A proper nutrition during the first two years of life is critical to reach the full potential of every human being. To the present day, this period is recognized as a critical window for promoting optimal growth, development, and good health. Therefore, adequate feeding at this stage of life has an impact on health, nutritional status, growth and development of children; not only in the short term but in the medium and long term. This paper provides recommendations on complementary feeding (CF) presented as questions or statements that are important for those who take care for children during this stage of life. For example: When to start complementary feedings; Exposure to potentially allergenic foods; Introduction of sweetened beverages; Use of artificial sweeteners and light products; Food introduction sequence; Food consistency changes according to neurological maturation; Number of days to test acceptance and tolerance to new foods; Amounts for each meal; Inadequate complementary feeding practices; Myths and realities of complementary feeding; Developmental milestones; Practice of Baby Led Weaning or vegetarianism.
La nutrición adecuada durante los primeros dos años de vida es fundamental para el desarrollo pleno del potencial de cada ser humano; actualmente se reconoce que este periodo es una ventana crítica para la promoción de un crecimiento y desarrollo óptimos y un buen estado de salud. Por tanto, cumplir con una alimentación adecuada en esta etapa de la vida tiene impacto sobre la salud, estado de nutrición, crecimiento y desarrollo de los niños; no sólo en el corto plazo, sino en el mediano y largo plazo. El presente trabajo ofrece recomendaciones de alimentación complementaria (AC) que se presentan en forma de preguntas o enunciados que consideran temas importantes para quienes atienden niños durante esta etapa de la vida; por ejemplo: inicio de la alimentación complementaria; exposición a alimentos potencialmente alergénicos; introducción de bebidas azucaradas; uso de edulcorantes artificiales y productos light; secuencia de introducción de alimentos; modificaciones de consistencia de alimentos de acuerdo a la maduración neurológica; número de días para probar aceptación y tolerancia a los alimentos nuevos; cantidades por cada tiempo de comida; prácticas inadecuadas de alimentación complementaria; mitos y realidades de la alimentación complementaria; hitos del desarrollo; práctica del Baby Led Weaning y del vegetarianismo.
A proper nutrition during the first two years of life is crucial for achieving the full potential of every human being. This stage is characterized by its rapid growth rate, which imposes higher energy and nutrients required, and gets infants who are exposed to faulty feeding practices into nutritional risk. It is now recognized that the period from birth to two years old is a critical window for promoting optimal growth and development, and good health.1 Longitudinal studies have consistently shown that this is the stage of more increased risk for growth and development deceleration, micronutrient deficiencies and common diseases such as diarrhea. In the long-term, early nutritional scarcities are related to poorer work capacity, intellectual performance, reproductive health, and overall health status during adolescence and adulthood. Inadequate breastfeeding and complementary feeding, along with the high prevalence of infectious diseases, are the leading cause of malnutrition in the early years of life. Also, it is now known that the caloric and the deficient or excessive consumption of some nutrients can influence early metabolic programming with long-term consequences, including on the onset of adult chronic disease.2
The United Nations International Children's Emergency Fund (UNICEF) has highlighted the crucial role of breastfeeding and complementary feeding as the preventive intervention with the most significant impact on child survival, growth and development.3 Complementary feeding (CF) is defined as the process that begins when breastfeeding is not enough for the nutritional requirements of the infant. Therefore, the introduction of new diet elements aside from breastfeeding is needed.
It is important to note that the cornerstone intervention for complementary feeding in any context is education and advice for caregivers on the use of locally available foods, in addition to considering the use of micronutrient supplementation or fortified foods when deficiencies are a common problem and regular food does not provide enough nutrients.4 This paper is the product of a consensus meeting for the discussion and development of complementary feeding recommendations by a multidisciplinary group of experts formed ad hoc, from different states and representatives of the leading pediatric and nutrition organizations.
The work is presented as questions or statements covering the most critical issues for caregivers of children during this stage of life. For example, the introduction to complementary foods; risk and benefits of introducing some types of food into the infant's diet; proper sequence of food introduction, among others.
Previously, research and review of the best evidence to answer such questions were conducted and discussed at the consensus meeting, which are presented in this document with the purpose to help child caregivers use the best recommendations for the CF.
2Criteria for starting complementary feeding: start at four or six months of age?There are numerous benefits of exclusive breastfeeding for the first six months for both the infant and the mother. Therefore, national and international institutions such as the World Health Organization (WHO), the American Academy of Pediatrics, the Mexican Association of Pediatrics, the National Confederation of Pediatrics in Mexico and the Ministry of Health in Mexico, among others, recommend exclusive breastfeeding for the first six months, and to start later with complementary feeding.
The recommendation to maintain exclusive breastfeeding for the first six months of age is supported because it promotes optimal growth and prevents comorbidities,5 influences cognitive development,6,7 and recent findings show that it participates in the early prevention of chronic diseases.
However, considering renal, immunological, gastrointestinal, and neurological development, institutions such as the European and North American Societies of Gastroenterology, Hepatology and Pediatric Nutrition (ESPGHAN, NASPGHAN) and the European Academy of Allergy and Clinical Immunology (EAACI) recommend initiating the introduction of complementary food between weeks 17 and 26.8
Regarding the prevention of chronic diseases, the effects of breastfeeding and its duration on overweight and obesity prevention are highlighted, although most evidence points toward a modest, protective effect. Furthermore, in the long-term, it depends on other variables related to family history of obesity and lifestyle of the children and adolescents.
Similarly, the higher risk of being overweight or obese due to the early exposure to complementary foods or the type of foods offered could be pointed out. The study by Burdette et al. in a population of the United States showed no differences in adiposity at five years old in exclusively breastfed children versus those not breastfed, or between the extent of the breastfeeding, or the introduction age of solids (before or after four months).9 Jonsdottir et al. randomly assigned to a group of 119 couples (mother-child) to practice exclusive breastfeeding for six months or four months before starting complementary feeding. They did not identify anthropometric differences in the follow-up (29-38 months) nor increased risk of overweight or obesity among groups.10
However, when complementary feeding starts before four months of age, it seems to have an adverse effect on children adiposity and an increased risk for overweight or obesity. Recently, Pearce et al. published the results of a systematic review to identify the association between the time of introduction of complementary foods and the risk for overweight or obesity in childhood. They concluded that the introduction of complementary foods before four months (versus after four months) might increase the percentage of fat mass and the risk of overweight in children.11
In the Netherlands, De Beer et al. evaluated data of body composition in 2,227 children (aged five to six years old) and recognized that breastfeeding and complementary feeding after six months (vs. <4 months) is associated with less fat mass (p <0.01).12
Daniels et al. completed a literature review (26 papers, mainly cohorts) to identify the association between the risk of obesity after twelve months of age and the time of inclusion to complementary feeding. They concluded that the introduction of solids before four months of age may increase the risk of obesity, but there is little evidence of a difference between introducing food between four and six months versus introducing food after six months.13
Another benefit of breastfeeding regarding the promotion of healthy eating habits was reported in the study of Lauzon-Guillain et al. (2013). Data from four European cohorts were analyzed and the consumption of fruits and vegetables in preschool children was evaluated. They identified a positive association between the extent of breastfeeding with the consumption of fruits and vegetables, regardless of the age of introduction of these foods and their intake in the maternal diet.14
Therefore, in our social and epidemiological context, the initiation of complementary feeding until six months of age should be recommended, as suggested by WHO, who encourages exclusive breastfeeding for six months.
3Exposure to potentially allergenic foodsIn the past, it was traditionally recommended to delay the introduction of potentially allergenic foods, considering the intestinal structural and functional immaturity and the increased permeability to proteins with the potential risk of sensitization. In recent years, this recommendation has been modified by the early introduction of these foods according to studies showing that the risk of sensitization and allergic response is the same, or even less, as a result of the emergence of immunological tolerance as of the fourth month of life.
The oral tolerance induction occurs between four and six months of age, and it is associated with colonization factors, genetic predisposition, exposure to breastfeeding and immunomodulatory agents such as proteins, lipids, fiber, and some vitamins.
