Clay bonded silica refractory was prepared by utilizing agriculture waste called rice husk ash (RHA) and refractory grog. Various samples were prepared with different compositions based upon partial replacement of quartz by RHA. Rectangular samples were prepared by following semi dry process prior to pressing in a uniaxial hydraulic press and sintering at a temperature of 1200°C in air atmosphere. Various physical, mechanical and thermal characterizations were done like X-ray diffraction (XRD), scanning electron microscope (SEM), apparent porosity (AP), bulk density (BD), cold crushing strength (CCS), refractoriness and thermal conductivity measurement. The sample utilizing 30% of RHA was considered most optimum composition which produced cold crushing strength of 38MPa and thermal conductivity of 2.08W/mK at 800°C with a considerable good refractoriness. Enhancement in the mechanical as well as thermal properties may be considered as attributed to the amorphous silica which has reacted more easily and efficiently with other material surrounding giving rise to the densification and produced stable crystalline phase to the refractory material. These promising characteristics suggests that the RHA may lead to be used as a potential material for the preparation of clay bonded high strength silica refractories.
Se preparó sílice refractaria unida a arcilla con residuos agrícolas conocidos como ceniza de cascarilla de arroz (rice husk ash [RHA]) y grog refractario. Se prepararon varias muestras con diferentes composiciones basadas en la sustitución parcial de cuarzo por RHA. Las muestras rectangulares se prepararon siguiendo un proceso semiseco antes de prensarlas en una prensa hidráulica uniaxial y sinterizarlas a una temperatura de 1.200°C en atmósfera de aire. Se realizaron diversas caracterizaciones físicas, mecánicas y térmicas, como la difracción de rayos X, el microscopio electrónico de barrido, la porosidad aparente, la densidad aparente, la resistencia al aplastamiento en frío, la refractariedad y la conductividad térmica. La muestra que utiliza el 30% de RHA se consideró la composición más óptima que produjo una resistencia al aplastamiento en frío de 38MPa y una conductividad térmica de 2,08W/m-K a 800°C con una refractariedad considerablemente buena. La mejora tanto de las propiedades mecánicas como de las térmicas puede atribuirse a la sílice amorfa que reaccionó más fácil y eficazmente a otro material circundante, lo que produjo la densificación y una fase cristalina estable al material refractario. Estas características prometedoras sugieren que la RHA puede justificar que se use como material potencial en la preparación de sílices refractarias de alta resistencia unidas a arcilla.
Silica refractories are generally known as refractory materials consisting more than 93% SiO2[1,2]. Silicon dioxide (SiO2) is most commonly found in nature as quartz which is also considered to be the most conventional source of silica to be used for refractory manufacturing [3]. Silica refractories are typically produced in the form of shaped products (bricks) as well as in bulk powder (castables). Silica refractory bricks possess excellent thermal shock resistance, particularly in certain temperature range. Regardless the reduction in their use in the recent decades; silica refractories are still successful, beneficial or exceptional material for some of the special applications such as glass tank furnace roof constructions, electro steel kilns, coke ovens, blast furnace air heaters and some construction elements in ceramic tunnel kilns and furnaces employed in the fabrication of non-ferrous metals [4–6].
As reported by a study, rice production around the globe reached 661 million tons in 2008 [7]. Around 20% of the rice paddy produces rice husk (RH) during its processing in rice mills and refinement. RH occupies large areas, has a very low nutritious significance and takes very long time to decompose due to which it is not appropriate for composting or manure. Thus it generates disposal problem [8]. To reduce the RH efficiently, one effective method used these days is to use it in the rice mills to generate steam for the parboiling process or as fuel in industries. RH generally contains 70–80% organic matter such as cellulose, lignin etc. and rest 20–30% mineralogical components such as silica, alkalis and trace elements [9–12]. Thus when this RH is burnt, ∼25% of its weight is converted into ash during the firing process and is called rice husk ash (RHA). This burnt RHA has different chemical, mineralogical and morphological characteristics depending upon the rice variety, soil chemistry, climatic conditions, and geographic localization of the culture as well as the process acquired during burning conditions of the husk [13]. If the combustion is incomplete, large amount of unburnt carbon is found in the ash while complete combustion results in gray to whitish ash with minimal amount of carbon contamination. This RHA contains around 85–90% of amorphous silica. However, the amorphous silica content depends upon the burning temperature and holding time. Optimum properties can be achieved when husk is burnt at temperatures around 500–700°C with shorter holding time [14].
In order to utilize this type of waste as silica precursor in ceramics, various studies were accomplished; particularly, in white ware [15], synthesis of colorants [16,17], glaze preparation [18,19] and other ceramics [20,21]. Utilization of RHA in the synthesis of borosilicate [22], cordierite [23], carbosil [24], alumina-silicate [25], and mullite [26,27] are some of the works worth mentioning. Furthermore, this amorphous silica has also been employed in the preparation of various valuable materials such as solar grade silicon [28], magnesium-alumina-silica [29], lithium-aluminum-silica [30], and silicon carbide [31]. Particularly; RHA derived silica refractories have also developed earlier [32,33] but the major objectives of these works were focused upon the application in insulation purpose. On the other hand, effect of incorporation of clay and refractory grogs in a RHA based silica refractory has also not been investigated yet. In this present study, we have prepared clay bonded high strength silica refractory with utilizing agriculture waste-RHA by gradually replacing quartz and studied the effects of this partial replacement with various characterization techniques.
