The independent occurrence of aneurysms in the thoracic aorta (TAA) and abdominal aorta (AAA), simultaneously (synchronous aneurysms – SA) or sequentially (metachronous aneurysms – MA) occurs in 20–25%.
Endovascular or open repair (OR) of SA may be simultaneous or staged, while interventions for MA always involves two procedures.
In both cases, an increase of spinal cord ischemia (SCI) rates was reported.
The present study analyzes our experience in the management of SA and MA.
MethodsIn a retrospective analysis, all the patients submitted to thoracic endovascular aneurysm repair (TEVAR) between March 2009 and February 2015, were identified. From these, those who had TEVAR+EVAR or TEVAR+OR of AAA in the same period of time (Group-1: synchronous) and those who had TEVAR and had previous repair of AAA (Group-2: metachronous) were selected.
All surgeries were performed under strict haemodynamic control, cerebrospinal fluid (CSF) drainage and pressure monitoring and the patency of the left subclavian artery was assured.
The endpoints were: incidence of SCI, stroke, acute kidney injury and mortality.
ResultsTEVAR was performed in 58 patients of which 5 had SA (Group-1: 8.6%) and 6 had MA (Group-2: 10.3%).
Group-1 included 3 patients treated with EVAR+TEVAR simultaneously, one patient who had a TEVAR and OR of a type-4 thoracoabdominal aneurysm (TAAA) in the same hospitalization and, finally, a fifth patient that underwent TEVAR due to a contained rupture of a proximal TAA. This patient also presented a type-4 TAAA, whose treatment was deferred due to poor medical condition, but ruptured 1 month after.
Group-2 included 6 patients. Five had OR of AAA in the past and underwent TEVAR. The sixth patient had a previous EVAR with an abdominal debranching. One patient was submitted to a supra-aortic debranching and another to a chimney procedure of the superior mesenteric artery. The median of the initial to current intervention time was 6.5 years.
There were no reports of SCI or early mortality but 1 patient in Group-1 died due to non-procedural complications.
ConclusionThe prevalence of SA and MA in all the TEVAR cases was 18.9%.
With implementation of a surgical and anesthetic protocol, there were no cases of SCI or surgical mortality.
A ocorrência independente de aneurisma na aorta torácica (AAT) e abdominal (AAA), no mesmo momento temporal (síncronos - AS) ou em alturas sequenciais no tempo (metácronos - AM), tem uma prevalência de 20-25%.
O tratamento endovascular ou aberto (CA) dos AS pode ser efetuado no mesmo tempo operatório ou em tempos diferidos; nos AM ocorre em momentos temporais diferentes.
Em ambos os casos, a literatura sugere maior risco neurolo¿gico.
O presente estudo pretendeu analisar os resultados do tratamento destes doentes.
MétodosForam identificados retrospetivamente os casos de TEVAR entre março de 2009 e fevereiro de 2015, selecionando-se os submetidos a TEVAR+EVAR ou CA+TEVAR no mesmo momento temporal (grupo-1) e os tratados por TEVAR com tratamento anterior de AAA (grupo-2).
Todos os doentes foram operados segundo um protocolo de anestesia geral, estabilidade hemodinâmica, drenagem e monitorizac¿a¿o da pressa¿o do li¿qui¿do cefalorraquidiano, e assegurou-se sempre a permeabilidade da artéria subclávia esquerda.
Os endpoints do estudo foram a taxa de isquemia medular, de acidentes vasculares cerebrais de lesa¿o renal aguda e da mortalidade.
ResultadosCinquenta e oito doentes foram submetidos a TEVAR. Cinco correspondiam a AS (grupo-1:8,6%) e 6 a AM (grupo-2:10,3%). No grupo-1 incluíram-se 3 doentes tratados por TEVAR+EVAR simultâneo, um submetido a TEVAR+CA por aneurisma toracoabdominal tipo-4 (ATA4) no mesmo internamento, um submetido a TEVAR por rotura de AAT, onde se diferiu o tratamento de um ATA4 que ocorreria de emergência por rotura 1 mês depois.