Breastfeeding plays a fundamental role in the immune tolerance since the antigens in the maternal diet are processed by herself, releasing immunological components (IgA, IL-10, growth factors and antigens) in breast milk. For this reason, food restriction is not recommended as a preventive measure for the sensitization of the infant.15
Recently, the role of lipids as immunomodulators to induce food tolerance has been suggested. Among these lipids, long-chain polyunsaturated fatty acids (omega-3) and cholesterol stand out as allergic reactions protectants, while medium-chain triglycerides and omega-6 fatty acids promote sensitization and allergic reactions.15
Fibre, in turn, is fermented by microbiota to short-chain fatty acids, whose immune function has been studied showing that it favors the emergence of regulatory T cells (Treg) with intestinal (Peyer's patches) and lung activity, protecting against airway inflammation with reduced risk of respiratory allergies.15
Adequate consumption of vitamin A has also been associated with the regulation of the Th1 response over Th2, consequently favoring oral tolerance and less airway inflammation. Vitamin D induced a similar reaction and also appears to protect against atopic dermatitis.15
To take advantage of the window to induce tolerance, several studies have shown that the early introduction of these foods is advantageous. In a study with 1,612 children, who were followed-up from birth until five years of age, the incidence of wheat allergy was assessed based on the exposure to cereals. It was found that only 1% developed allergies, and an increased risk was present in those children with exposure after six months, even after controlling by family history.
The study of Koplin et al. (2010) evaluated egg introduction at four, six, 10, 12 and >12 months in 2,589 Australian infants and showed that the more delayed the introduction, the higher the risk of developing an allergy. Moreover, denaturation of proteins by the cooking method affects the allergenic risk; those exposed to cooked egg showed a lower risk than those who received it in baked products between four and six months of age.16
The United Kingdom study LEAP (Learning Early About Peanut Allergy) evaluated the effect of early exposure to peanuts (before eleven months old) and allergy in high-risk children, finding that the frequency of cases of allergy to this food decreases when introduced early.17
In the PATCH cohort (Prediction of Allergy in Taiwanese Children) exclusive breastfeeding for at least four months compared to partial breastfeeding or the introduction of other foods before four months of age significantly decreased the risk of cow's milk proteins sensitization at two years of age (OR=0.2, 95% CI 0.07-0.5). Also, children who were exclusively breastfed showed lower eosinophil count in peripheral blood during monitoring, and lower levels of total IgE in plasma during the first three years of life, although the difference was not significant.18
A systematic review of 74 studies noted that a delay in the introduction of solids beyond four months of age does not confer benefits in preventing allergies, regardless of a high-risk population. This review was critical to the development of prevention guidelines for the EEACI (European Academy of Allergy and Clinical Immunology) that favor the introduction of solids after four months of age.19,20
Based on the aforementioned and recent studies, it is resolved that the initiation of potentially allergenic foods should not be delayed. Furthermore, it is recommended to start its introduction since six months of age according to the cultural and social context.
4Gluten exposure and breastfeeding, mixed breastfeeding or with breast milk substitutesThe Nutrition Committee of the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) recommended avoiding early introduction (before four months) or delayed (after seven months) of gluten to reduce the risk of developing allergies and autoimmune diseases such as diabetes or celiac disease. This recommendation was based on previous, predominantly observational studies. However, recently published randomized trials suggest that this association does not exist.
In 2012, De Palma et al. suggested that infants with risk factors for celiac disease had different microbiota composition, with more extensive colonization of Bacteroides fragilis and Staphylococcus spp, and to a lesser extent of Bifidobacterium spp and and Bifidobacterium longum. However, breastfeeding favored the presence of Clostridium leptum (unusual in formula-fed infants), reducing the difference in the microbiota composition.21
Recently, Konincks et al. (2015) consistently concluded that the extent of exclusive breastfeeding or the time of complementary foods introduction is not associated with the development of the celiac disease. Moreover, in children with genetic risk, early or late introduction does not modify the risk of presentation. Researchers recommend gluten introduction from five to six months of age.22
Another study which confirms these findings is the randomized trial of 944 at-risk children (HLA-DQ2 or DQ8 positive and with at least one relative with celiac disease), who received 100mg of gluten or placebo between 16 and 24 weeks of age. Antibodies were periodically measured and the presence or absence of celiac disease with a biopsy at three years of age was confirmed. They concluded that the introduction of small amounts of gluten did not reduce the risk of celiac disease at three years of age.23
Similarly, Szajewska et al. recently presented a systematic review (ESPGHAN, Amsterdam 2015), showing that the development of celiac disease in childhood is not affected by breastfeeding or the age of gluten introduction.24
Moreover, Lionetti E. et al. noted that the loss of gluten tolerance is a dynamic process in which neither breastfeeding nor the delayed introduction can protect against celiac disease.25
Therefore, it can be stated that the maintenance of exclusive breastfeeding and the introduction of gluten in the window of opportunity—from four to seven months of age—do not protect nor increase the risk of celiac disease. However, gluten introduction is not recommended before four or after seven months of life.
5Introduction of sugary drinksIt is known that the taste for sweet flavors is innate. At six months of age, breastfed children prefer sweet, as well as salty and umami flavors.26 This preference may increase due to their exposition, as it is associated with endorphins and dopamine release.27 Therefore, the early introduction of sugary drinks can have addictive effects on the sweet taste preference through dopamine signaling on hypothalamic structures (nucleus accumbens, amygdala and lateral hypothalamus) together with other neurotransmitter systems such as glutamate28 for reward pursuit (pleasure).
Some studies suggest that the energy intake of drinks has no effect (or very little) on satiety compared with solids. Therefore, it has been pointed out that they induce a lack of compensatory dietary response; in other words, the energy input is not detected, and no further appetite regulation adjustments are made.29
According to the analysis of Pan et al. in the FPS II (Feeding Practices Study) cohort, sweetened beverage consumption during the first year of life doubles the risk of obesity at age six. Those children who ingested sugary drinks before six months presented a 92% higher risk of obesity than non-consumers. However, there was no difference in the risk of developing overweight or obesity when compared to children who drank these beverages after six months.30
In the Project Viva at Harvard, 1,163 participants were studied evaluating the association of the consumption of fruit juice (natural) and water at twelve months of age with the consumption of juices, sugary drinks and body mass index at three and seven years old. They reported that the consumption of 8-15 oz/day or more than 16 oz/day of fruit juice was associated with increased intake of juice, sugary drinks, higher BMI, and increased adiposity during the follow-up. They concluded that the early consumption of fruit juices can trigger deeper intake of sugary beverages in later years, increasing the risk of overweight and obesity.
The WHO guidelines on children and adults sugar ingestion point out that simple sugars should not exceed 5% of the total diet energy intake since they can promote a positive energy balance, beyond what is necessary for children. Following this recommendation, sugar intake between six and 24 months of age should not exceed 30 to 45kcal.
This consensus does not recommend the introduction of sugary drinks of any kind in children younger than 24 months old.
6Use of artificial sweeteners and light productsA study showed that rodents exposed to acesulfame-K in utero or through breastfeeding showed a more significant preference for the consumption of sweeteners (caloric and noncaloric). In an animal model exposed to breast milk and acesulfame-K, a more significant dose-dependent preference for sweet flavors was observed in adult life. Furthermore, alterations were identified in the expression of the leptin receptor, suggesting that there may be a role of this hormone in the development of the sweet taste preference.31 Other data indicate alterations in metabolic programming in animals exposed to aspartame during pregnancy.
It is considered that non-caloric sweeteners ingestion during breastfeeding is safe. The LactMed database mentions that sweeteners may not have adverse effects in infants; however, the content of these in breast milk is not sufficiently demonstrated. Sylvetsky et al. showed that breast milk of women contained acesulfame-K 20, saccharin and sucralose, even in women who reported no intake. The effects of this exposure are unknown, but it is suggested that they can affect microbiota, the preference for sweet tastes, and perhaps promote metabolic abnormalities and obesity.32
Sweet taste is an oral stimulus that works as a predictor of energy intake and active hormonal, neurological and metabolic pathways. The use of sweeteners can reduce this response, which favors changes in these pathways, probably increasing the risk of metabolic disorders. There is no scientific evidence for its use in children under two years old. Therefore, this consensus discourages their intake in children under 24 months of age, considering that there is no need for sugary drinks consumption neither for non-caloric sweeteners.