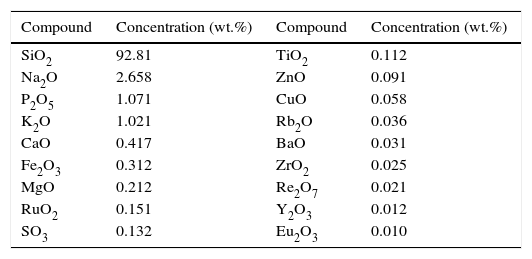
Materials and experimentalMaterials and materials characterizationIn this work, RHA was collected from rice mills where rice husk is often used as a fuel. Typically in these mills, temperature rises above 500°C due to which this RHA contains a very negligible amount of volatile matter as well as carbon content. However, to obtain highly pure amorphous silica, this RHA was further heat treated at 600°C for 2h to eliminate the possibility of any carbon contamination. The chemical analysis was carried out by using the X-ray fluorescence (XRF) spectrometer according to ASTM C114-00 [34] shown in Table 1. According to XRF results, RHA contains more than 90% of SiO2, which is almost similar to the amount of SiO2 present in quartz. So, in this work complete and partial replacement of quartz by RHA was investigated in the preparation of silica refractory. The quartz was purchased by Lobachemie Pvt. Ltd., India having analytical grade quality and used without any further treatment.
XRF results for RHA calcined at 600°C.
| Compound | Concentration (wt.%) | Compound | Concentration (wt.%) |
|---|---|---|---|
| SiO2 | 92.81 | TiO2 | 0.112 |
| Na2O | 2.658 | ZnO | 0.091 |
| P2O5 | 1.071 | CuO | 0.058 |
| K2O | 1.021 | Rb2O | 0.036 |
| CaO | 0.417 | BaO | 0.031 |
| Fe2O3 | 0.312 | ZrO2 | 0.025 |
| MgO | 0.212 | Re2O7 | 0.021 |
| RuO2 | 0.151 | Y2O3 | 0.012 |
| SO3 | 0.132 | Eu2O3 | 0.010 |
Microstructural analysis of RHA heat treated at 600°C was carried out using a SEM analysis for which results are shown in Fig. 1. It can be observed that the RHA particles possess an expressed relief structure. The interior of the RHA was found to be porous, contributing to the high specific surface area. This high specific surface area can lead as an active surface during the sintering reactions and may produce better phases involving accelerated reaction kinetics. As per the SEM observations, there was no evidence of volatile matter or carbon content present in this RHA.
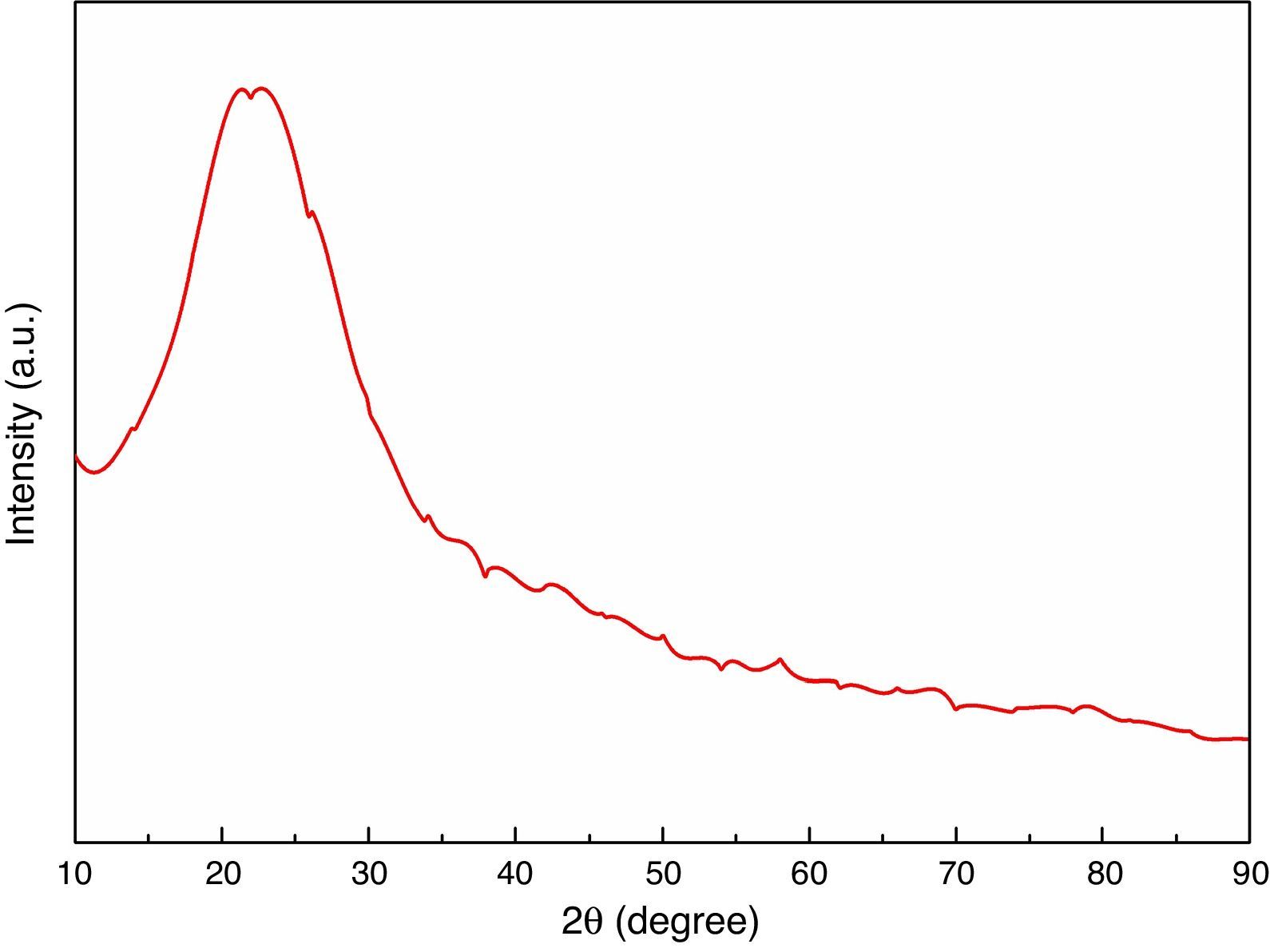
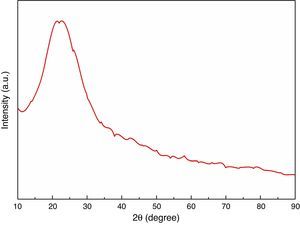
The XRD analysis was carried out for heat treated RHA powder and the results are shown in Fig. 2. The results has shown absence of any sharp peak which indicates that the silica in RHA exists in the amorphous form only. The amorphous silica present in RHA, is also called active silica which is considered to be more reactive than the crystalline form because it lacks the long-range order characteristic of a crystal. Amorphous materials have an internal structure made of interconnected structural blocks. Even amorphous materials have some sort of short-range ordering at the atomic length scale due to the nature of chemical bonding. However, this absence of long range ordering allows them to react more easily and efficiently with other material surrounding so that a better densification is achieved.
