Hard porcelain is constituted in the alkali oxides-alumina-silica ternary system, and produced by a mixture of clay-feldspar and silica. The most important properties of this porcelain are high mechanical strength, translucency and whiteness. These properties depend on quality of raw material, firing temperature and soaking time. In bone porcelain bone ash was added to body composition up to 50wt.%. Generally hard porcelain and bone porcelain scrap cannot be reused in body composition. Whereas using these scrap could help natural resources. In this research using bon porcelain scraps in hard porcelain body have been investigated. Results show, this substitution decrease firing temperature, linear expansion and increase glass, probability of deformation and total shrinkage. Using 6wt.% bone porcelain scraps to hard porcelain body composition besides improving some properties, increases 1340°C firing mechanical strength two times and helps natural resources.
La porcelana dura está compuesta por el sistema ternario de óxidos alcalinos-alúmina-sílice y se produce por una mezcla de arcilla-feldespato y sílice. Las propiedades más importantes de esta porcelana son alta resistencia mecánica, translucidez y blancura. Estas propiedades dependen de la calidad de la materia prima, la temperatura de cocción y el tiempo de remojo. En la porcelana de huesos se añadió ceniza de hueso al 50% del peso de la composición del cuerpo. Por lo general, la porcelana dura y los restos de porcelana de huesos no pueden reutilizarse en la composición del cuerpo, aunque el uso de estos restos podría ayudar a los recursos naturales. En este estudio se ha investigado el uso de restos de porcelana de huesos en el cuerpo de porcelana dura. Los resultados muestran que esta sustitución disminuye la temperatura de cocción y la dilatación lineal, y aumenta la probabilidad de deformación y la contracción total del vidrio. Utilizar el 6% del peso de restos de porcelana de huesos en la composición del cuerpo de porcelana dura, además de mejorar algunas propiedades, aumenta al doble la resistencia mecánica a la cocción a 1.340°C y ayuda a los recursos naturales.
Hard porcelain is composed of the triple oxide of silica, alumina, and alkaline oxides (K2O), obtained through firing a combination of Kaolin, Feldspar, and Quartz, whose important characteristics include high whiteness, translucency, and mechanical strength. These characteristics are fully dependent on the quality of raw materials, the firing atmosphere, the maximum temperature, and soaking time at the maximum temperature [1–3].
In bone porcelain, the bone ash which contains hydroxyapatite (Ca10(PO4)6(OH)2) is added to the composition of the porcelain body by as much as 50%. The bone ash present in the bone porcelain is degraded to β-tricalcium phosphate [Ca(PO4)2], lime (CaO), and water (H2O). Produced lime reacts with metakaolin obtained from the clay and creating anorthite [4]. The structure of hard porcelain usually consists of glass, cristobalite, and mullite phases, whereas the phases constituting bone porcelain contain cristobalite, anorthite, and whitlockite [5]. Their firing curves are also different with each other: hard porcelain usually sintered within 1320–1350°C during 4h, while bone porcelain is fired within 1220–1260°C during 11h [6,7].
Sintered scraps, in both types, cannot be recycled. Their reuse helps the environment from their disposal and less usage of natural resources but may influences the quality of production. Many attempts have been made to use the scraps in body composition. However, in production of porcelain, a slight change in the composition might cause some problems, leading to uneconomical production. Mukhopadhyay et al. [8] have used bone porcelain scraps in bone porcelain formulation and concluded that application of these scraps can result in increased strength and density, and decreased firing temperature. Marinoni et al. [9] has used the scraps of soda-lime glass in sanitary ware as ceramic flux. He has shown that this results in decreased firing temperature, diminished fuel consumption, and removal of scraps from the environment. Similar to this work has been carried out by Oulaseyi et al. [10] to use soda-lime glass in hard porcelain. Moreover, Pagani has recommended application of hard porcelain scraps at high percentages in sanitaryware [11].
Gouvêa et al. [12] has employed bone ash as an additive in sintering of bodies and has shown it can be used as a flux in porcelain composition. Developing of anorthite-based porcelain [13] and improving the porcelain characteristics of body with bone ash encourage us to verify the effect of bone porcelain scraps (BPS) on the characteristics of hard porcelain. It can help us for environmental scope and improving the hard porcelain properties.
Hard porcelain with 0, 3, 6, 9, 12, 18 percent BPS was prepared and fired at temperature 1030, 1100, 1250, and 1340°C and the main properties of hard porcelain was investigated.
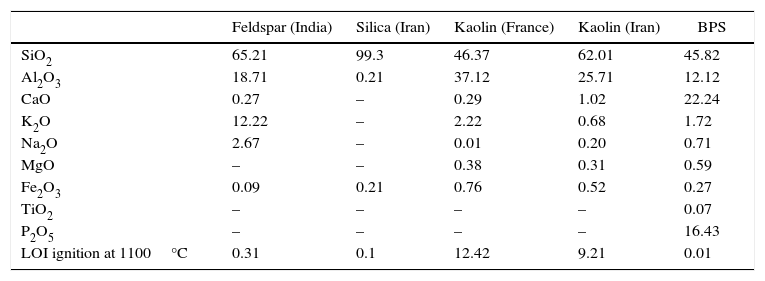
Materials and methodsA mixture of 80wt.% clay (65wt.% Iranian Zenoz clay+15wt.% French Imerys clay), 7wt.% Indian feldspar, and 13wt.% silica has been considered as the base body. The chemical analysis of the all of the raw materials and the bone porcelain scraps consumed in the study is provided in Table 1. The BPS was milled in a disk mill (Retsch, PRDM01) to reach a particle size of lower than 1% on a 63-micrometers sieve. Different percentages of the BPS (0, 3, 6, 9, 12, 15, and 18wt.%) were added to the composition of the base body. In each experiment, 2500g of raw materials were grounded in a 5-L Jarmill for 390min with 1250ml water and 2.5g Dispex N40 (Allied Colloids, UK), as deflocculant, until reaching a particle size of 58% under 15μm. The slurry obtained from seven bodies (B0, B3, B6, B9, B12, B15, B18) was transferred to storage tank, passed through a 105-micrometers sieve and permanent magnet. The density was adjusted at 1.8g/cm3, and the viscosity was regulated at 180° with Torsion viscometer (Anderen Ltd, UK). The slurry was dewatering up to 20% on plaster tablets, and then converted to bars with a cross-section of 1cm×2cm and length of 10cm using an experimental extruder (Netzsch, D-95100). Thereafter, it was dried in a dryer at a temperature of 100±5°C. A number of the dried bars were used for measurement of the green and firing strength (using Netzsch, Bending strength tester 401) at a rate of 2mm/min, whereas some others were used for measurement of the firing shrinkage. (Three samples were used for mechanical strength and nearing results were averaged.) The rest was then powdered and converted to tablets with a diameter of 5cm and height of 5mm using a hydraulic press. All of the samples were fired in a cubic shaped industrial kiln with dimensions of 2m×2m×2m at a rate of 3°C/min using gas fuel with 2h of soaking time at a temperature of 1030±5°C (the temperature of the first firing of the hard porcelain, 1100±5°C (the temperature of the second firing of the bone porcelain), 1250±5°C (the temperature of the first firing of the bone porcelain), and 1340±5°C (the temperature of the second firing of the hard porcelain). As the process of the firing of the body is highly dependent on the position of the sample in the kiln [14], all of the samples were loaded in the middle of the kiln.