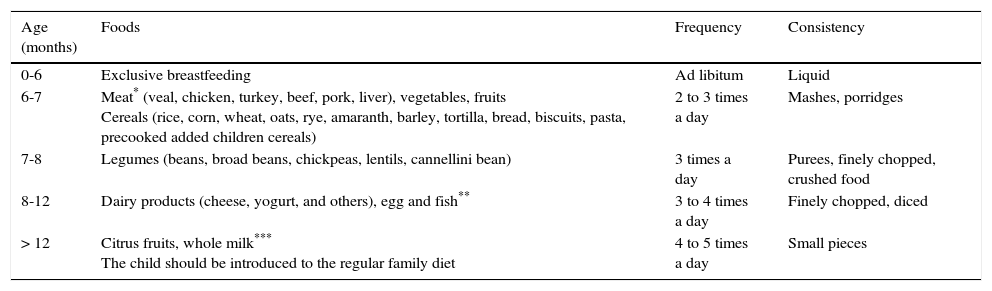
7Can a food introduction sequence be recommended?In Mexico, recommendations on what kind of foods to incorporate during complementary feeding have changed over the years. Thus, the recommended weaning pattern by the Weaning Mexican Consensus in 2007 was the introduction of fruits, vegetables, cereals, legumes and meats,33 similar to the Mexican Official Norm 043 from 2005.34 On the contrary, the update of this norm encourages the introduction of meat, vegetables, fruits and iron-fortified cereals at six to seven months of age, legumes from seven to eight months, dairy products (cheese, yogurt, and others) from eight to 12 months, egg and fish from eight to 12 months, and citrus fruit and whole milk after 12 months of age (Table 1).35
Supplementary feeding scheme.35
| Age (months) | Foods | Frequency | Consistency |
|---|---|---|---|
| 0-6 | Exclusive breastfeeding | Ad libitum | Liquid |
| 6-7 | Meat* (veal, chicken, turkey, beef, pork, liver), vegetables, fruits Cereals (rice, corn, wheat, oats, rye, amaranth, barley, tortilla, bread, biscuits, pasta, precooked added children cereals) | 2 to 3 times a day | Mashes, porridges |
| 7-8 | Legumes (beans, broad beans, chickpeas, lentils, cannellini bean) | 3 times a day | Purees, finely chopped, crushed food |
| 8-12 | Dairy products (cheese, yogurt, and others), egg and fish** | 3 to 4 times a day | Finely chopped, diced |
| > 12 | Citrus fruits, whole milk*** The child should be introduced to the regular family diet | 4 to 5 times a day | Small pieces |
According to a systematic review published by Pearce et al. (2013), the introduction of complementary foods into the infant's diet can be considered according to macronutrient intake, type or food group, or based on dietary guidelines.36
Regarding the breastfed infant, it is considered that children older than six months require extra energy and critical nutrients supply to continue an optimal growth and development and to reduce the risk of slowing growth or specific nutritional deficiencies. Early introduction of animal products has been emphasized in recent years since they are a suitable source of micronutrients such as iron and zinc, plus high biological value proteins, which decrease the risk of these disorders.
Olaya et al. (2013)37 conducted a study in Colombia with 85 term breastfed infants, which were assigned to two groups. In the intervention group, red meat was recommended more than three times a week (≥ 5 servings per week), and fruits and vegetables were recommended daily. The control group received only general recommendations. At 12 months of age, the intervention group consumed more meat (5.4 vs 3.5 days/week, p <0.001) and had better hemoglobin (0.41 vs -0.13, p=0.01), and hematocrit (1.04 vs -0.15; p=0.03) values, although no differences in serum ferritin and zinc were identified.
In another controlled clinical trial conducted in China, with 1,471 infants between six and eight months old, a meat-based mash was offered up to 12 months of age in the intervention group, and iron-fortified cereal in the control group. During the follow-up, linear growth was moderately higher in the meat-supplemented group and showed less length decrease for age (Z score) over time.38
Data from Tang et al. (2014)39 strengthened the recommendation about introducing meat as the first food. These authors assessed the growth effect of meat protein as a complementary food for breastfed infants in Denver, Colorado. The higher protein intake was associated with better linear and ponderal growth without adiposity excess, suggesting that the potential risk associated with a high protein intake may differ in infants breastfed from those fed with milk formula. Some reports have found an association between high protein intake in the period of two to 12 months of age and body mass index (BMI) or body fat in childhood, similar to the high-energy intake.
Regarding the introduction of food groups, FITS (Feeding Infants and Toddlers Study) identified dietary patterns that provide an excessive supply of energy, such as using a bottle, a deficient intake of fruits and vegetables, and excessive consumption of sweet foods, sugary drinks, and saturated fat. In Mexico, the analysis of the National Health and Nutrition Survey (2012) revealed a low intake of fruits, vegetables, and iron source food in infants. Only 18% of children between six and 12 months of age reported consuming meats and 4%, iron-fortified cereals. However, 42% stated soft drink consumption at 12 months, a figure that increased to 63% at 24 months of age.40 These eating habits have been reported in other countries like Australia, where Amezdroz et al. observed not recommended food choices, such as soft drinks, cookies, sweet bread, granola bars and canned meat. Although Australian recommendations are one of the most updated and complete guides, a high percentage of parents do not follow them.41
In addition to adherence to complementary feeding recommendations, the authors recognize the importance of parent's education prior to their children's birth, breastfeeding attachment, focusing on hunger and satiety signs, right food choices, and selection of the appropriate portion size to prevent an early overfeeding.42
Recently, the formation of intestinal microbiota in the early stages and the impact of a different diet on it have been studied. A study in Kenya with 115 infants who consumed foods with added iron showed that supplementation with this metal was associated with increased pathogen and elevation of inflammatory markers.43
Salvini et al.44 performed a double-blind controlled trial in 20 randomized infants who were fed with a formula containing a mixture of prebiotics, or formula with maltodextrin as placebo. The group receiving prebiotics resulted in a higher amount of bifidobacteria (p <0.0001) and lactobacilli (p=0.004). These differences were maintained during the second half of the first year of supplementation. Therefore, they concluded that early colonization of the intestine might have effects on the composition of the intestinal microbiota in the long-term.
In 2006, Scholtens et al. showed the same effects with the addition of prebiotics (fructo oligosaccharides and galacto oligosaccharides FOS/GOS) complementary foods for six weeks in infants between four and six months old.45
As a recommendation, the selection of foods to offer to the infant when the intake of complementary foods begins can be analyzed in two ways: by specific nutrients for age (iron, zinc, essential amino acids, fatty acids, long chain) or by the type or food group. Regardless of the kind of food to offer, it is important to introduce foods with adequate nutrients for the child’s needs when starting complementary feeding. No scientific evidence exists on the benefits of the introduction of complementary foods at any specific sequence. However, the data presented confirm that, in addition to extra energy, the source of iron and zinc foods such as meat and grains are the best choice to start this process. It is also recommended that the first solid foods are individual, boiled, without salt, sweeteners, flavors or preservatives. No controlled studies have addressed some practical aspects of the introduction of complementary foods. However, the introduction of one food at a time at intervals of two to three days with observation suggests tolerance and acceptance. Emphasis should be placed on the importance of the combination of food groups that national and international guidelines recommend. With the introduction of one food at a time and the integration of the different food groups, it is considered that in a short time an infant will be consuming the primary recommended food groups (meat, cereals, vegetables, fruits, and legumes). Thus, at seven months of age, the infant will consume three foods from each group, which will provide enough nutrients in combination with the human milk.