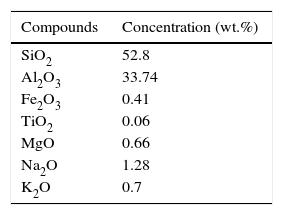
Fired clay refractory grogs have been used here having high percentage of silica and alumina. When refractory grogs are incorporated in any composition, their particle size is taken coarse than the other raw materials. Refractory grogs when incorporated in the batch may contribute to reduce shrinkage and also add strength to the sample. Ball clay has been used here for proving the bonding strength to the fired refractory. When clay is mixed with water, it becomes malleable, plastic or liquid, allowing it to be shaped. After drying, clay sets and recovers its cohesive properties, and so can bind the materials together. The chemical analysis of clay were determined using XRF spectrometer [34] and results are shown in Table 2.
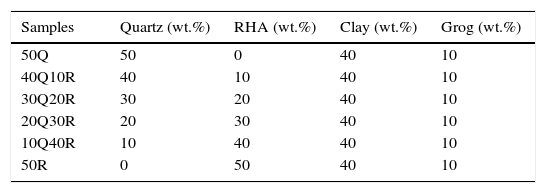
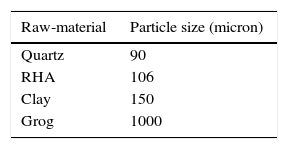
Sample preparationThe preparation of samples were done from several different compositions containing rice husk ash, clay and refractory grog tabulated in Table 3. Particle size distribution of raw materials is shown in Table 4. Firstly, all of the raw-materials were weighed according to batch composition and then mixing was done manually for 30min in dry condition with the help of an agate mortar and pestle. Afterward, least amount of water was added by following semi dry process. Samples were prepared by putting the batch material in to a rectangular die having dimension of (x, y)=(40mm×10mm). Uniaxial pressing was done by hydraulic press at a pressure of 120MPa. These green samples were allowed to dry in an electric air oven at a temperature of 110°C for 24h followed by a sintering in a muffle furnace (Bysake & Co. Electric Furnace; Model No-7054) with a heat schedule of room temperature to 500°C in 4h and thereafter up to 1200°C in 5h. The soaking period was kept 2h after which the furnace was allowed to cool down to room temperature in 8h.
Various techniques were used to investigate the physical, mechanical, thermal and morphological characteristic of sintered samples. X-ray diffraction (XRD) analysis was done by Rigaku Desktop Miniflex II X-Ray Diffractometer equipped with Ni filter and Cu Kα radiation (λ) 1.54056Å (Serial no: HD20972, Rigaku Corporation, Tokyo, Japan) and data was collected at a scanning rate of 5°/min for 2θ in a range from 10° to 90°. Scanning electron microscopy (SEM) experiments were performed on a ZEISS, EVO 18-2045 for the morphological study of RHA as well as refractory samples. The examination of apparent porosity and bulk density was done by