscopy. Pilocytic astrocytomas show a lower creatine peak compared with that found in ependymoma and medulloblastoma radiology. A tendency of higher tCho levels in adults has been reported compared with paediatric age patients, who in contrast usually have a higher tCr peak.4
The most frequently differential diagnoses include relatively well circumscribed tumours such as: dysembryoplastic neuroepithelial tumour, rosette-forming glioneuronal tumour of the fourth ventricle, pleomorphic xanthoastrocytoma and multipform glioblastoma.6
Treatment for the patient with pilocytic astrocytoma is total resection, which may be performed in a high number of patients, even when it is in an area of complex access.15
The patient is considered cured when resection is complete. If incomplete, chemotherapy and radiotherapy are helpful.16 The site may determine whether resection is total and whether the condition progresses or reappears.14
When radical surgery is not possible, particularly when the site is profound or in a critical position, radiosurgery plays an essential role in cases of minor residual tumour.17
Pilocytic astrocytomas usually follow an indolent clinical course, with an extremely high rate of survival, above 90% after 10 years.13 They are benign and slow growing, thus leading to good prognosis,6 although they are more aggressive in adults,14 which is why follow-up in this group is considered essential.9,14
A more favourable clinical course is likely when the lesion is superficial and if only one lobe is affected, compared with more profound or midline lesions.2
Local reappearance is rare, as is cerebrospinal fluid dissemination or malignant transformation.18 Although the mechanism is unknown, improvement has been demonstrated when it is associated with a type I neurofibromatosis, mainly in children, but also in an adult with pilocytic astrocytoma with cerebrospinal fluid dissemination.10,18
Malignant transformation to anaplastic astrocytoma in patients with pilocytic astrocytoma has been described, particularly in adults who have received adjuvant radiotherapy, but without this association being definitive.19
Routine molecular analysis of adult pilocytic astrocytoma has been recommended, combined with histopathological analysis and neuroimaging, to sharpen prognosis.20
ConclusionsThe presented case is interesting since, in general, a pilocytic astrocytoma is not suspected when the lesion is supratentorial. The age, tumour site, radiologic characteristics and presentation form should help us to keep this diagnosis in mind.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Moreno-Jiménez S, Miranda-Fernández KA, García Gutiérrez M, Vázquez-Estrada N. Müller-Grohmann S, Flores-Vázquez F. Astrocitoma y epilepsia. Caso clínico. Cir Cir. 2017;85:419–423.