8Does a recommendation on what food to offer according to each diet (human, mixed or formula milk) exist?It has been observed that feeding practices in infants fed human milk and infant formula are different. For example, a cross-sectional study conducted in the UK examined the characteristics of infants and their mothers, which were associated with the introduction of solids. They included 756 infants between six and 12 months old and an application or a questionnaire for mothers to know the main reason why they decided to introduce solids to the diet of their children. Breastfeeding was associated with a perception of hunger in their children or the need to give something more than their milk, unlike children fed formula milk.46
The World Health Organization (WHO) and the Pan American Health Organization (PAHO) have specific recommendations depending on the type of food supply of the infant during the first six months. Recommendations for non-breastfed children between six and 24 months are very similar to the feeding guides for breastfed infants, but in some cases they require some adaptation. The document refers to those infants fed whole cow's milk (or from other mammals), evaporated, or fermented milk. It notes that the amount of milk the infant should have depends on the type complementary foods he will receive. The article emphasizes the inclusion of animal source foods (ASF) as a source of key nutrients, such as iron and zinc, as well as fat. Thus, an infant who receives a daily intake of ASF should consume between 200 and 400ml/day of milk. In contrast, an infant who does not eat ASF, would require 300 to 500ml/day. In the case where the intake of ASF is not regular, the infant will require fortified products or supplemental nutrients. Accordingly, a specific recommendation is made regarding the consumption of plain water in children who are not breastfed: in temperate climates, children require 400 to 600ml/day of an additional liquid and on warm climates, from 800 to 1,200ml/day. This is due to the higher load of renal solutes associated with whole cow's milk consumption or other products, and an increased water consumption associated with breastfeeding.47
In breastfed infants, the energy density of foods and some meals will depend on the intake amount of breast milk (low-mixed or average). Thus, in children with poor appetite and insufficient breastfeeding, porridge should have a higher energy density, and conversely, in children with a good appetite, it is necessary to balance the intake with porridges with low and high density.1,48
Breastfeeding is strongly recommended. It is clear that the biological elements contained in breast milk are not found in formula milk or other products, which is a disadvantage for the infants feeding of the latter. Besides, it can be assumed that the process to introduce complementary feeding will have an increased risk of rejection of certain foods and flavors in non-breastfed children. Breastfed children accept more food because of the taste of the milk changes according to the mother's alimentation. Thus, this panel of experts recommends that the introduction of complementary food in infants fed milk formula should be monitored regarding acceptance and tolerance towards new foods. It is vital that dietary recommendations start from pregnancy. Another suggestion is to offer information and skill's development to the mother to improve her alimentation.
Due to the low content of iron in human milk, there are recommendations to give supplementary iron to breastfed children, starting at about six months old, and to following recommendations regarding an early introduction of foods rich in this mineral.49,50
Although combined feeding (breast milk and formula milk) is the most common in our country, recommendations on complementary feeding for these specific cases are rare. It will depend on whether which feeding is predominant, breastfeeding or formula. It should be considered if children have a complete supply of nutrients until six months old. It is prudent to assess each infant regarding chronological, anthropometric and neurological development parameters. The WHO document emphasizes the consideration of the energetic density of complementary foods depending on the intake of breastmilk and the infant's appetite.
The protein intake consumed from formula and complementary foods must be monitored since evidence shows that excess protein by the formula can cause increased adiposity in infants.39 Additionally, it must be recommended to give milk formula with added long chain fatty acids. As mentioned above, in the case of breastfed children, the amount of these fatty acids depends on the alimentation of the mother, which should be monitored as well.
9Modifications of food consistency according to neurological developmentThe official standard NOM-043-SSA2-2012 recommends that food textures should be gradually changed, from liquid to pureed, mashed, minced, and diced.35 The WHO also recommends to increase the consistency of food as the child grows, and specifies that at six months of age infants can eat pureed and mashed food, and at eight months old they can eat food which they can grab with their fingers. At twelve months old they can have the same type of food as the rest of the family. In addition, avoiding foods that may cause choking is also suggested.51
Butte et al. as well as Pardío-López et al.52,53 describe the feeding skills that infants acquire while growing and developing. Thus, infants establish a pattern of suck-swallow-breath, and their tongue moves forward and backward. When they sit with assistance, they may start pushing food out of their mouth as a reflex, which gradually disappears to swallow food. They also recognize the spoon and open their mouths when it is near. When the infant feeds by himself, it can keep thick purees in its mouth, pass the food from one hand to another and drink from a cup with the help of someone else. As they begin to crawl, they will also start to move the tongue from side to side to grind food, to use the jaw and tongue to chewing, to drink from a cup without assistance and to hold small items between their thumb and forefinger. As they begin to walk with assistance, they can begin to drink with a straw, to feed easily using their fingers, to hold the cup with both hands, and they will be more skilled to chew, for which they will demand to feed by themselves. As they start to walk independently, their chewing and swallowing skills are sufficiently developed. Thus, they can start to eat with a fork and spoon.
It is important to mention that neuromuscular development depends partly on imitation. Therefore, the feeding habits of the family have a significant influence. It is desirable that during the food introduction, the child observes how the family is fed and the process in which food is chewed and swallowed.48
New techniques for supplementary feeding have recently arisen, and they break the scheme described above. The baby-led weaning (BLW) is an alternative to the introduction of complementary foods that emphasizes self-feeding instead of spoon-feeding by caregivers, offering food pieces instead.54
In the UK, Townsend and Pitchford (2012) analyzed dietary preferences, exposure to certain foods and weaning technique used. They found that the BLW impacts on food preferences. BLW-fed children learn to regulate food intake in a better way, which is associated with lower BMI and to prefer healthy foods such as complex carbohydrates. The authors conclude that these practices may be relevant as a way to combat obesity in contemporary societies.55
A cross-sectional study in New Zealand families examined feeding practices with technical BLW and the traditional method. The authors of this study gathered 199 mothers, whom mostly introduced food in the form of purees. Only 21% provided food that the child could take with his fingers (BLW), which was the only way of providing food in 8%. Different links between the feeding method (BLW) and health behaviors were found, such as to give breastmilk exclusively and start complementary feeding until six months old, eating the same food as the rest of the family since the beginning of the food introduction, and to consuming less commercially prepared foods. However, it was alarming to find that these children were not provided cereal fortified with iron in the first meals.56
Afterwards, a modification of the BLW was proposed in a pilot study, where it was compared with the baby-led introduction to solids (BLISS). This is an approach to self-feeding that encourages the consumption of iron and energetic food without the risk of choking. It was noted that BLISS was accepted and applied by parents in this pilot study. This approach resulted in an increased consumption of foods containing iron and fewer children with risk of choking, comparing with foods that babies are offered at six months in the BLW. Although no statistically significant differences were identified in the amount of iron in complementary foods by BLISS (4.9mg/day) and BLW (2.2mg/day) in participants who completed dietary records, it should be considered that the sample size of the study was small. Interestingly, it was pointed out that the BLISS group offered a more considerable amount of red meat (20.1g/day) than the BLW group (3.2g/day) (p=0.014).57
It has been shown that there is a window of opportunity to introduce a variety of flavors, smells, textures, and meals in this period of life. To achieve this in the first two years of life can lead to the acceptance of a wide variety of foods later on, which highlights the importance of a varied diet from the very start, setting schedules and eating patterns. The appearance of teeth is not decisive for modifying the consistency of the food in early stages. However, later on, they are required to cut and grind more complex foods. The neurological development and maturity has a key role in advancing the consistency of food, which should be adapted based on the needs dictated by the skills acquired. National and international recommendations agree that textures offered by complementary feeding should be changed gradually. The stages of skill development (neurological maturation, consistency of food) depend on the neuromuscular training performed with the infant, as well as the use of appropriate tools. The decision of when to change texture depends on the maturation and development of the children and the stimulation for the gaining of skills.
It is of great importance to “train” the brain to receive different kinds of textures continuously. One of the main reasons why studies have been published proposing new trends in food consistency is the problem in accepting thick textures in early stages of complementary feeding.
New approaches, such as the BLW and BLISS, have been shown to be advantageous in the regulation of infant feeding, independence in feeding and stimulation for neurological maturation. However, this panel recommends that the use of these techniques must be evaluated individually, depending on the neurological maturation of each infant, as well as to monitor the type of food and nutrient being granted (energy, protein, and iron). It is suggested that parents or guardians must know how to act in the case of choking or suffocation.
As suggested by Butte et al., the preparation and acceptance of different textures of food seem to depend on the stage of development and experience before a texture in particular: infants learn to eat foods if they are exposed to them in the right stages of development. It is prudent to guide the child to consume foods with solid texture in a progressive way (from the introduction of complementary foods until around ten months of life), which may decrease the risk of rejection, choke or vomit.52
10How many days to test acceptance and tolerance to new foods?It is recommended to counting intervals from 2 to 7 days to assess the acceptance and tolerance to a new food in the infant's diet.52 According to tolerance (biological factor) and acceptability (hedonic factor), the introduction of another new food should continue. In practical terms, this period could be reduced to 2-3 days.