Archimedes method according to ASTM C20 [35]. Linear shrinkage was measured according to the method described by ASTM C356-10 [36]. Cold crushing strength was measured according to the method described in ASTM C133 [37]. The thermal conductivity of the samples was measured by Hot Cross-Wire method using “Thermal conductivity measurement apparatus, VBCC, LOC: 441A102-0005”. Refractoriness was measured according to ASTM C24-09 using standard pyrometric cone equivalent method [38].
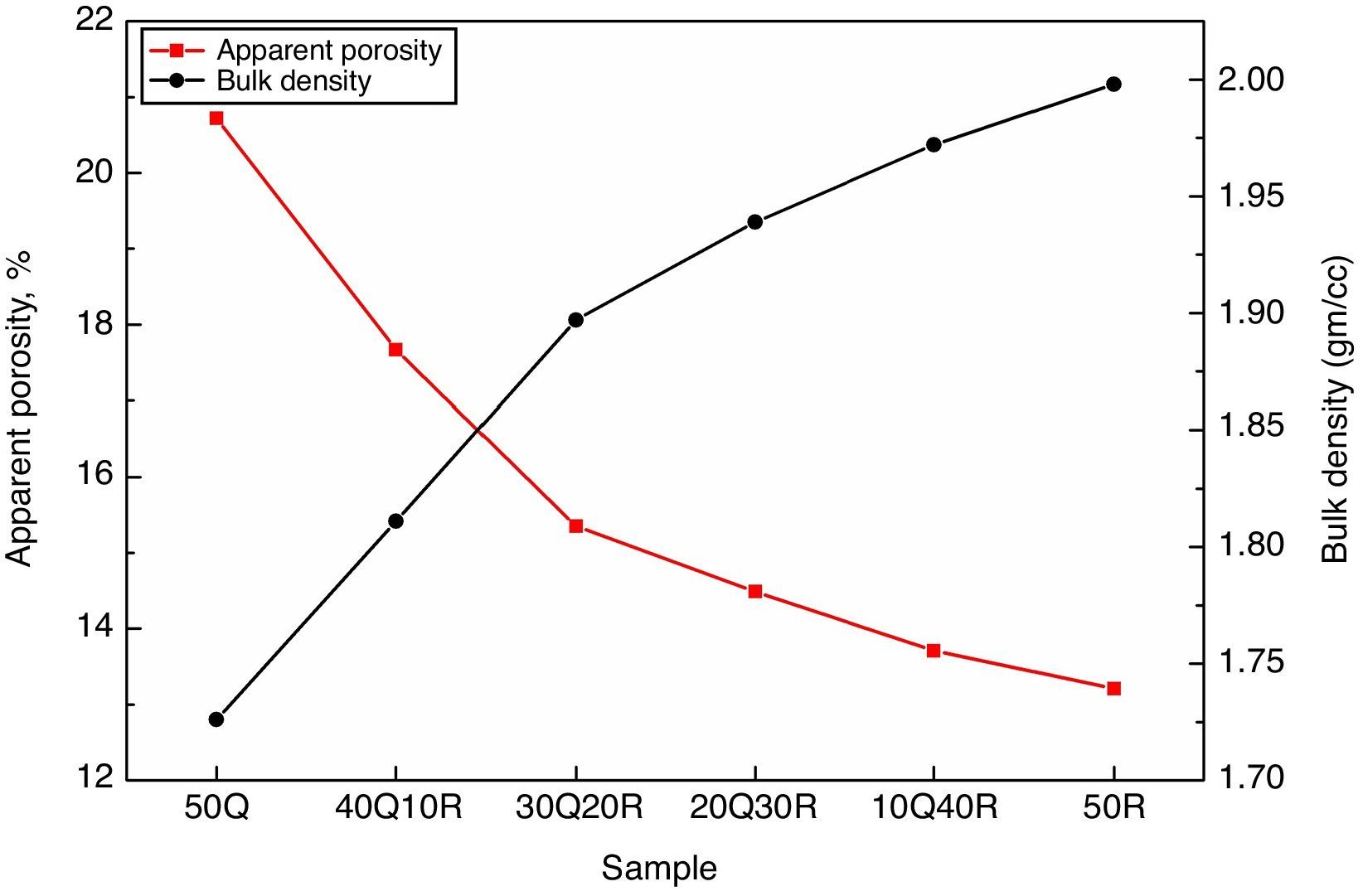
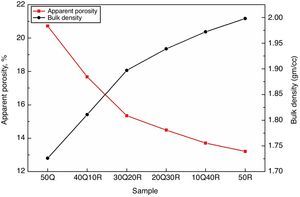
Results and discussionThe apparent porosity (A.P.) and bulk density (B.D.) measurements were performed for all sintered samples and results are shown in Fig. 3. It has been observed that incorporating RHA by replacing quartz in the composition results in a significant decrease in A.P. while at the same time B.D. shows a gradual improvement. These effects came out as a result of quartz replacement through RHA in the refractory sample can be considered due to the active silica contained in RHA which shows more reactivity than quartz which is a conventional source of silica. This active silica has reacted with the surrounding material more effectively which further eliminates the inter-granular spaces and micro pores leading to more packing efficiency and better densification during sintering.
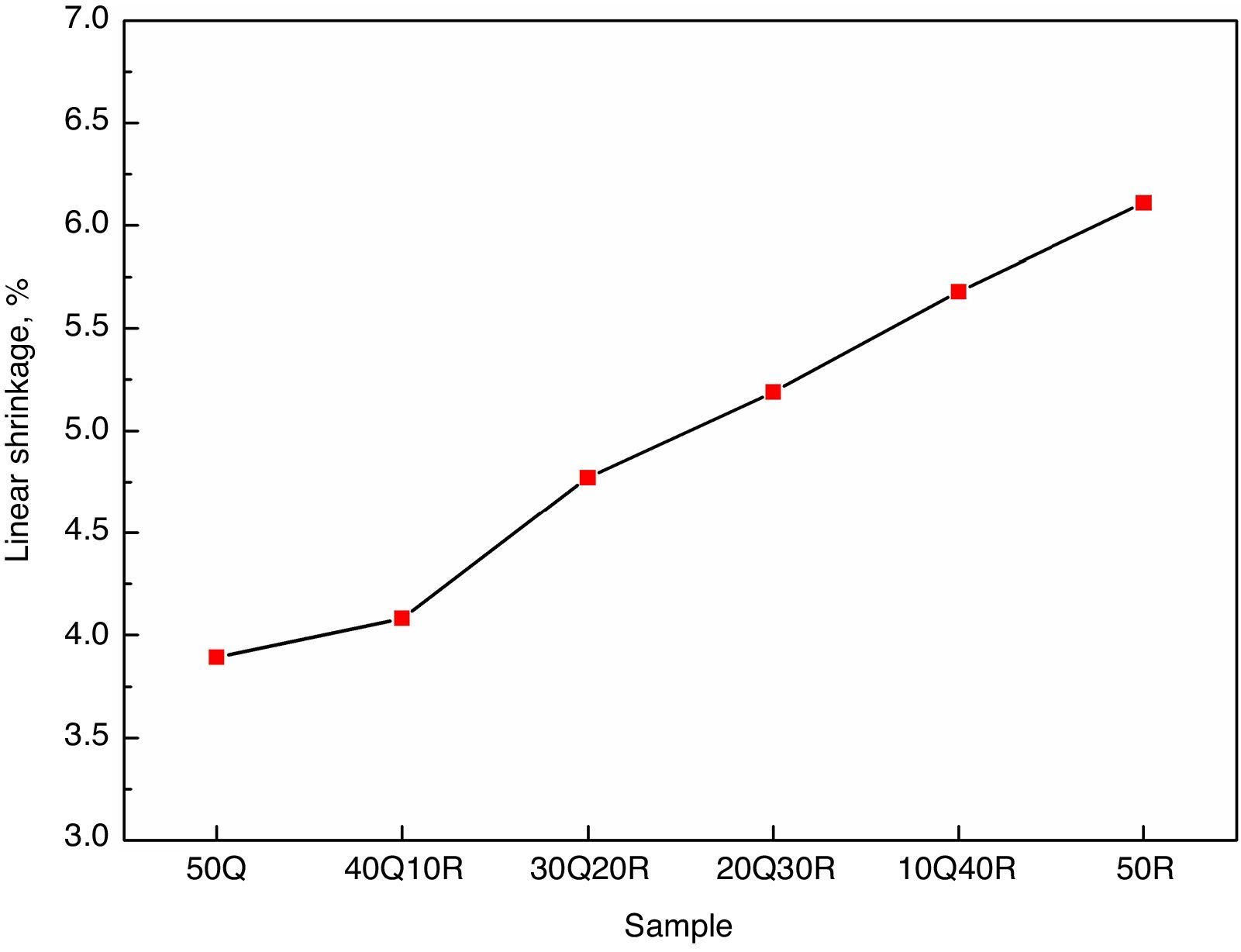
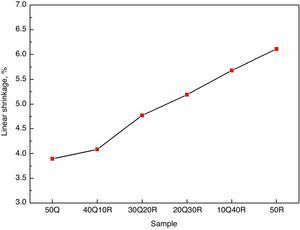
The change in linear dimension that has occurred in the test specimen before and after sintering is called linear shrinkage and the measurement results are shown Fig. 4. It has been observed that the overall shrinkage in the entire refractory samples were quite less. These results can be attributed to the fire clay refractory grogs incorporated in the composition. The refractory grog must be considered as a non-shrinking material as it has been fired already at the temperatures above the sintering temperature of these samples. However, a slight increase in the linear shrinkage was observed when quartz is gradually replaced with RHA which may be explained as a result of removal of pores and inter-granular spaces during the sintering. Another hypothesis which must be considered in this regards is that the amorphous silica present in RHA goes into crystalline form when these samples were subjected to sintering. This amorphous to crystalline transition which is a rearrangement of atoms in a defined order to produce a defined crystal phase, often results in shrinkage in the material hosting this transition.