Chemical analysis of the raw materials used.
| Feldspar (India) | Silica (Iran) | Kaolin (France) | Kaolin (Iran) | BPS | |
|---|---|---|---|---|---|
| SiO2 | 65.21 | 99.3 | 46.37 | 62.01 | 45.82 |
| Al2O3 | 18.71 | 0.21 | 37.12 | 25.71 | 12.12 |
| CaO | 0.27 | – | 0.29 | 1.02 | 22.24 |
| K2O | 12.22 | – | 2.22 | 0.68 | 1.72 |
| Na2O | 2.67 | – | 0.01 | 0.20 | 0.71 |
| MgO | – | – | 0.38 | 0.31 | 0.59 |
| Fe2O3 | 0.09 | 0.21 | 0.76 | 0.52 | 0.27 |
| TiO2 | – | – | – | – | 0.07 |
| P2O5 | – | – | – | – | 16.43 |
| LOI ignition at 1100°C | 0.31 | 0.1 | 12.42 | 9.21 | 0.01 |
The fired bars were used for measuring the firing strength, firing shrinkage, and thermal expansion coefficient. The melting viscosity was calculated with the measurement of the degree of bending in the bars with a length of 10cm in the kiln, when one of their heads was free. The fired tablets were used for measuring water absorption, bulk density (Sartorious densitometer LA230S).
X-ray diffraction (XRD) patterns were recorded on a diffractometer Unisantis (XMD-400) with a power of 30kV×20mA. Monochrome beam of CuKα has been used with a wavelength of λ=0.154060nm. The radiation range has been chosen between 10 and 90 with an accuracy of 1°. Percentage of crystalline species is found through the calculation of peak area by Xpert highs core and Sigma plot software.
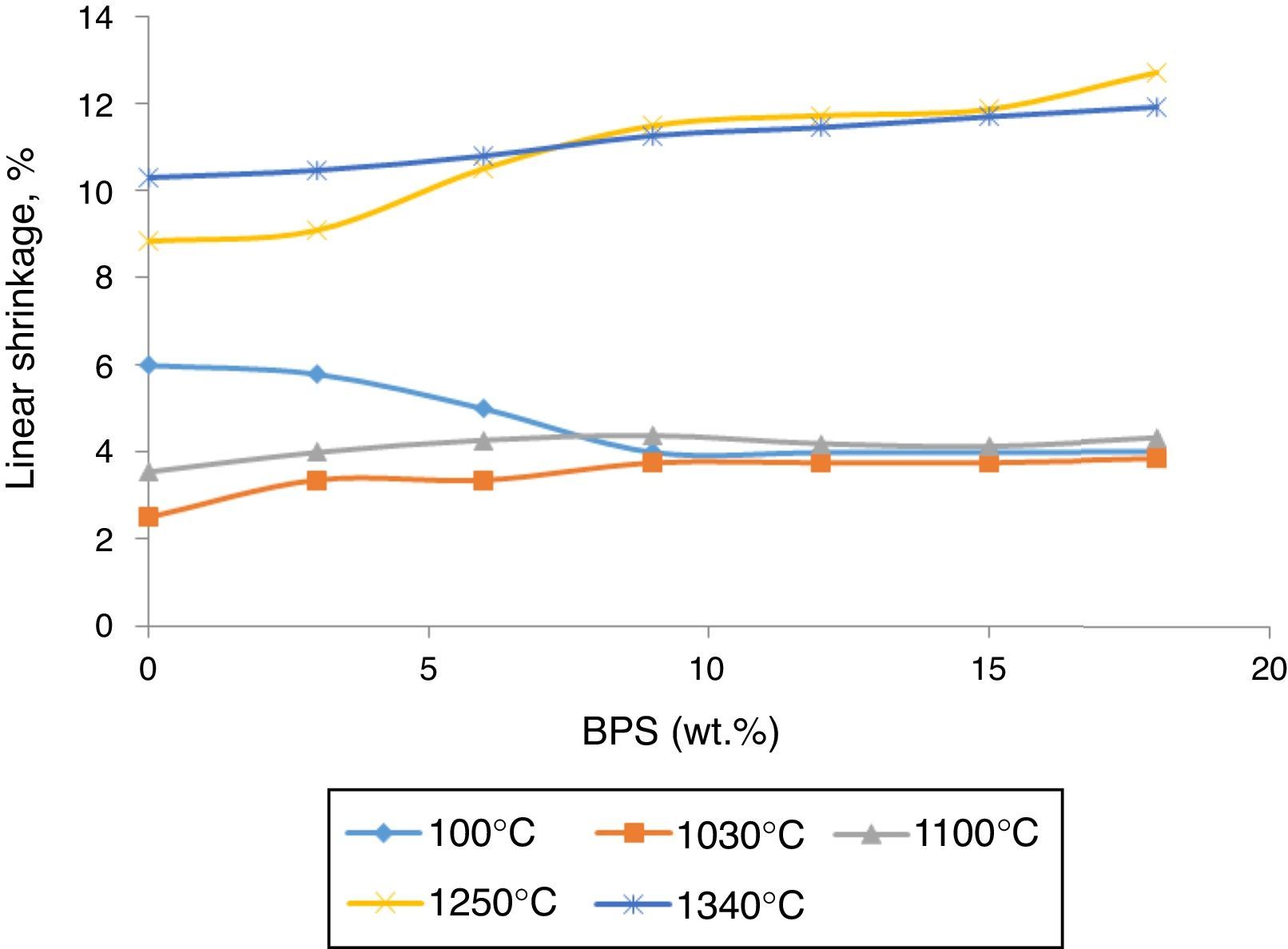
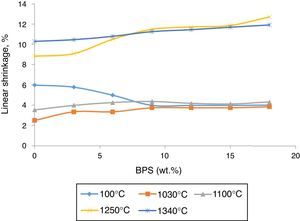
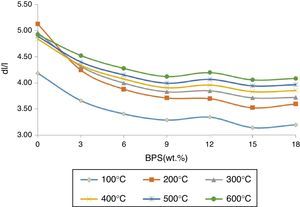
Results and discussionBy increasing BPS, the wet-to-dry shrinkage (at 100°C) declines due to the increase in non-clay compounds and the dry-to-firing shrinkage (at 1030, 1100, 1250, and 1340°C) increases due to existence alkaline earth ceramic fluxes (CaO) in the scraps (Fig. 1).