11How much for each meal?At the beginning of complementary feeding, the duration of meal times is determined by different factors. Feeding actions will be more efficient over time, as well as the motor skills of the child and infant interaction with the caregiver. At the start of complementary feeding, the duration limits can be 5 to 15minutes.58 Afterwards, an appropriate length for food consumption is 20-30minutes. However, this can be influenced by specific child behaviors, capabilities, and other variables.59
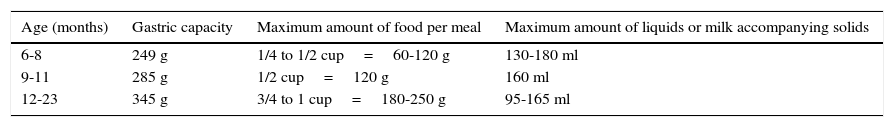
11.1Progressive increase in the quantities of food: portion sizesTo calculate the food portions that an infant can consume, the gastric capacity can be considered as 30g/kg of the reference weight. Table 2 shows data on gastric capacity and the maximum amount of food and fluids per meal.
Maximum amount of food and liquids recommended for each meal.1
| Age (months) | Gastric capacity | Maximum amount of food per meal | Maximum amount of liquids or milk accompanying solids |
|---|---|---|---|
| 6-8 | 249 g | 1/4 to 1/2 cup=60-120 g | 130-180 ml |
| 9-11 | 285 g | 1/2 cup=120 g | 160 ml |
| 12-23 | 345 g | 3/4 to 1 cup=180-250 g | 95-165 ml |
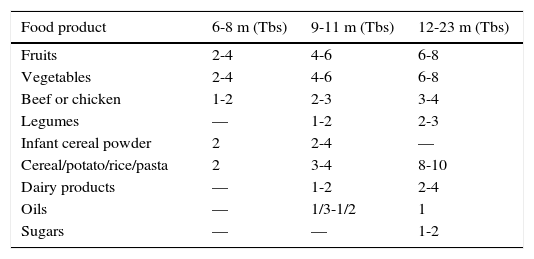
It has been reported that ingestion of solid food increases 30g approximately during the first week to 80g in the first month of complementary feeding, and 120g, six weeks from the start.58Table 3 shows the approximate amount of food servings per group according to age.
Portions increasing per group of food according to age range (months).37,51
| Food product | 6-8 m (Tbs) | 9-11 m (Tbs) | 12-23 m (Tbs) |
|---|---|---|---|
| Fruits | 2-4 | 4-6 | 6-8 |
| Vegetables | 2-4 | 4-6 | 6-8 |
| Beef or chicken | 1-2 | 2-3 | 3-4 |
| Legumes | — | 1-2 | 2-3 |
| Infant cereal powder | 2 | 2-4 | — |
| Cereal/potato/rice/pasta | 2 | 3-4 | 8-10 |
| Dairy products | — | 1-2 | 2-4 |
| Oils | — | 1/3-1/2 | 1 |
| Sugars | — | — | 1-2 |
Tbs, 1 tablespoon=15g of fruit or vegetables; 7-9g of legumes; 8-10g of powdered infant cereal; 9-10g of rice, noodles, potato; 5g of meat or poultry; 10-15g dairy products;10-15g of oil or sugar.
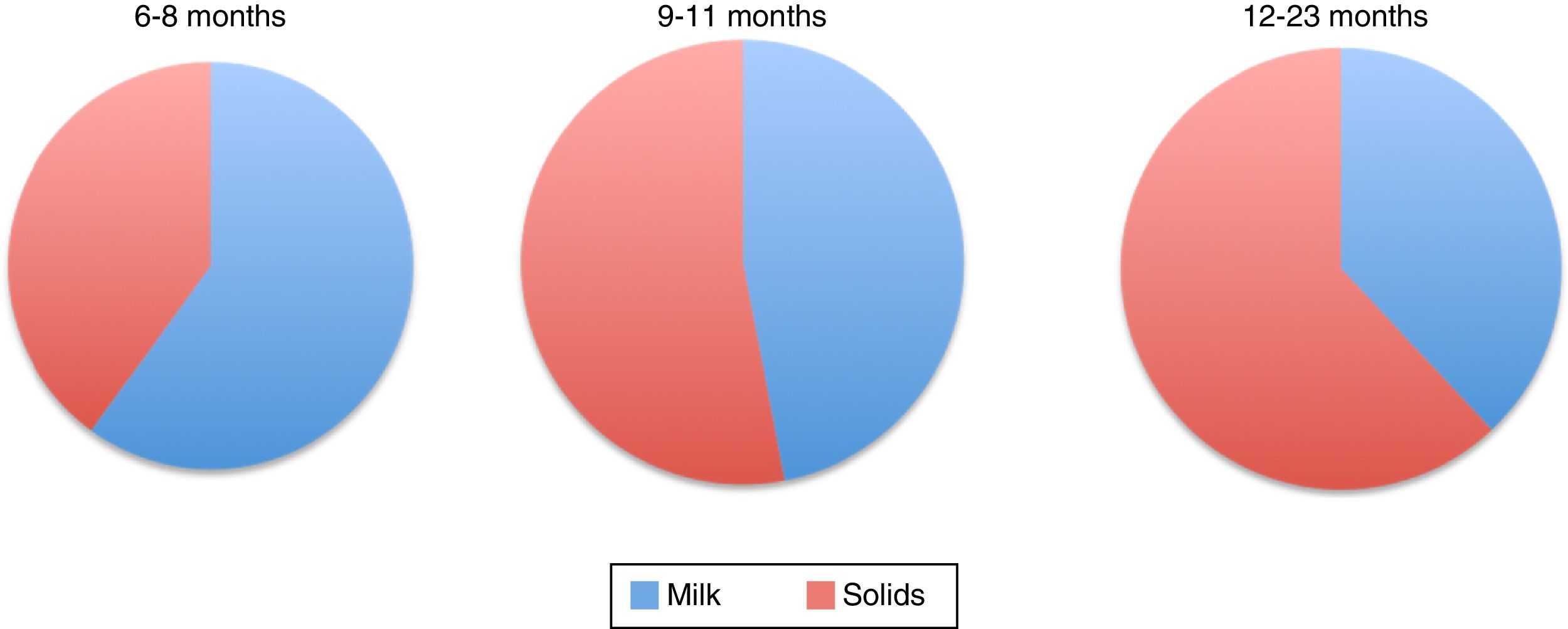
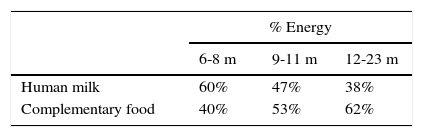
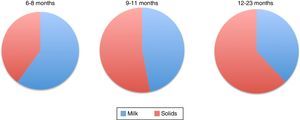
Figure 1 shows the percentage of total energy consumption to be covered by breastmilk or formula and the portion covered by complementary foods starting from six months. As it can be noted, decreasing energy input by breast milk increases the energy input of complementary food (Table 4).1,51
Energy consumption distribution of by breastfeeding and complementary feeding in infants.1
Percentage of energy consumption by breast milk/formula and complementary foods for infants of different ages.1
| % Energy | |||
|---|---|---|---|
| 6-8 m | 9-11 m | 12-23 m | |
| Human milk | 60% | 47% | 38% |
| Complementary food | 40% | 53% | 62% |
| 6-8 m | 9-11 m | 12-23 m | |
|---|---|---|---|
| Infant formula | 50% | 44% | 39% |
| Complementary food | 50% | 56% | 61% |
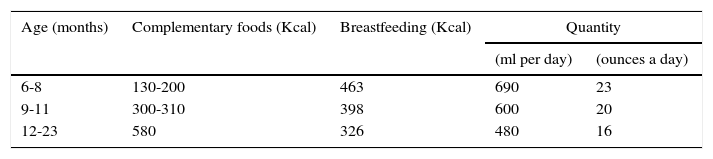
Table 5 shows the amount of energy in food supplements according to age. Gradual incorporation of meal times is recommended beginning from six months; 2-3 times between six and eight months; 3-4 between nine and eleven months and 4-5 between twelve and 23 months of age.60 In addition, it is recommended that the energy density of complementary foods is at least 0.8kcal/g, and up to 1.2kcal/g in healthy infants.