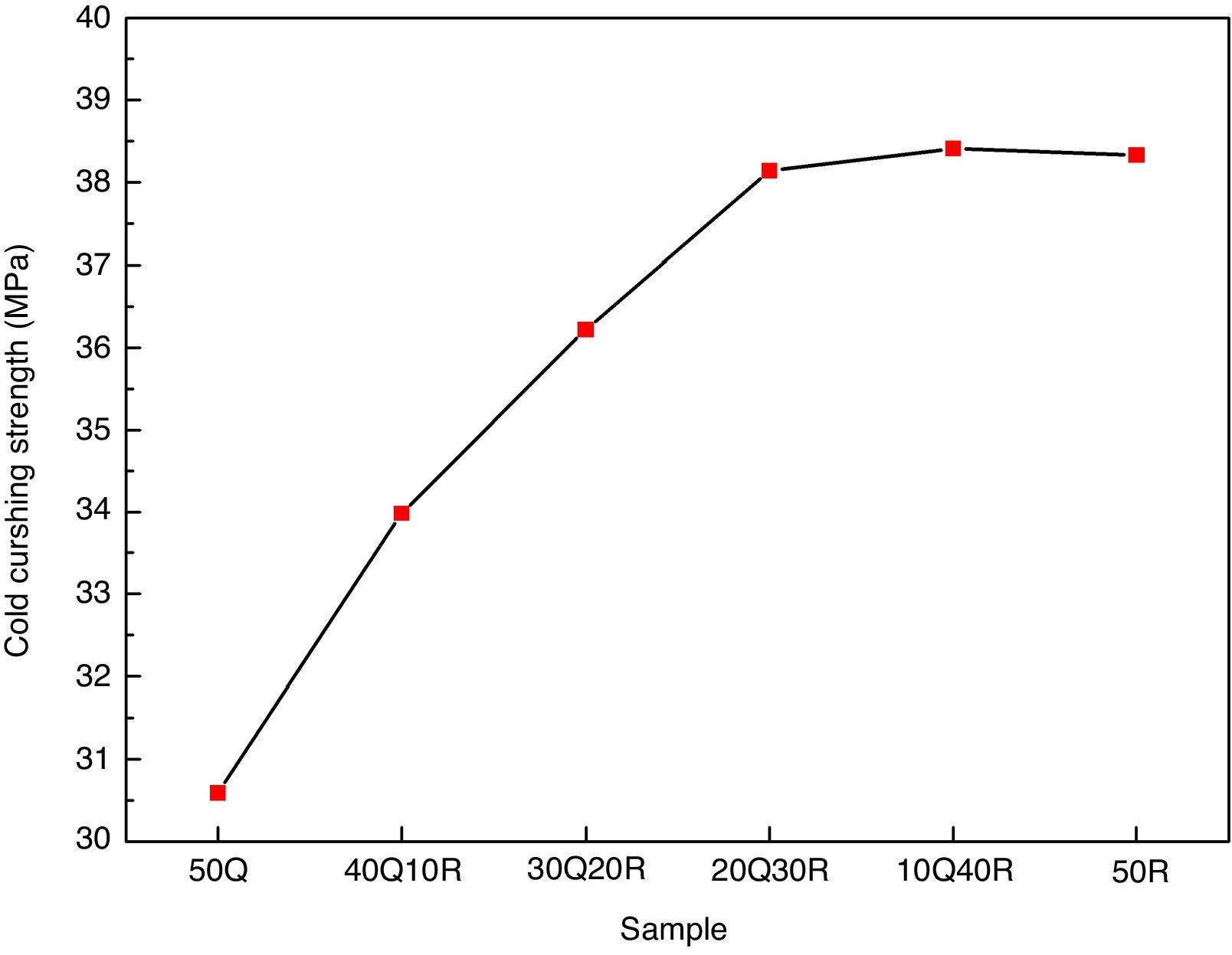
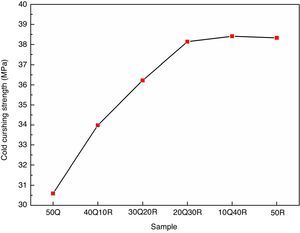
The results for cold crushing strength (CCS) measurements are shown in Fig. 5. It was found that these refractory samples has shown very good mechanical strength. These results may be attributed to the incorporation of refractory grogs and clay into the composition. Refractory grogs behaves as a non-shrinkage material further enhancing the stress withstand capability of the refractory. Ball clay used here, contributed to good fired bonding strength. Initial replacement of quartz by RHA has resulted in a significant improvement in the CCS as RHA has an active silica in amorphous form which provides better bonding with other ingredients of the composition during sintering and the final compactness of the samples has increased prior to high cold crushing strength. However, reaching the replacement of quartz by RHA up to 30% in the composition 20Q30R, a saturation in the CCS increment is observed. This saturation can be explained as after obtaining the compactness leaded by the pore removal, the RHA and quartz both result in the same phase in the final sintered product which do not lead to any differential strengthening capabilities.