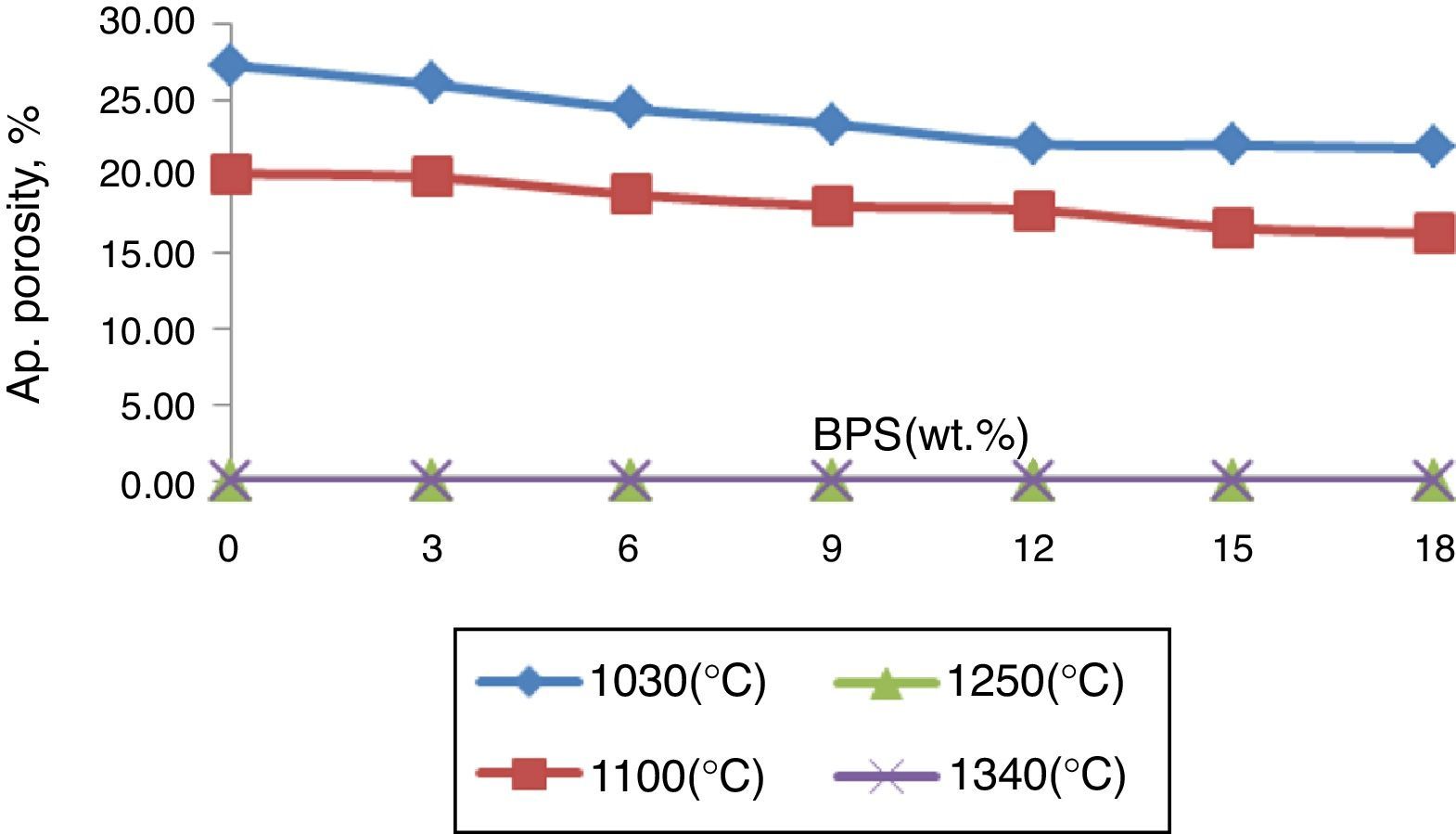
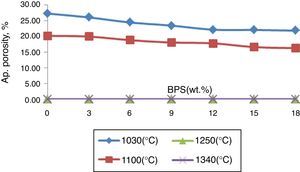
All of the samples have had an apparent porosity lower than 0.5% at 1250°C (Fig. 2), and have been fully sintered. However, at lower temperatures, the apparent porosity has declined with the increase in the scraps (Fig. 2). The reduction of the apparent porosity suggests decreased firing temperature in the bodies. This shows that presence of milled scraps causes decreased glass transition temperature, with melting of the bodies containing the scraps taking place at lower temperatures, possibly due to increased extent of alkaline and alkaline earth oxides especially CaO. Further, presence of glass phase in the scraps (since it has gone through the firing process once) has a major role in early melting of the body containing scraps.
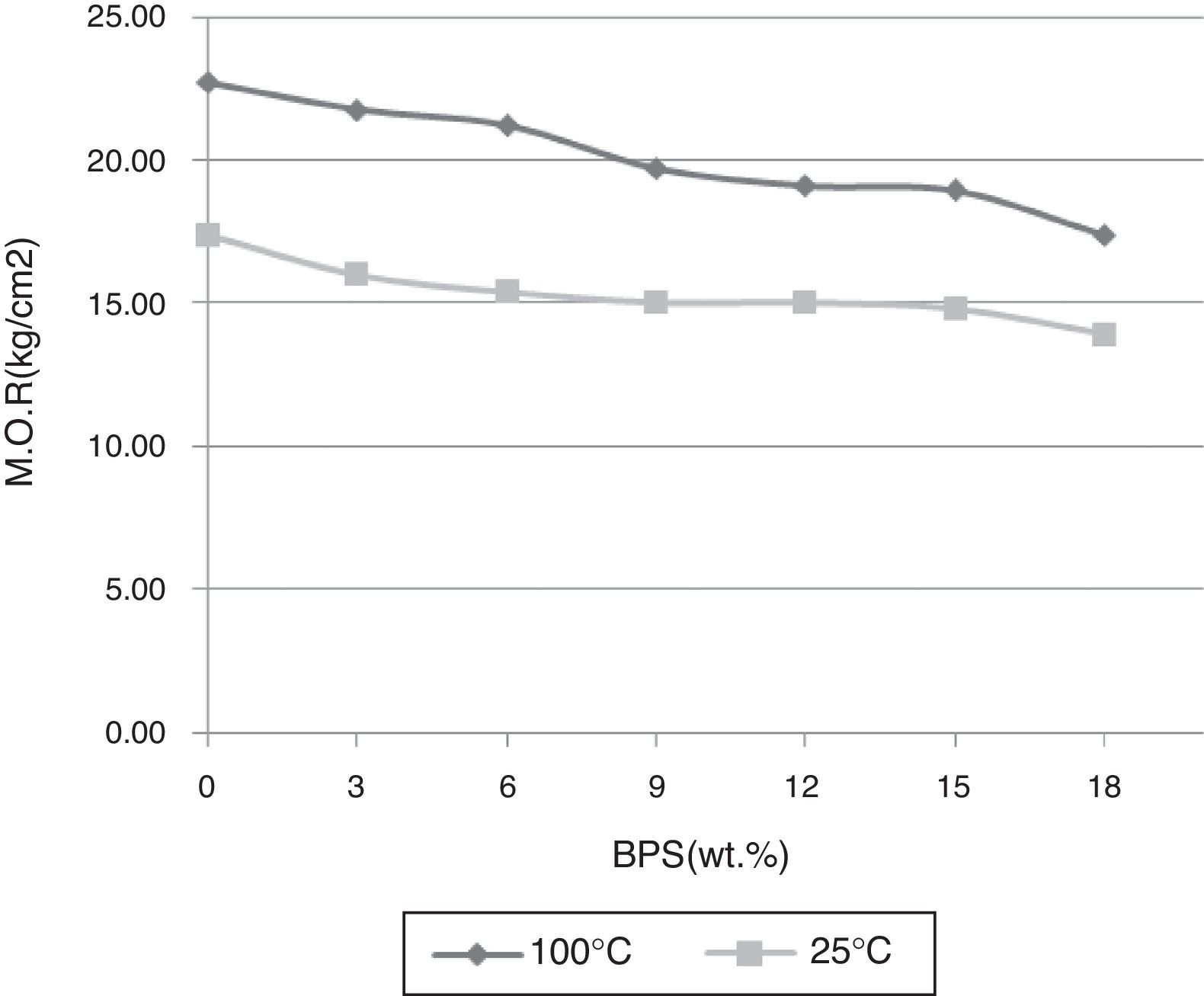
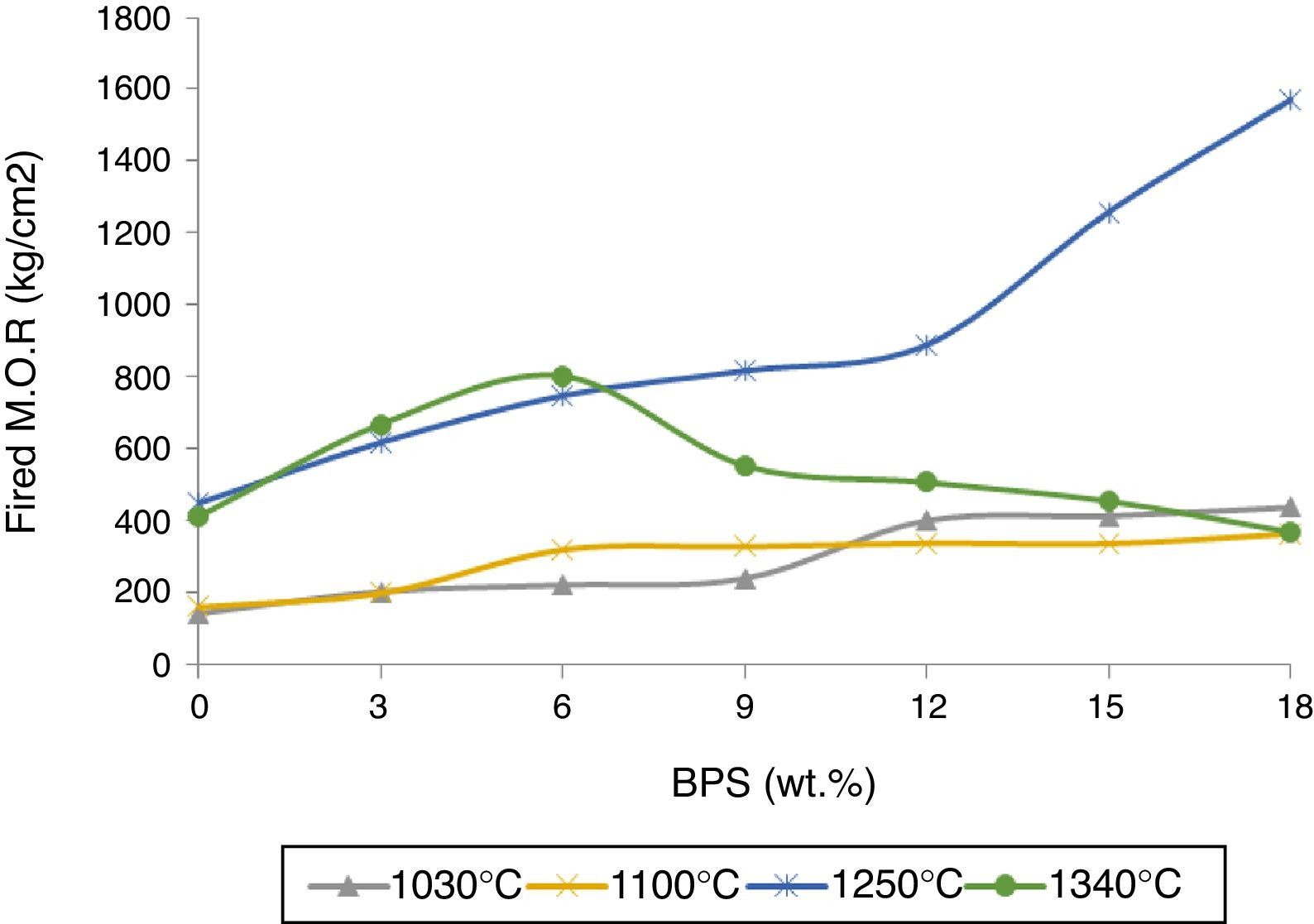
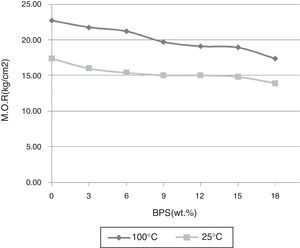
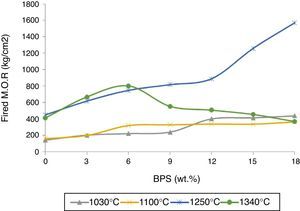
By increasing BPS, the green strength declines at temperatures of 25 and 100°C, possibly due to increased amount of non-plastic compounds devoid of green strength (Fig. 3). In relation with the B0 body, this reduction in strength reaches around 22% in both temperatures, which can result in increased amount of raw scraps during production. Further, with the increase in the amount of scraps, the firing strength grows at temperatures of 1030, 1100, and 1250°C (Fig. 4). The temperature of 1030°C is the biscuit temperature of the bodies, where with the increase in the strength, the degree of biscuit scraps in production line will diminish. This increase is double at 1030°C for B12, while this elevated the mechanical strength at 1250°C for the B12 and B18 grows twofold and fourfold, respectively. The changes in the strength of sintered body at the hard porcelain firing temperature, i.e. 1340°C show a different behavior. With the increase in the amount of scraps up to 6wt.%, the strength rises. However thereafter the strength starts to decline, such that at 12wt.% it reaches its initial value at 0%, and continues to decline.