Recommendations for daily energy consumption by complementary foods and milk feeding.60
| Age (months) | Complementary foods (Kcal) | Breastfeeding (Kcal) | Quantity | |
|---|---|---|---|---|
| (ml per day) | (ounces a day) | |||
| 6-8 | 130-200 | 463 | 690 | 23 |
| 9-11 | 300-310 | 398 | 600 | 20 |
| 12-23 | 580 | 326 | 480 | 16 |
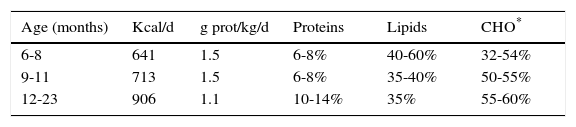
Table 6 shows the recommendations for total energy intake (average recommendation for children) and protein by age range, as well as the distribution of macronutrients as a percentage of the total energy consumption.
12Inadequate complementary feeding practicesEarly complementary feeding introduction. Early introduction of complementary foods is considered when it starts before week 17 of life.4,8 Prior to this age, the immaturity of different systems, such as gastrointestinal, renal, neuromuscular and immune systems, can cause health risks in the short- and the long-term. Short-term risks are the interference of exclusive breastfeeding; an increase in gastrointestinal infections caused by the reduction of the protective effect of human milk5 and by the introduction of contaminated food; a decrease in growth and malnutrition due to a decreased consumption (volume) of breast milk and formula with an introduction of poor nutritional density food;5 an increased risk of atopic dermatitis, wheezing or allergy to milk cow protein;61,62 an increased risk of iron deficiency and anemia, and zinc deficiency due to a decreased consumption of human milk and formula not covering the Recommended Dietary Allowances (RDA) by age; an introduction of food that are not suitable sources of these nutrients; an increase of respiratory diseases; possible renal damage caused by an increase in the solute load. Long-term risks are an increased adiposity;63 a predisposition to develop hypertension and obesity;64 the modification of healthy food preferences and future nutrition of the individual; the development of unhealthy eating habits; and food allergies.63
It is important to emphasize that Mexico presents an alarming percentage (10%) of early consumption (< 6 months) of sweetened drinks and other sweet foods, when none of these products should be included as part of a healthy diet. The percentage of the Mexican population consuming these foods increases rapidly at four years old when 90% consume sweet products, and 78% consume sugary drinks40. This preference for sweet foods and drinks that develops at an early age is a risk factor for the development of obesity.
Late complementary feeding introduction. It is considered when complementary feeding is introduced after week 26 of life,4,8 which can have negative effects, such as a decrease in the growth rate and malnutrition since exclusive breastfeeding does not satisfy the requirements of energy and protein after six months old; iron and zinc deficiency and anemia due to the late introduction of foods such as red meat and fortified cereals, which are sources of these minerals;8,65 eating disorders in infants, such as rejection of solid food, vomit caused by aversion to food and apparent choking.66,67
Inadequate practices and risk of anemia. Anemia remains as a public health problem in Mexico. The National Health and Nutrition Survey (2012) reported that 23.3% of children under five years old have anemia. In children under one-year old, 38.3% of all cases of anemia are associated with iron deficiency.68 It is considered that the most common causes are a decrease in the practice of breastfeeding and a reduced intake of iron-rich foods when starting complementary feeding. Foods that should be introduced to avoid and treat anemia are red meat,69 fortified cereals,70 and plant foods (Table 7). Between six and 12 months old, fortified cereals can contribute up to 30% of the iron according to the RDA.71 Fortified cereals without added sweeteners are suggested to cover these needs.70
Foods containing heme iron, non-heme iron, and vitamin C.
| Heme-iron sources | Non-heme Iron sources | Vitamin C sources |
|---|---|---|
| Red meat, black pudding, liver, organ meats, fish | Legumes: beans, soy beans, lentils, chickpeas Vegetables: spinach, chard, quality, purslane, huazontle, chaya, tomatillo, poblano, zucchini, Brussels sprouts, rosemary, cabbage, watercress, fungi Oilseeds: Almonds, sunflower seeds, nuts Fortified infant cereals Iron-fortified flour products made with whole grains base | Sweet peppers, broccoli, Brussels, cauliflower, cantaloupe, guava, blackcurrant, kiwi, strawberry, citrus fruit, mango, red tomato |
Iron bioavailability in animal foods is better than those of plant origin due to their heme iron content. To improve absorption of non-heme, it is suggested to accompanying them with foods rich in vitamin C (Table 7).48
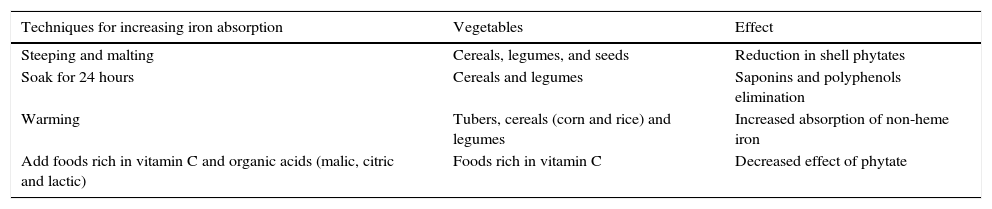
Preparation techniques of plant foods that enhance the bioavailability of non-heme iron are presented in Table 8.
Food preparation techniques to improve non-heme iron bioavailability.
| Techniques for increasing iron absorption | Vegetables | Effect |
|---|---|---|
| Steeping and malting | Cereals, legumes, and seeds | Reduction in shell phytates |
| Soak for 24 hours | Cereals and legumes | Saponins and polyphenols elimination |
| Warming | Tubers, cereals (corn and rice) and legumes | Increased absorption of non-heme iron |
| Add foods rich in vitamin C and organic acids (malic, citric and lactic) | Foods rich in vitamin C | Decreased effect of phytate |
Inadequate salt intake. It is recommended to include iodized salt in the diet since the first year of life.72 The suggested amount is 1500mg daily, which covers iodine RDA (65μg/day).71 This is the recommended amount since the first year of life and until adulthood.73 Daily-consumption of other non-iodized salts such as “light” salt or gourmet salts are not recommended.
Inadequate practices of complementary feeding which cause zinc deficiency. In Mexico, the prevalence of zinc deficiency in children under five years is 32%.40 The introduction of unfortified cereals along with a late introduction of red meat is one of the causes of zinc deficiency.69 A portion of infant fortified cereal (15g) provides 42% of zinc RDA.71 Another food that can be included at the beginning of the complementary feeding is the tortilla dough, which contains zinc. A piece of tortilla provides 10% of zinc RDA74,75 and it should be accompanied by food such as red meat or fortified cereals.
Intake of sugary beverages. The FITS study in Mexico40 shows excessive consumption of sugary drinks since an early age; 8% of children under five months of age already consume sweetened drinks. This percentage increases quickly to reach 78% at three years old. It has been shown that consumption of these beverages increases the risk of obesity.76
The recommendation is not to promote the consumption of juices and sugary drinks before two years old. Sweeteners that are common in Mexican cultures, like brown sugar, syrups, honey, corn and maple syrups, should not be offered either. The use of these sugars increases the energy content of drinks and favors the sweet taste creating a bad habit from childhood to adulthood.1
Due to the high consumption of sweetened beverages, the consumption of milk and food with better nutritional quality has decreased, which has resulted in a decreased intake of calcium and vitamin D. Most sweetened beverages available on the market contain corn syrup (fructose) as the primary sweetener. Fructose absorption is slower and may be associated with diarrhea caused by malabsorption,77 which increases the risk of growth deceleration. Furthermore, fructose consumption is associated with insulin resistance, hyperinsulinemia, hyperleptinemia, and dyslipidemia. All these alterations promote obesity and the risk of developing type 2 diabetes mellitus, nonalcoholic fatty liver disease or metabolic syndrome in the long term.78,79
12.1Other inadequate complementary feeding practicesWrong use of the baby bottle. The baby bottle should only be used in the absence of breastfeeding and should be promoted exclusively as a container for formula milk and not for other beverages. It should be gradually withdrawn since the seventh month of life, in an inverse relation to solid intake. It must be annihilated at one year of age to encourage the use of a conventional glass.
