To assess ocular involvement in the pathophysiology of autosomal dominant compelling helio-ophthalmic outburst syndrome (ACHOOs).
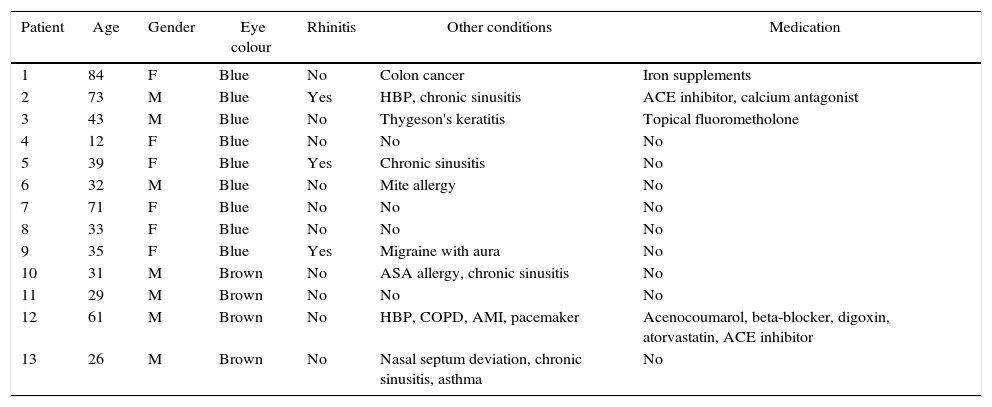
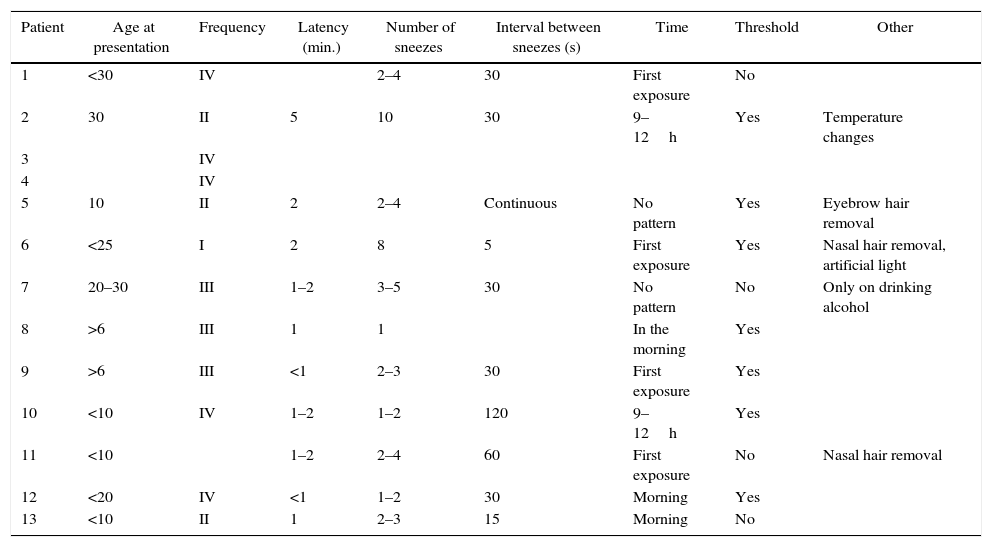
MethodsAn interview was conducted with a Caucasian family that showed clinical features of ACHOOs. Twelve of them had photic reflex and were recruited. A complete eye evaluation was made.
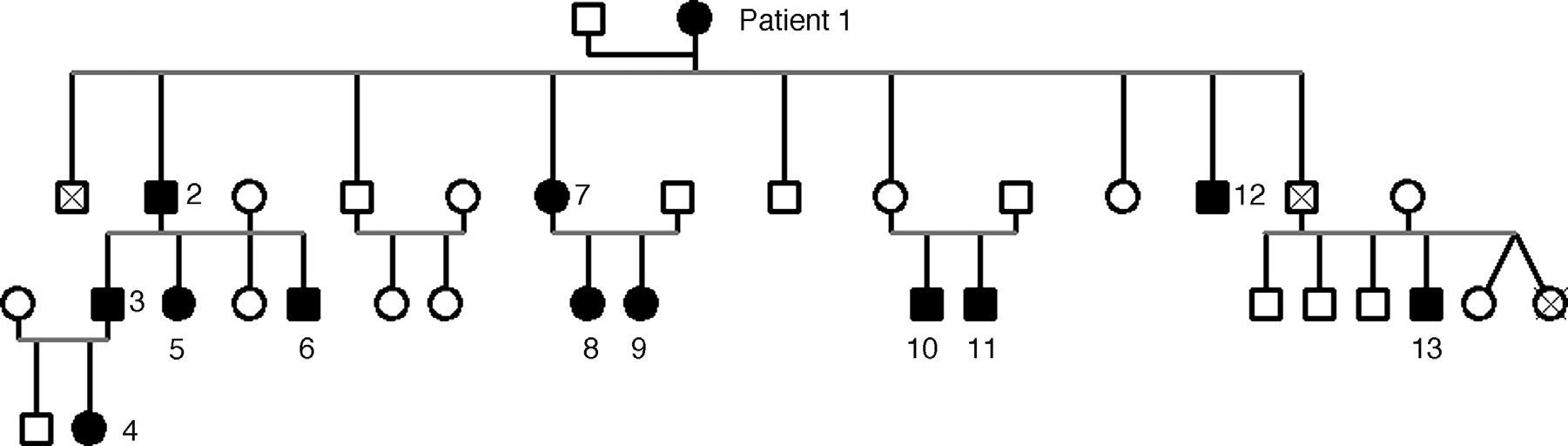
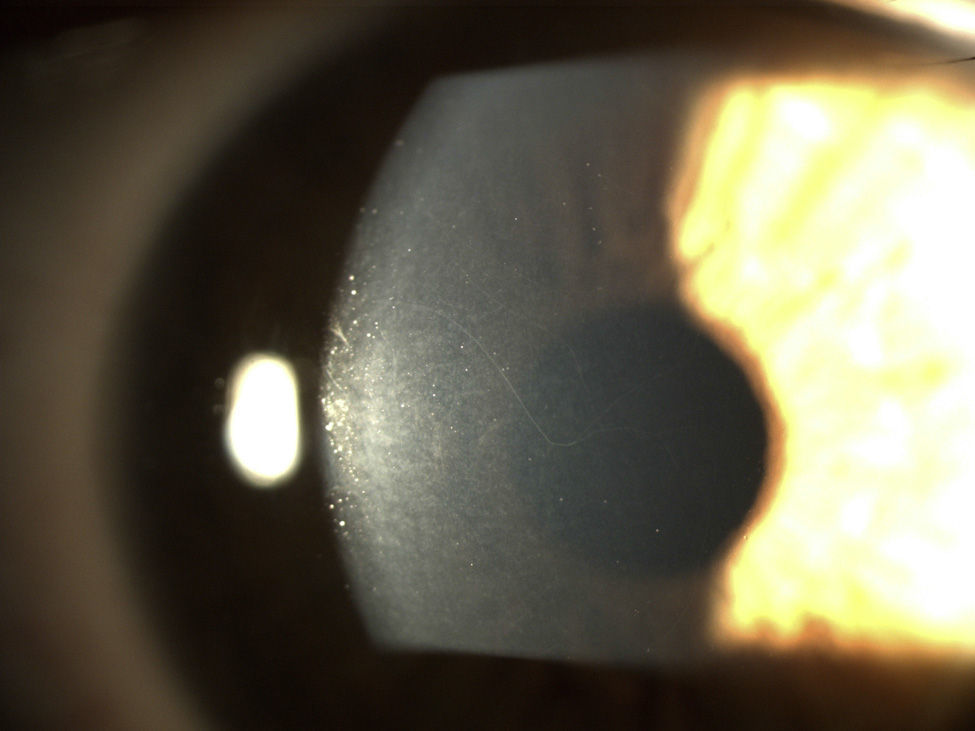
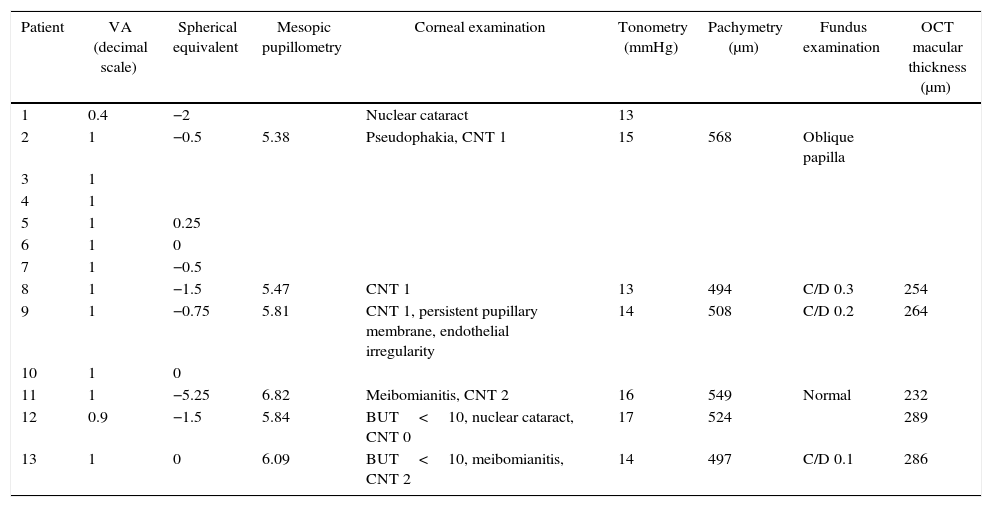
ResultsA dominant autosomal inheritance with mild penetrance was demonstrated, with 67% of the studied subjects showing some degree of prominent corneal nerves. No other eye changes were found.
ConclusionsProminent corneal nerves may be associated with ACHOOs. The other eye structures studied do not seem to play a role in ACHOOs. Further studies are needed to understand the physiology of the ACHOOs.
Evaluar la implicación ocular en la fisiopatología del síndrome helio-oftálmico de estornudos compulsivos autosómico dominante (ACHOOs).
MétodosUna familia de raza caucásica, que muestra las características clínicas de ACHOOs, fue interrogada. De toda la familia, 12 pacientes presentan reflejo fótico y fueron seleccionados. Se realiza una evaluación oftalmológica completa.
ResultadosSe encuentra una herencia autosómica dominante con penetrancia parcial. El 67% de los sujetos estudiados mostró algún grado de prominencia en los nervios corneales. No se encontraron otras alteraciones oculares.
ConclusionesLos nervios corneales prominentes pueden tener asociación con el ACHOOs. Las otras estructuras del ojo estudiados no parecen desempeñar un papel en el ACHOOs. Se necesitan más estudios para comprender la fisiología del ACHOOs.