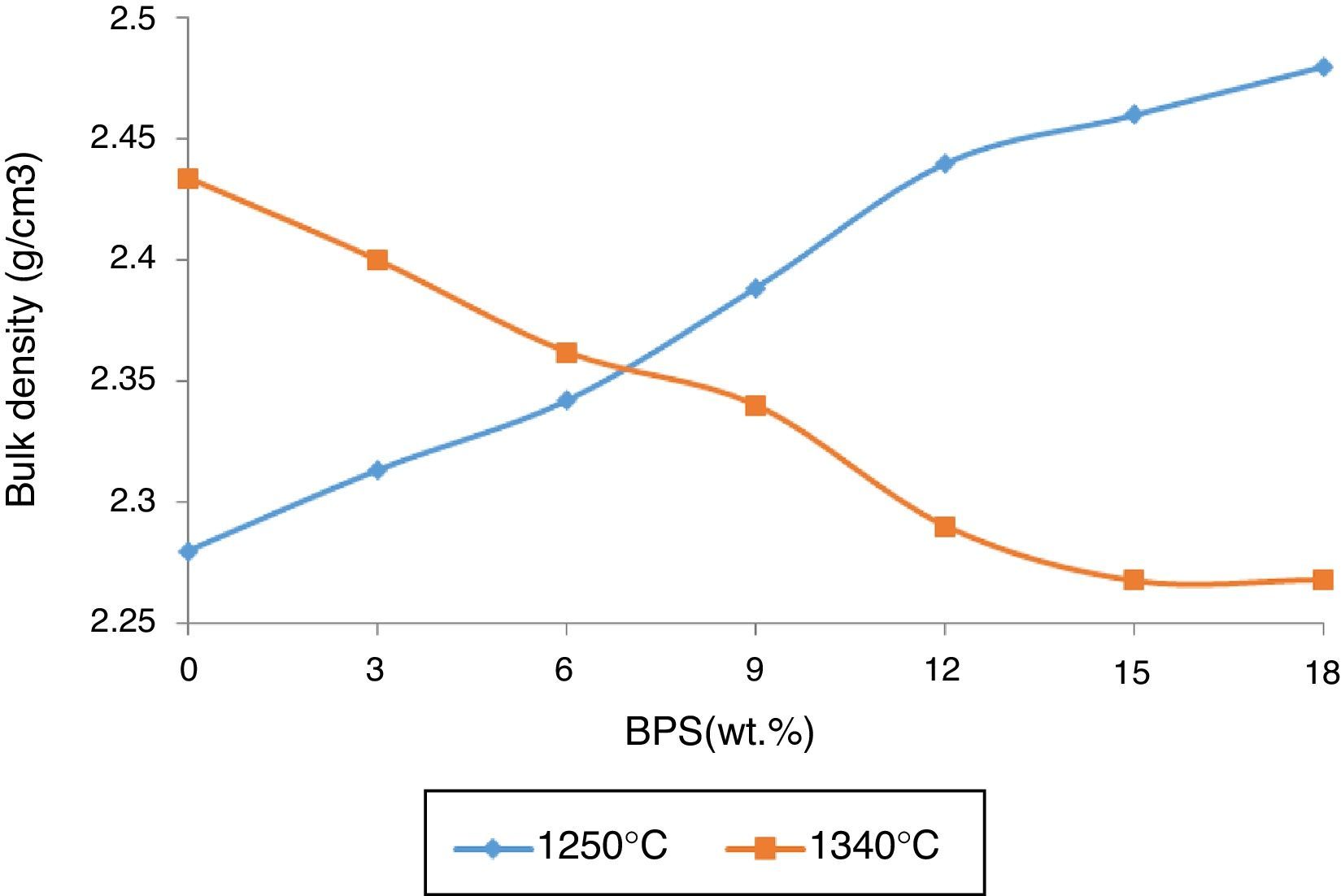
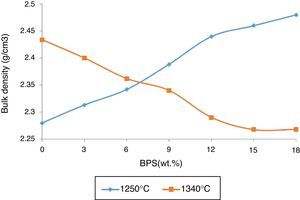
The results are congruent with the results obtained from apparent porosity curves (Fig. 2). The mechanical strength increases with the decrease in the apparent porosity. Reduction of porosity depends on the amount of the liquid phase in the firing temperature. At glass transition temperature, open pores close and develop closed porosity. Due to existence of surface tension in liquid phase, closed pores start to decline in size, until the pressure of the gas inside the pore reaches equilibrium with the surface tension. Then the lower the apparent porosity and the shrinkage of closed pores cause the greater bulk density (Fig. 5). The greatest bulk density, 2.46g/cm3, is observed in B18 sample containing 18wt.% of scraps fired at 1250°C. The maximum bulk density has been obtained in conditions where the mechanical strength is also at its maximum (Fig. 4). This has also been observed by Bragança et al. [15], though it has been worked in another system.
However, at 1340°C, the result is different. The bulk density of the sintered body, fired at 1340°C, starts to decline in response to elevated amounts of scraps. This suggests decreased density of the body as a result of development of glass phase or development of a bubble in the fired body.
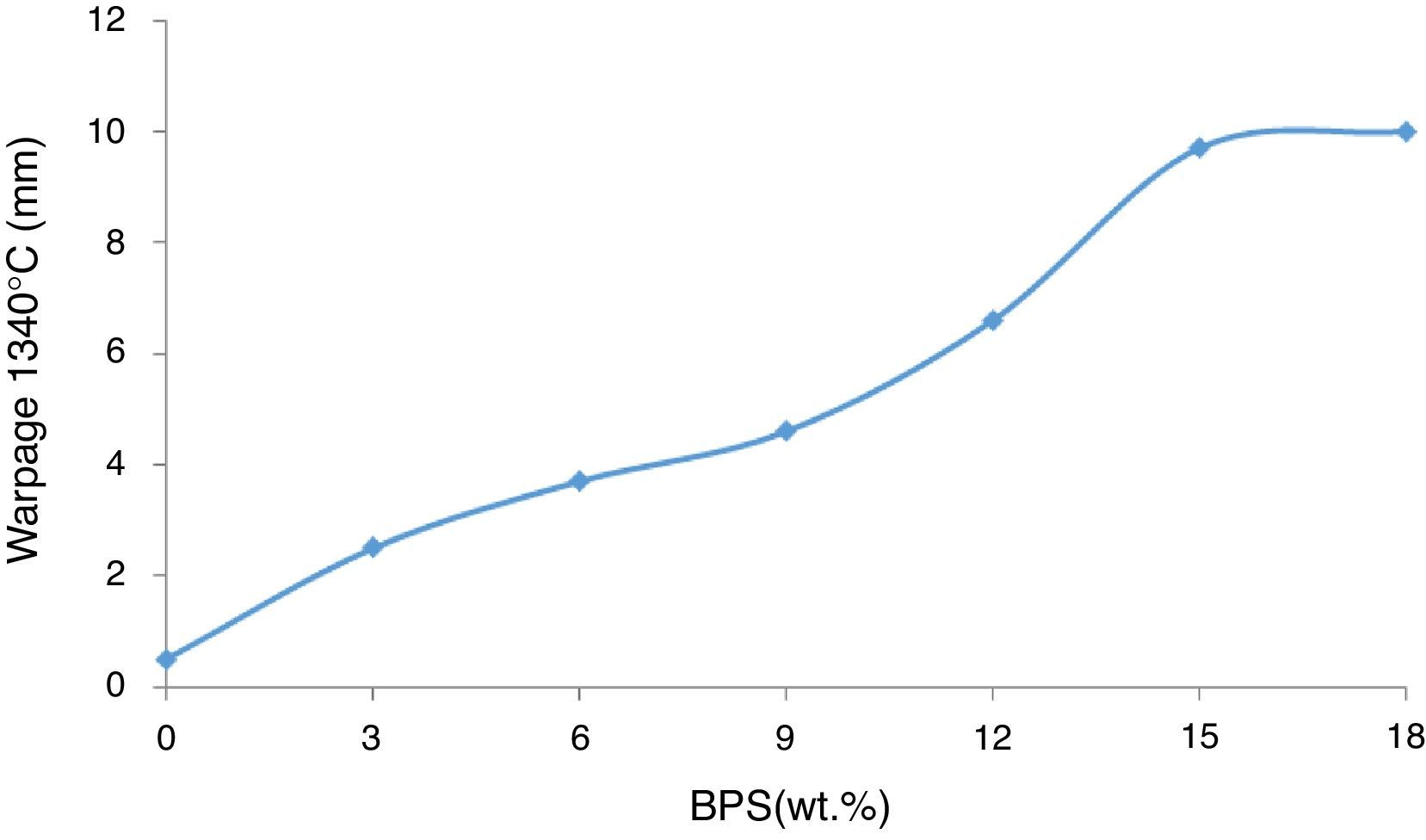
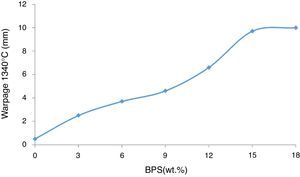
The increase in BPS results in elevated degree of deformity in the samples (Fig. 6). The degree of glass transition temperature is in line with the volume of the formed liquid and its viscosity. The liquid phase is essential during the sintering process for compression of the body, but this liquid phase causes deformity of the pieces in response to weight force and due to facilitated movement of crystals on each other.