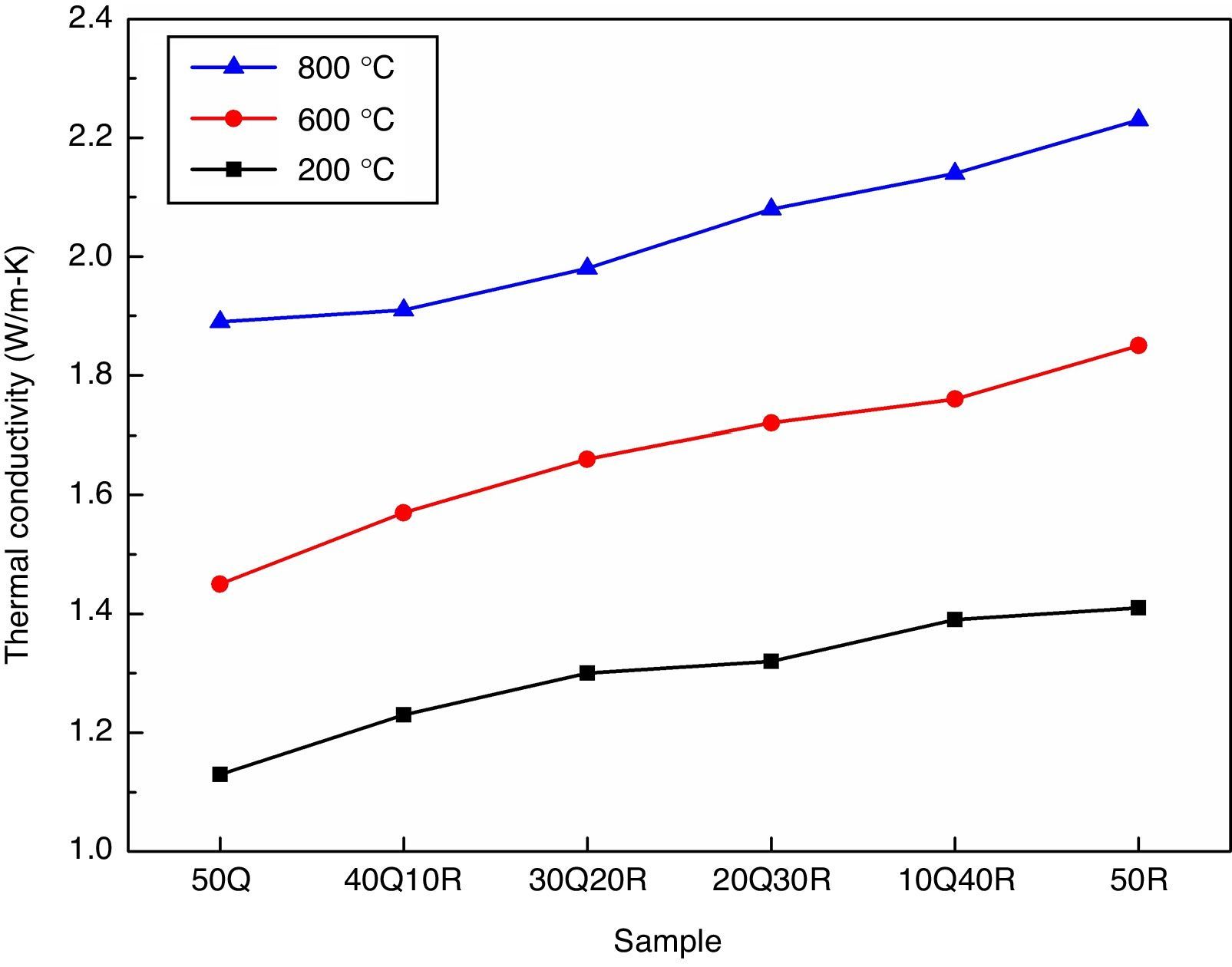
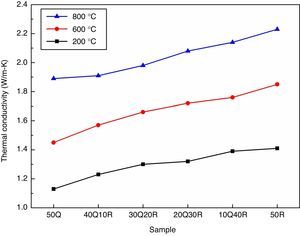
The thermal conductivity measurements are particularly important for refractories where the thermal gradients from the hot face to the cold face dictate the use of a refractory material for the specific uses. The thermal conductivity measurement of various samples were performed at temperatures 200°C, 600°C, 1000°C, respectively and results are shown in Fig. 6. The thermal conductivity of refractories basically depends upon the chemical and mineralogical compositions as well as the glassy phase contained within the refractory. However, the temperature at which the refractory is being used also plays an important role. The way in which thermal conductivity of refractories varies with temperature is subjected to the nature of the material under examination. One mode of heat transfer through a solid occurs via energy transfer between vibrating atoms. At low temperatures (up to about 400°C), energy travels through the material predominantly via lattice vibrations to be called phonons with the speed of sound. Lattice vibrations increase in frequency and amplitude as temperature increases until they get scattered. This scattering may occur either through phonon–phonon collision or at lattice imperfections. In general, with increasing temperature, phonon conductivity is observed to be decreasing in crystalline materials as the extent of scattering increases. At higher temperatures, photon conductivity to be called radiation, becomes the predominant mechanism of energy transfer. This is considered to be a rapid sequence of absorptions and emissions of photons that travel through the speed of light [39]. It was observed that the entire refractory samples has an increasing thermal conductivity with increasing measurement temperature. RHA incorporation by replacing quartz in the refractory has not raised any significance effect in the thermal conductivity. However, the pore elimination and increased compactness has delivered an improvement in the conductivity. Noble thermal conductive refractories may benefit when used in furnace lining by providing homogeneous heat transfer throughout the area.