Inadequate food consistencies and textures according to age. Porridges and purees should be given only during the first two months of initiation of complementary feeding. From the eighth month of life, fine and soft lumps should be introduced. Subsequently, consistency should be progressively increased to chopped and firmer but easy grinding food. The caregiver who perpetuates the use of baby food and purees until the infant is one-year-old will favor difficulties accepting food with greater consistency.66,80
13Myths and reality of complementary feedingIn Mexico, as in Latin America, it is frequent to include certain solid food and liquids that do not provide the nutrients required for optimal growth and development. Its part of the Latin American culture, which is transmitted from generation to generation without questioning its real benefit. Even some nutritional and healing benefits have been attributed to many of these foods. These beliefs remain despite the numerous information campaigns developed by government and private institutions. Health personnel should be trained to properly guide the caregivers to eradicate these myths. Some of the foods most commonly used to try to improve child nutrition are the following.
Gruel. By any means, it does not replace breast milk or formula designed for infants. In addition, it has a higher energy density, mainly for the content of sugar and starch. Also, it does not contain the nutrients needed to promote proper growth and development. It should not be routinely consumed since, depending on its cooking process, it might predispose children to obesity or malnutrition. If it is prepared with milk and sugar, it will have a high energy density. In contrast, if it is prepared with water, it will be deficient in proteins, lipids, and micronutrients. As the gruel recipes are heterogeneous, the real nutritional contribution is unknown.
Herbal infusions (teas) and coffee. Their consumption in infants is not recommended, as they may replace breast milk, formula milk, or even recommended solid foods. Some teas can become toxic (e.g., star anise). Moreover, no health beneficial effect has been yet demonstrated (chamomile, peppermint, lemon tea, orange blossoms). Also, sweeteners are usually added, which increase health risks.
Broths. Their nutritional benefits of broths are a common myth in Mexican and Latin American populations. There is a misconception that the most important nutrients of the ingredients of the broths remain in the liquid after cooking. However, the potages do not contain iron or vitamins as popularly believed. It is health personnel duty of demystifying this false belief.
Smoothies. The consumption of smoothies, a drink made from whole milk and one of the following: fruit, unsweetened chocolate powder, raw egg, amaranth, oats, granola, nuts, or other, is a typical Mexican habit at breakfast. However, it is not recommended since it cannot replace a balanced and healthy breakfast. They are also heavily energy drinks that can promote obesity. Raw egg consumption should not be encouraged since the white contains a protein called avidin, which inhibits biotin absorption.81
Fermented dairy products. Lactobacillus casei Shirota and Lactobacillus paracasei are the most commonly used probiotics in fermented beverages, whose effect on the intestinal microbiota and health are still unknown. The dairy industry recommends a portion of these products a day. However, it should be noted that the pleasant taste (usually sweet) induces children to consume more servings than recommended, promoting high sugar intake. It should be considered that a portion of 80ml (55kcal) contains from 10 to 12g of sugar.
Swiss cheese. Swiss cheese consumption (petit cheese) is not recommended in children under one year of age due to its high energy and sugar content. A 90g portion signifies 12% of the total daily energy requirement (104kcal) for a one-year-old. It seems that the dye contained in these cheeses is associated with attention deficit hyperactivity disorder.82,83
13.1Demonized but adequate foodsCitruses. No scientific evidence to support the delay in citrus consumption until the year of age exists. Citruses can be introduced since the sixth month of age. The intake of citruses in combination with other foods is essential to enhance the absorption of non-heme iron (Table 7).
Strawberry and kiwi. Allergy to these fruits is uncommon. Depending on the population culture, their consumption can start since the sixth month of life. In the case of strawberries, washing and disinfection before eating is very important.
Pork meat. The consumption of pork is recommended from six months old, as other red meats. The pork was stigmatized since the early 70s due to an increased cysticercosis in Mexico. Although their presence is rare today, the pork should be cooked thoroughly before eating.
Chocolate. There is no scientific evidence to support that chocolate causes health problems in children. In Mexico, chocolate has been consumed for hundreds of years. Its health benefits are well known but have been demonstrated only in adults. However, it is very likely that these same benefits are also observed in children. It is imperative to consume it without adding any sugar or sweetener. Nor it should be consumed as milk or other beverage flavoring, especially before two years of age.
Egg. Its consumption is appropriate since the sixth month of life, complete and without separating the yolk from the white. The delayed introduction is associated with increased risk of allergy.
Beans. Their introduction is recommended since the seventh month of life considering that the skin fiber does not cause gastrointestinal problems.
“Cold” and “hot” food. In different parts of Latin America, the popular belief states that there are “cold” and “hot” foods, which help or harm health depending on the age and condition of the child. No food should be forbidden based on these beliefs.
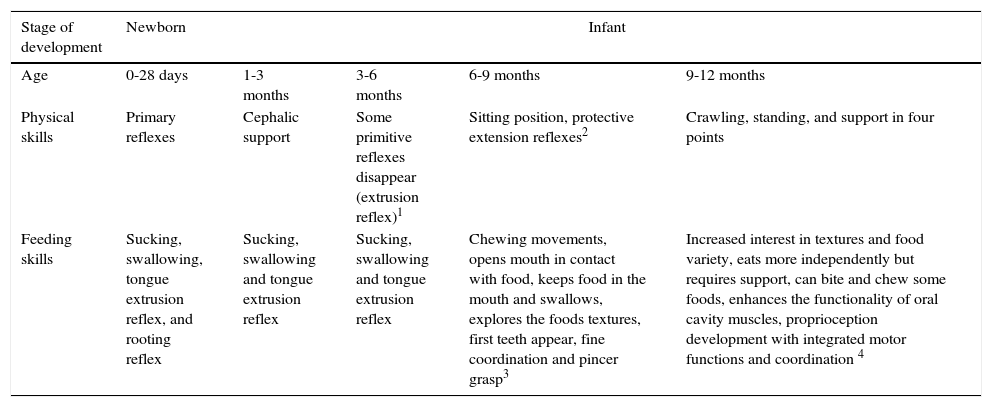
14Developmental milestonesNeurodevelopmental achievements relate to the capability to begin complementary feeding.84–87 Infant important results are displayed during the different stages of life and how the progressive skills management relates to food intake. For example, the suitable age/maturation to use a high chair, to facilitate food manual handling and to facilitate the use of cutlery (Table 9).
Participation of development gains in acquiring the ability to receive complementary foods.84–87
| Stage of development | Newborn | Infant | |||
|---|---|---|---|---|---|
| Age | 0-28 days | 1-3 months | 3-6 months | 6-9 months | 9-12 months |
| Physical skills | Primary reflexes | Cephalic support | Some primitive reflexes disappear (extrusion reflex)1 | Sitting position, protective extension reflexes2 | Crawling, standing, and support in four points |
| Feeding skills | Sucking, swallowing, tongue extrusion reflex, and rooting reflex | Sucking, swallowing and tongue extrusion reflex | Sucking, swallowing and tongue extrusion reflex | Chewing movements, opens mouth in contact with food, keeps food in the mouth and swallows, explores the foods textures, first teeth appear, fine coordination and pincer grasp3 | Increased interest in textures and food variety, eats more independently but requires support, can bite and chew some foods, enhances the functionality of oral cavity muscles, proprioception development with integrated motor functions and coordination 4 |
About the caregiver reaction in the presence of selective responses of like and dislike and the relationship between infant and the feeder, we should note the following concepts.88–91
- 1)
Labor division must be established: the child decides “how much” to eat and parents dictate “what,” “when” and “how.”
- 2)
The caregiver should eat the food that displeases the child, which improves their willingness to try.
- 3)
The child must be accompanied when eating.
- 4)
Caregivers should see and taste the food to enhance the acceptance.
- 5)
Food must be exposed from eight to 15 times for better acceptance.
- 6)
A greater control from caregivers (pressure, control, and restrictions) has negative consequences on the infant's behavior.
- 7)
Meal intervals depend on the self-regulation of children and the environment and parent-child bond.
- 8)
Parents influence their infant's eating habits, not just the genetic pool.