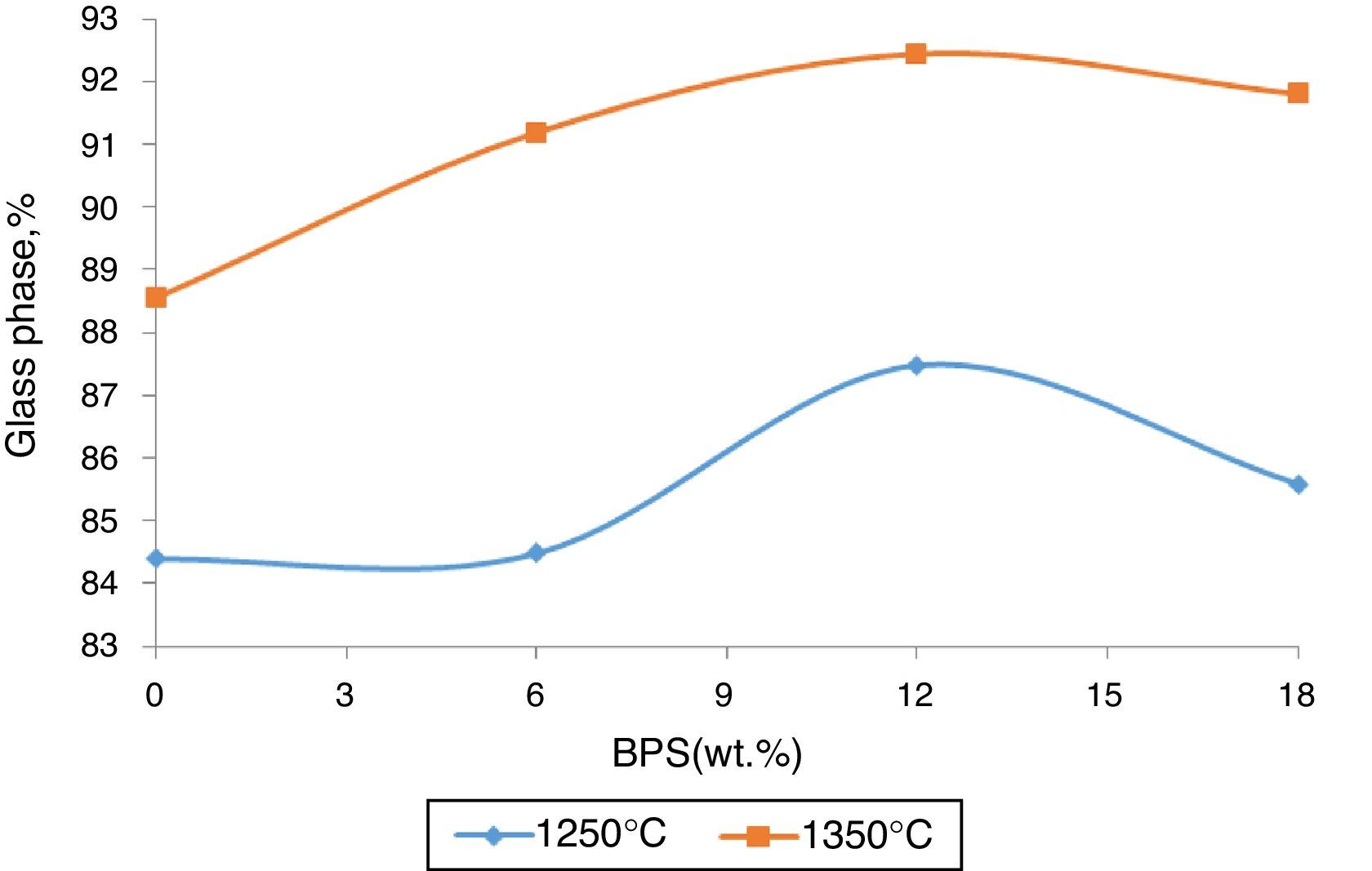
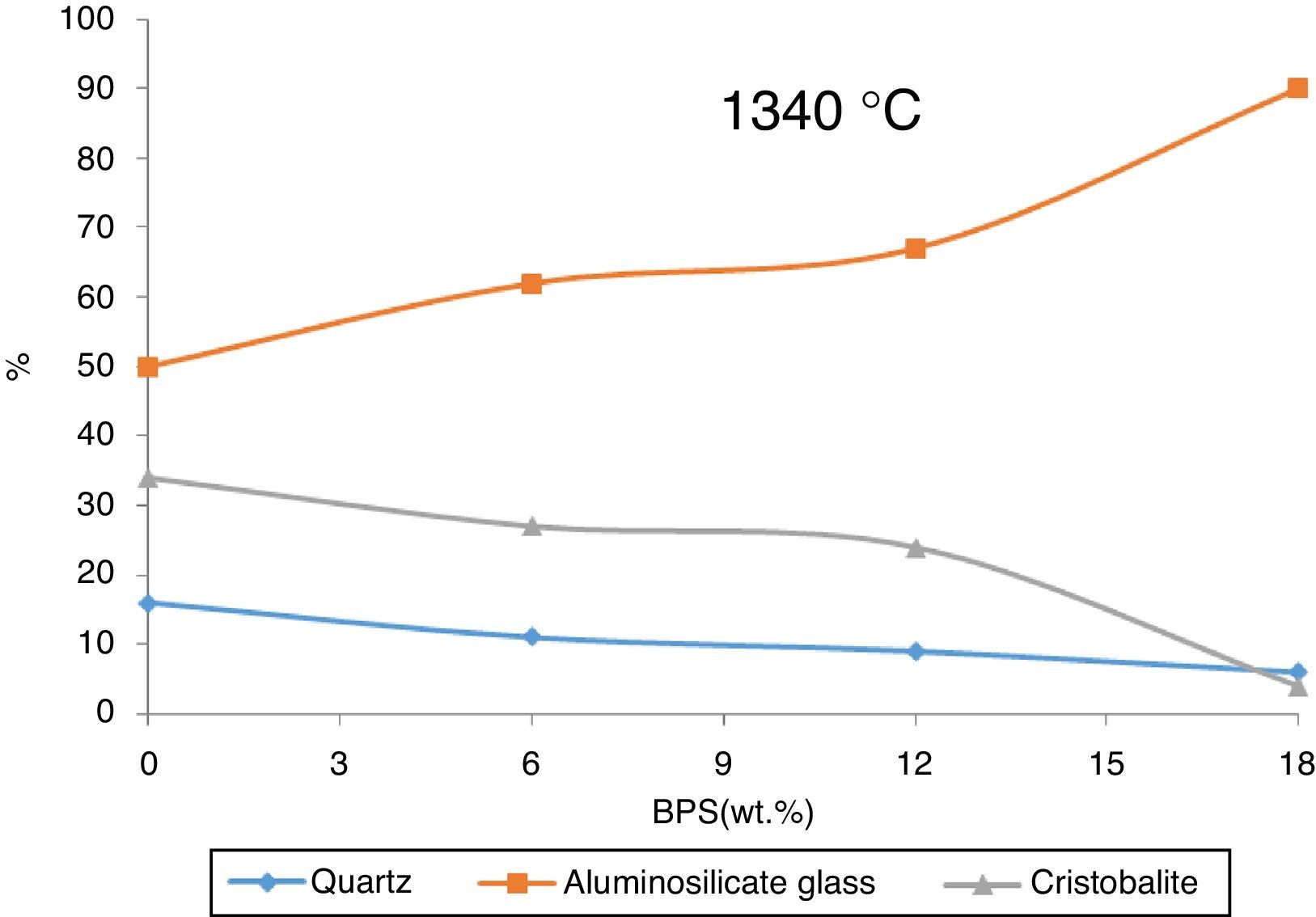
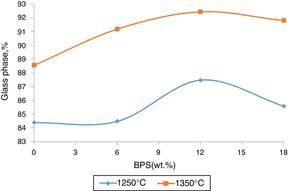
The value of the glass phase from the selected bodies at two different temperatures has been shown in Fig. 7. The results show that the hard porcelain body is highly non-crystal, where over 80% is liquid phase and their low crystal phase makes them non-resistant against impact [16]. The increase in the glass phase in response to increased amount of BPS signifies increased transparency and lack of resistance against impact.