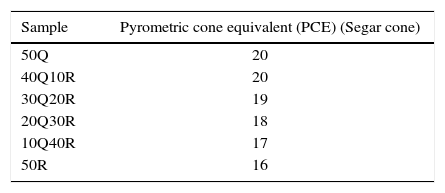
Results for refractoriness measurements are tabulated in Table 5. When RHA was incorporated gradually by replacing quartz into the refractory composition, a slight decrease in refractoriness was observed. It may be considered as a result of the active silica contained in RHA has low melting point (1440°C) than quartz due to which the refractory formulated with RHA can easily be densified at certain lower temperatures. The low temperature withstand capability of active silica can be explained by its open network structure having small range ordering and absence of crystalline phase. However, once the amorphous material acquires the crystalline phases of cristobalite and tridymite after sintering; it does not produce any significant differential properties. In our experiments, the conventional silica refractory formulated by quartz produced a maximum PCE temperature of 1580°C whereas sample 50R having complete substitution of quartz by RHA shown a PCE temperature of 1470°C due to the reduction in refractoriness dramatically.
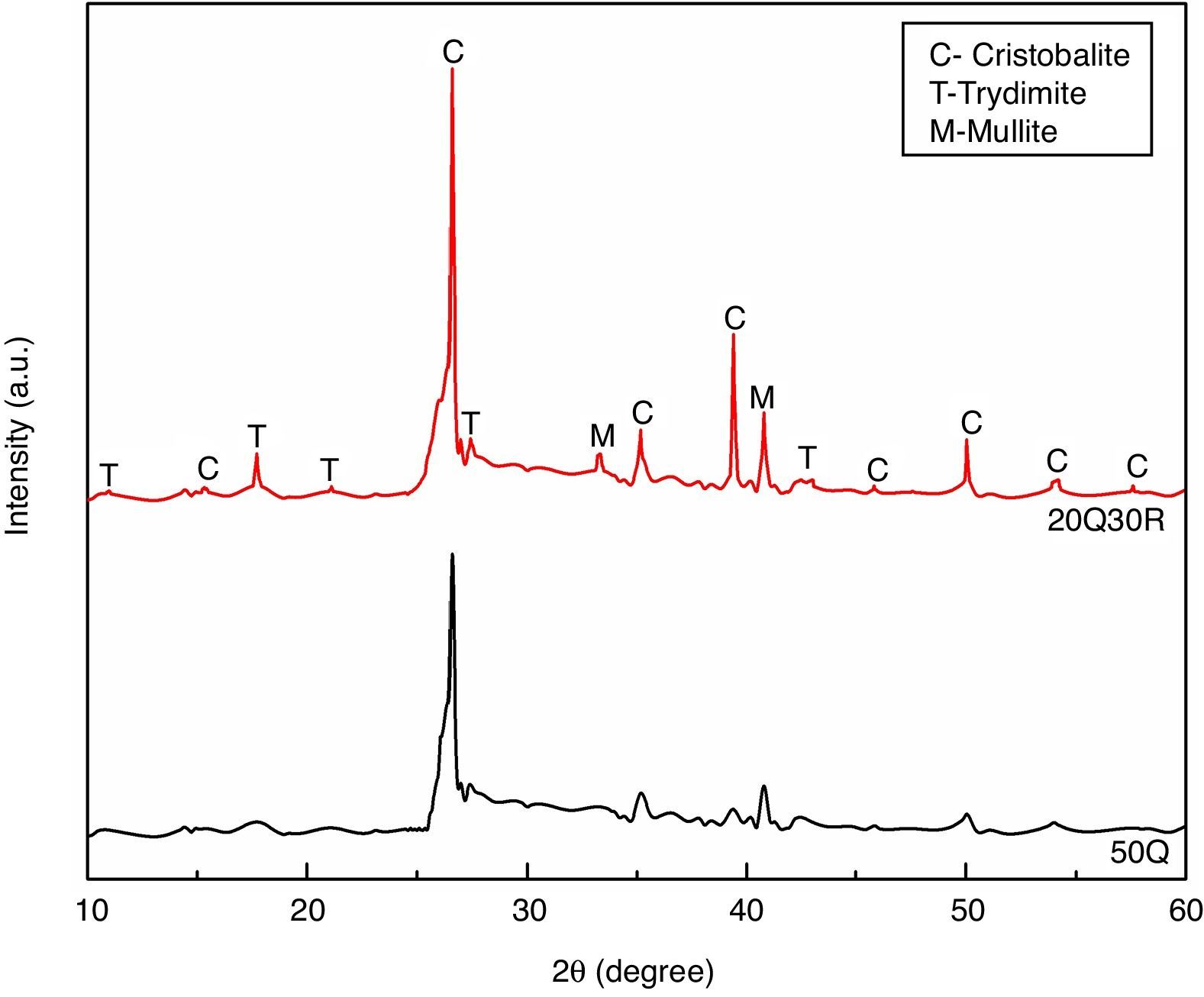
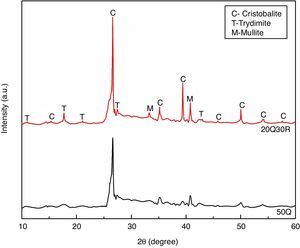
The standard sample 50Q and most optimum sample 20Q30R containing 30wt% RHA sintered at 1200°C was analyzed by X-ray diffraction and the results are shown in Fig. 7. The experiment was performed in a 2θ range of 10–60° and results were studied using JCPDS to determine the various phases developed after sintering. Comparing both the XRD patterns, we may observe that the intensity of crystallinity has increased significantly for sample 20Q30R comparing to the sample 50Q. However both the sample consist of crystalline silica (SiO2) as the major phase in its two popular polymorphs i.e. cristoballite and trydimite. In sample 20Q30R, crystalline silica was produced when the parent amorphous silica starts to convert into crystalline form above 800°C. The conversion continues upto 1000°C to obtain completely crystallized structure [40].