- 9)
Food preference decreases in children who are pressured to eat certain food types.
In the relationship between the feeder and the baby, a friendly environment is necessary for the beginning of the complementary feeding. Both should enjoy the experience. It should encourage a friendly atmosphere with enough time to coexist with the baby. The baby must be sitting straight either on the feeder's lap or a safety seat. Talking to the baby is key during the entire process of feeding.59
Ideally, the food should not be delivered directly from the container/vessel in which it was prepared but on the baby's plate. It is necessary to provide confidence that he can eat on his own, taking the spoon by himself.
Before starting with the complementary feeding, it is important that the baby has strengthened the orofacial cavity with suction-swallowing—mainly by the breastfeeding process—as it stimulates the growth of the jaw, enhances the development of bones and muscles and the tongue positioning and performance; tooth balance is achieved and rhythmic movements of the jaw, tongue, and lips are acquired.92
Accordingly, it should be remembered that when complementary feeding begins, there are different objectives, such as the display and support the experience of trying different foods considering colors, flavors, textures, consistencies, and temperatures to promote sensory stimulation (sensory inputs and orofacial cavity motor stimulating), and the arousal of psychosocial development (emotional bond).93
Moreover, from the speech and language development point of view, the experience of complementary feeding is invaluable to strengthen the perceptive and expressive language, acting as a bridge between pre-linguistic and linguistic communication and considering that the experienced proprioceptive and kinesthetic feedback are critical for speech and feeding skills.94
15Baby-led weaning practiceAs stated before in section 6 “Food consistency modifications according to the neurological development”, BLW is an alternative method of complementary food introduction, in which children feed themselves with their hands instead of being spoon-fed by an adult. In BLW, adequately prepared food pieces are offered for the infant to feed by himself. Although this method has been popular in recent years, scientific information is limited.56 Its advantages and disadvantages are described below.55,95,96
Advantages. It has been described that BLW improves relationships during family meals since the meal is shared; baby's autonomy is promoted; saves time and money; encourages healthier foods intake; motivates the interest for complex carbohydrates but not sugars; fosters the recognition of the satiety feeling, which reduces the possibility for overweight; promotes healthy eating styles; decreases commercial food consumption (p=0.002); promotes a family diet incorporation (p=0.018); promotes an energy intake autoregulation and the development of fine and gross motor skills.
Disadvantages. It does not improve family eating style; there is a risk of inadequate iron intake, depending on the type of food being offered (p=0.001), and deficient food and energy intake that favors underweight. There is a choking risk (30% of infants have a choking episode with solids, and parents do not distinguish choking from nausea). The adherence to BLW is not complete. Currently, no randomized clinical trials exist.
Interestingly, it has been noted that mothers who apply the BLW method have a minor score on anxiety and obsessive-compulsive disorders questionnaires.
16Veganism and vegetarianismBeing a vegetarian means consuming only foods of plant origin. Although it has been a common practice before, vegetarianism has become a popular practice in the last decade.97 Despite the vegetarian diet is characterized by the consumption of plant foods, it has some variants (Table 10).
Types of vegetarian diets.101
| Diet | Characteristics |
|---|---|
| Semi-vegetarianism | Birds and fish are included |
| Lacto-ovo-vegetarians | Includes dairy products and eggs |
| Lacto-vegetarians | Includes dairy products |
| Vegans | No food of animal origin |
| Macrobiotic | The cooking of food is as natural as possible, trying not to lose its properties. No processed foods are included. It is based on the Japanese philosophy of ying and yang. Some people may add some meat or animal derivatives |
Vegetarian diets are often associated with health benefits. Some studies show that consumers of this diet have a lower cardiovascular risk, serum cholesterol within normal parameters, a reduced hypertension and type 2 diabetes mellitus risk.98,99
The Academy of Nutrition and Dietetics and the American Academy of Pediatrics argued that a well-planned vegetarian diet can promote a proper growth and development.98 However, the World Health Organization suggests that an unfortified plant-based diet does not meet the needs of particular micronutrients in the first months of life. Therefore, they recommended to include dairy products, meat, poultry, fish or eggs as often as possible.
In a Tennessee community from the United States, a cohort study was conducted with 404 children who followed a vegetarian diet, to assess the impact on growth from four months to ten years of age. At the end of the follow-up, vegetarian children were only 0.7cm and 1.1kg on average below the reference median. The researchers concluded that vegetarian children showed an appropriate growth.100
It should be considered that a poor food education, the lack of training by an expert, and the lack of adequate planned diets can lead to nutritional deficiencies, especially of micronutrients as iron, zinc and vitamin B12. Also, the high consumption of fiber and phytates inhibits the absorption of such micronutrients. Moreover, the lipid consumption can be deficient and compromise the total energy input, which consequently could produce children growth and development deceleration.101
If parents by conviction submit their children to this type of diet, it is suggested to have a nutritionist guide and supplement with the aforementioned micronutrients.98
The recommendations suggested to reduce the risk of poor nutrition are the following:
- 1)
To offer exclusive breastfeeding during the first six months of life101
- 2)
If breastfeeding is not possible, soy formula is an option for vegan infants102–104
- 3)
The complementary food must be introduced in the same way that not vegetarian infants, with specific recommendations to prevent nutritional deficiencies
The following of macro and micronutrients is important:98
Proteins. Proteins of plant origin are usually less digestible, and lack of one or more essential amino acids.105 Legumes and cereals are good sources of protein but need to be eaten together, as legumes are poor in sulfur-containing amino acids (methionine and cysteine), and cereals in lysine (wheat, rice and corn), tryptophan (corn) and threonine (rice). For this reason, an increased protein intake is recommended (up to 35%).106 It is recommended to introduce foods such as tofu, fortified cereals, legumes, soy yogurt, and tempeh at six months of age.98,101
Docosahexaenoic acid (omega-3). Omega-3 fatty acids are essential polyunsaturated fatty acids, which promote brain and retinal development among other functions.101 Vegetarian diets containing high levels of omega-6 fatty acids but low levels of omega-3. Additionally, the bioavailability of omega-3 is poorer in a plant-based diet.107 Foods rich in omega-3 fatty acids are the linseed, avocado oil, fish, tofu, and fortified soy formula.101
Vitamin B12. It is an essential factor for DNA synthesis and brain and nervous system development. Deficiency manifestations can be very subtle and even unnoticed since most vegetarians consume amounts of folic acid that mask deficiency symptoms. However, in the case of a major deficiency, patients may have neurologic manifestations. Consumption of fortified cereals, soy formulas, and fortified rice are recommended. If the infant is breastfed, the supplement should be 0.4μg/day from birth to six months and 0.5μg/day after six months, unless mother's diet meets the requirements. Supplementation is not necessary for children with a lacto-ovo-vegetarian diet.108
Zinc. Requirements on children exclusively breastfed usually met in the first six months but in plant-based diets, consumption can be below the recommendations.109 Complementary feeding should begin with fortified cereals, legumes, whole grain cereals, wheat germ and tofu. The bioavailability of zinc diminishes when ingested with high amounts of phytates found in whole grains and legumes. On the contrary, whole grain bread with yeast and fermented soy products increase its bioavailability.110
Iron. Different studies have shown that infants have an adequate iron intake if a well-planned vegan diet is followed.111 Vitamin C and other components of vegetables enable the absorption of non-heme iron found in leafy green vegetables. However, dietary fiber, phytates, and tannins can inhibit its absorption. Therefore, an appropriate balance is important.103 The use of iron-fortified cereals can increase its overall intake, as other sources of nutrients, including beans and dry peas.109
Infant's growth and neurodevelopment should be closely monitored since these diets provide a high fiber content, producing early satiety and conditioning availability of micronutrients, energy, and essential fatty acids that can affect growth and normal development of the child.
No safety studies were identified for raw-vegan, macrobiotic or frugivore diets. Therefore, they are not recommended for infants.
FundingFunds received from Nestle Infant Nutrition Mexico for the consensus meeting.
Conflict of interestThe authors declare no conflict of interest of any nature.
Please cite this article as: Romero-Velarde E, et al. Consenso para las prácticas de alimentación complementaria en lactantes sanos. Bol Med Hosp Infant Mex. 2016;73:338–356.