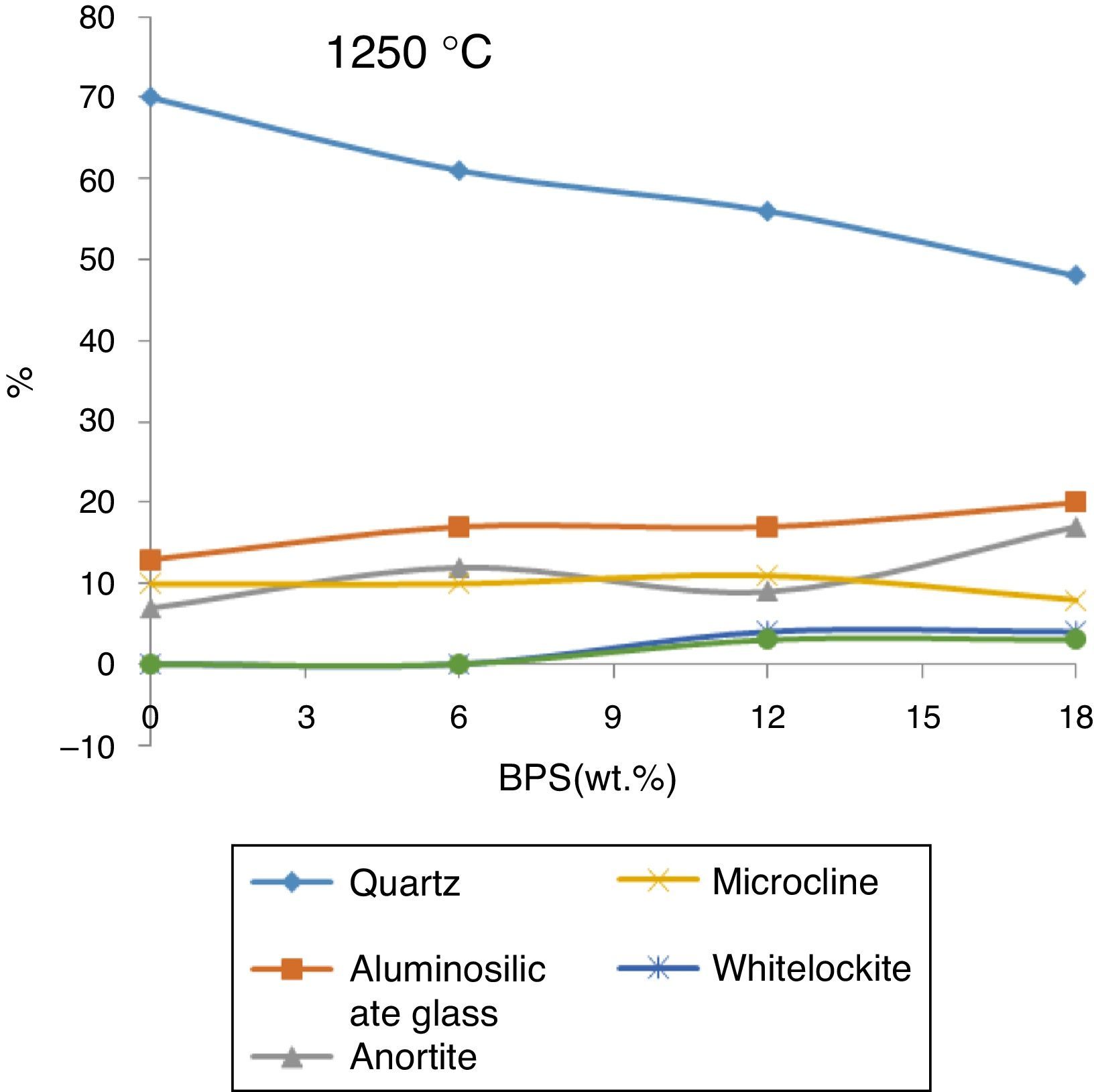
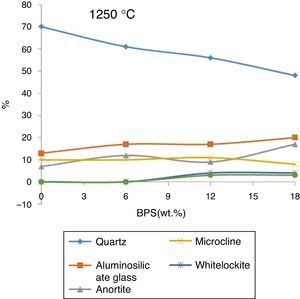
In order to investigate the residual crystal phase, XRD analysis of the selected bodies fired at 1250 and 1340°C is shown in Figs. 8 and 9. At 1250°C, α quartz, Mullite (Al6Si2O13), Anorthite (CaAl2Si2O8), and microcline (KAlSiO8) are the main phase present in the fired body with some amount of Cristobalite and whitlockite (β-Ca3(PO4)2) phase (Fig. 8).