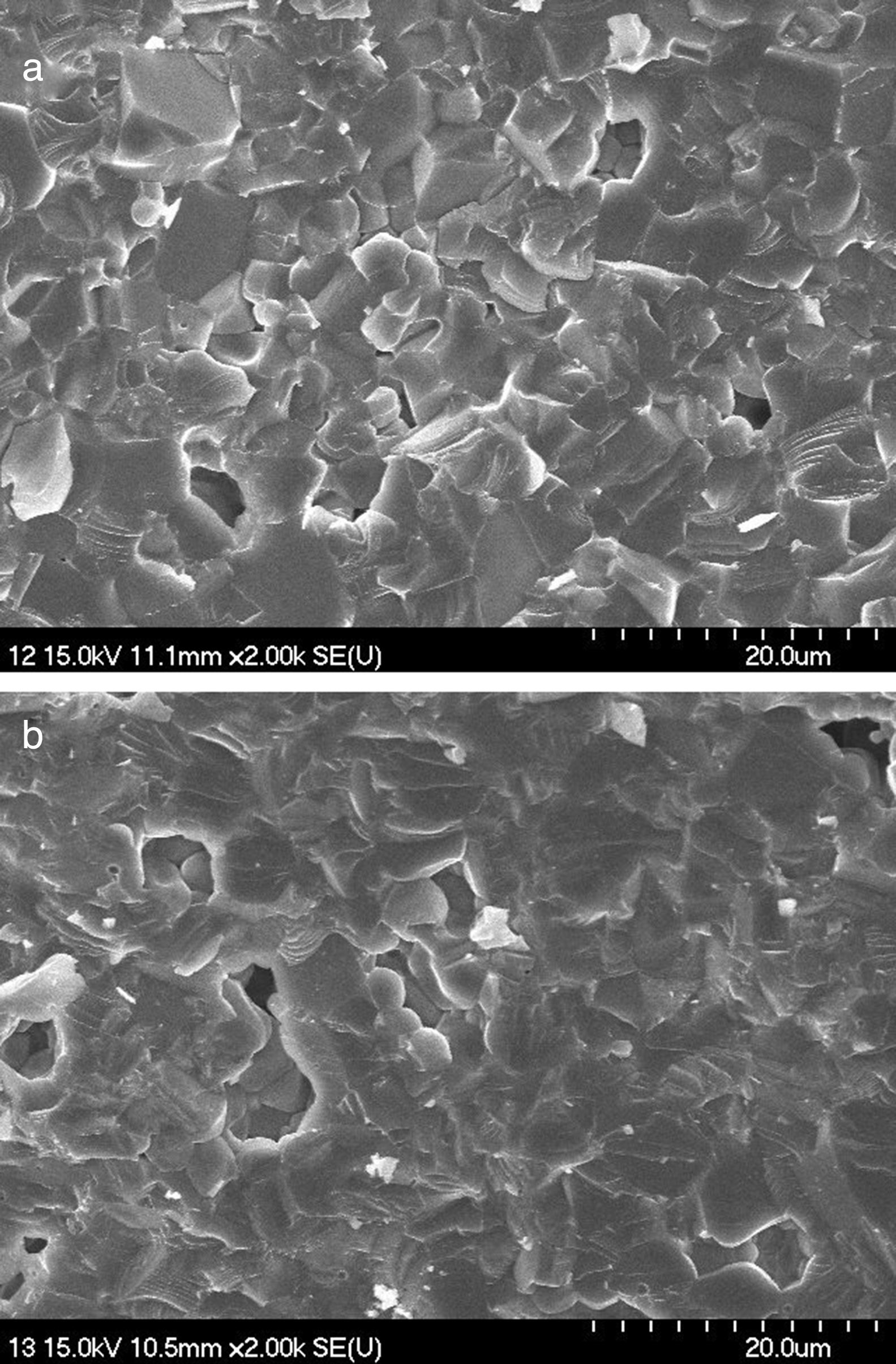
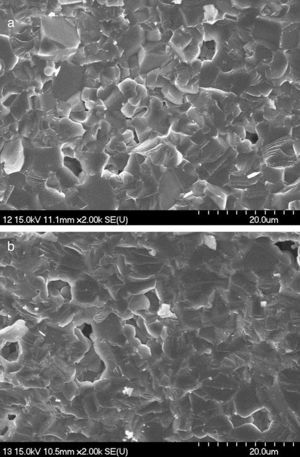
The surface morphological study of the sintered samples was performed by scanning electron microscopy. Fig. 8(a) and (b) shows the SEM images of the fractured cross sectional surface of sample 50Q and 20Q30R respectively. In Fig. 8(a), sample 50Q has shown clearly visible grain boundaries unlike sample 20Q30R in Fig. 8(b). Sample 20Q30R has shown an even surface with a slight enlargement in grains. These variations may be explained as the amorphous silica contained in RHA has transformed into crystalline form during sintering and the grains developed through this crystallization are slightly enlarged as well as are evenly distributed producing a smooth surface. Besides these variations both of the samples has acquired similar microstructural morphology after firing at a temperature of 1200°C as the resulting phase of the entire samples are same as confirmed by XRD analysis.
ConclusionThe clay bonded high strength silica refractory was successfully prepared by utilizing agriculture waste rice husk ash and refractory grog. The partial replacement of quartz by RHA has leaded to a better densification and high strength as observed in the bulk density and cold crushing strength measurements as amorphous silica in RHA has reacted more efficiently in comparison to quartz. As a result of reduced porosity, thermal conductivity has also increased slightly. The sample utilizing 30% agriculture waste-RHA is to be considered most optimum composition which showed CCS of 38MPa, thermal conductivity of 2.08W/mK at 800°C with a considerable good refractoriness suggesting that this refractory material can be used in most furnace lining applications where silica refractories are generally used.
The authors gratefully acknowledge all the faculty and staff of Department of Ceramic Engineering, Indian Institute of Technology (B.H.U.), Varanasi, India and the Ministry of Human Resource Development, Govt. of India for providing appreciable support.