These results confirm the results obtained by Iqbal et al. [17], who investigated the details of the structure of bone porcelain body fired at various temperatures. Observation of large amounts of α-Quartz phase at the firing temperature of 1250°C suggests that either additional quartz has been introduced into the complex or this quartz has not been dissolved at this temperature.
The structure of bone porcelain contains β-TCP, anorthite and α-Quartz, calcium aluminosilicate glass [5]. These phases are also observed in the body fired at 1250°C. The analysis of the phases in Fig. 8 demonstrates that with the gradual increase of the scraps, β-TCP also grows gradually, reaching its maximum (4%) at B12, where the glass phase also reaches its maximum. Further results indicate that a slight increase in the amount of anorthite takes place with the elevation of the amount of scraps (for B0 and B12 around 7% and 17%, respectively), the quartz phase has been observed across all of the analyzed samples. This phase declines with the increase in the scraps (from 70% in B0 to around 48% in B12). Further the mullite phase grows from 13% in B0 body to around 20% in B12. Presence of anorthite phase, which is not observed in the body of normal porcelain, can be due to the great tendency of CaO liberated from bone with the produced meta-Kaolin. Iqbal et al. [17] has claimed that the ratio of Al2O3/SiO2 in anorthite and meta-Kaolin is 1:1, therefore production of anorthite from meta-kaolin is prioritized to mullite when the Al2O3/SiO2 ratio becomes greater than 3.2%.
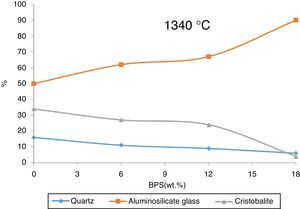
Compare to Fig. 8, Fig. 9 demonstrates that with the increase in the firing temperature, the microcline, whitlockite, and anorthite phases are dissolved in the glass phase and perish away. Furthermore, some of the quartz and cristobalite phase are also dissolved, forming glass phase. This increase in the amount of the glass phase and the reduction in the crystal phase lead to increased degree of distortion.
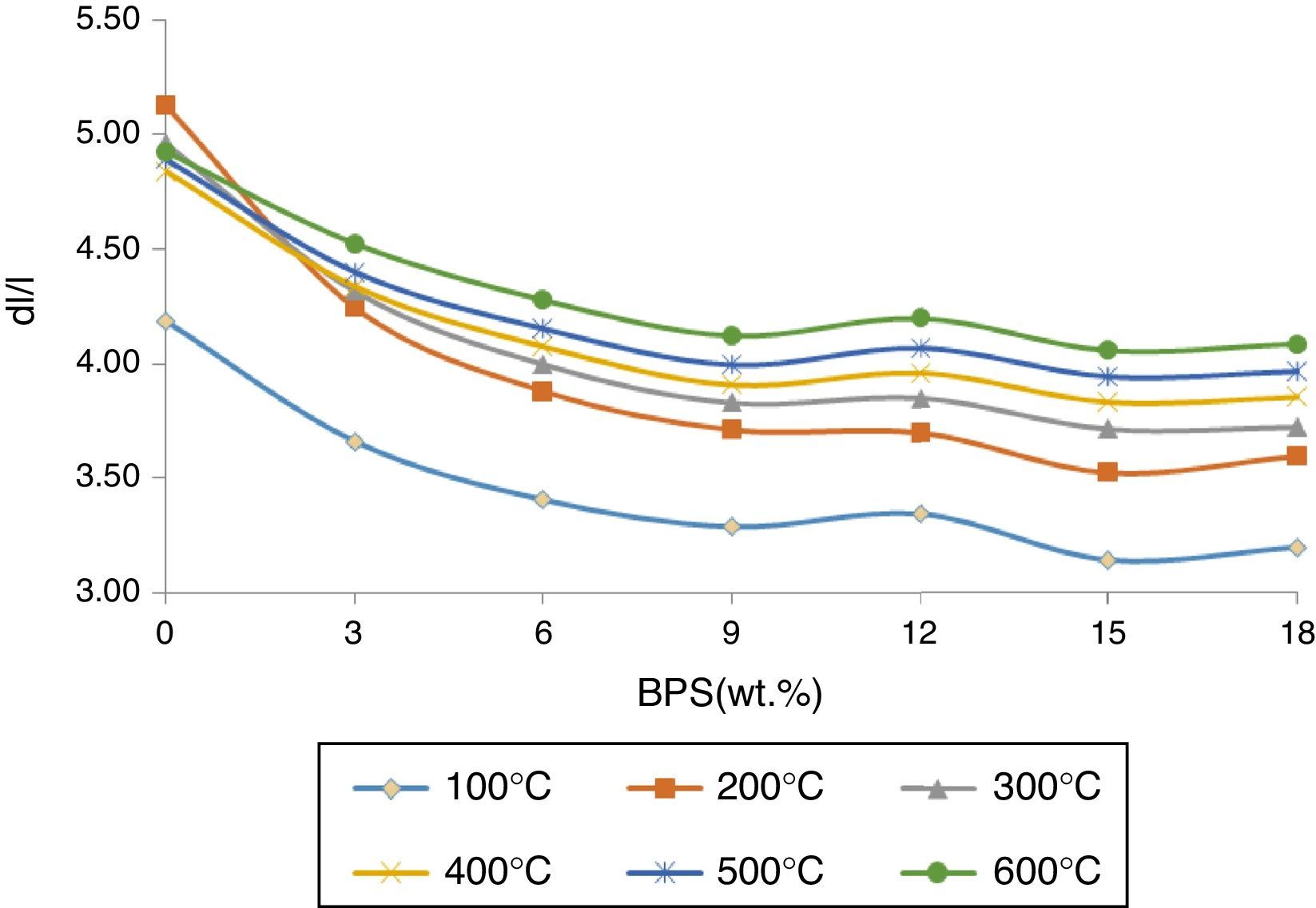
The changes in the thermal expansion coefficient α30–600 for bodies fired at 1340°C are shown in Fig. 10. At all temperatures, with the increase in the BPS, the thermal expansion coefficient has diminished. The reduction in the thermal expansion coefficient can be due to the reduction in the amount of crystal phase, which has a larger thermal expansion coefficient.
ConclusionApplication of bone porcelain scraps (BPS) in hard porcelain resulted in decreased firing temperature, green mechanical strength, and thermal expansion coefficient and increased glass phase, deformity, and firing shrinkage. The influence of the BSC on bulk density and mechanical strength depended on firing temperature. The BPS decreased bulk density of sintered body at 1340°C and increased the same properties of sintered body at 1250°C. The BPS increase the mechanical strength of sintered body at 1250°C several times. The increase in BPS up to 6wt.% leads to two-fold elevation of the strength of fired body at 1340°C. These results help us to choose the optimum point for adding the BSC to gain our purpose. This selection fully depends on firing temperature and the desired characteristics. Therefore, it is proposed that 6wt.% of BSC is used in the hard porcelain bodies in order to gain improvements in the mechanical strength of hard porcelain through application of the scraps without any undesirable change in other properties.
The author is very grateful to the Maghsoud Porcelain Company for equipment and laboratory support for this work. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.