No grupo-2 incluíram-se 5 doentes com CA no passado e submetidos a TEVAR, e um doente tratado no passado de debranching abdominal e EVAR.
Neste grupo-2 foi efetuado debranching dos troncos supra-ao¿rticos num doente e chimney de artérias abdominais noutro. A mediana do tempo intervenc¿a¿o inicial-atual foi 6,5 anos.
Não houve casos de isquemia medular ou de mortalidade. No grupo-1 ocorreu um óbito tardio por complicac¿o¿es na¿o ciru¿rgicas.
ConclusõesA prevalência de AS e AM numa população de doentes submetidos a TEVAR foi de 18,9%.
Utilizando um protocolo sistemático de manuseamento perioperato¿rio anestésico e ciru¿rgico, na¿o se verificou nenhum caso de isquemia medular ou de mortalidade.
Multianeurismatic aortic disease represents a therapeutical challenge. Its treatment involves various segments of the aorta and is associated with an increased risk of complications due to the exclusion of multiple side branches.
The presentation of aneurismatic disease involving several anatomic segments may be continuous, as thoracoabdominal aneurysms, or it may occur separately, on different aortic levels, with spared regions in-between. In fact, it is known that thoracic aortic aneurysms (TAA) will be found in 5–13% of patients with an abdominal aortic aneurysm (AAA) and, on the other hand, AAA are present in 10–29% of patients with TAA.1
The temporal occurrence of these aneurysms allows their classification as metachronous (when they appear at different time moments) or synchronous when they are identified on the same time period.
The therapeutical approach of synchronous aneurysms is controversial and there is much debate whether or not to treat them on the same surgical procedure.1–3
Historically, the treatment of these patients has been more frequently undertaken in two staged open procedures since two separate interventions seem to be less invasive, leading to less morbidity and mortality, than one simultaneous approach to the thorax and the abdomen.1
The introduction of endoluminal therapies allowed easier simultaneous approaches to both aneurysms and the publication of series of patients treated by staged, simultaneous and hybrid (open and endovascular) strategies.
The occurrence of acute renal failure, acute coronary syndromes and respiratory complications are inherent to any aortic surgery, but it has been the spinal cord ischemia (SCI) and its associated neurological deficits (paraparesis or paraplegia), which have been the target of greatest concern and debate.
The incidence of SCI in the perioperative period of patients undergoing simple thoracic endovascular aortic repair (TEVAR) is around 2.7%.4–6 However, in cases of TEVAR with prior or simultaneous abdominal aortic surgery, the literature suggests a risk increase which may be higher than 10%.7,8 Exclusion in both procedures of thoracic and abdominal aortic side branches which contribute to the spinal cord blood supply has been appointed to be the main cause of the reported increase rate of SCI.7
Numerous studies have also shown that the factor that seems to have the greatest impact on the occurrence of spinal cord dysfunction is the length of aortic exclusion, either by open repair or by endovascular treatment.4
In the present study we report the results of our experience regarding the incidence of SCI in the treatment of patients with synchronous and metachronous aortic aneurysms using TEVAR and either endovascular or open techniques for the abdominal aorta.
Material and methodsWe retrospectively identified all cases of TEVAR between March 2009 and February 2015 in our institution, corresponding to 58 patients. Of these, we selected 11 patients (18.9%) who had multilevel aortic disease with noncontiguous aneurysms in more than one aortic segment.
Patients were divided into two groups:
Group 1 included 5 patients with synchronous thoracic aortic and abdominal aneurysms, that were treated in the same time period (same surgical procedure, same hospital stay or close hospital admissions) as both aneurysms were already diagnosed. The strategy included endovascular treatment of the thoracic aortic aneurysm and endovascular (EVAR) or open repair (OR) for the abdominal aorta.
Group 2 included 6 cases with metachronous aneurysms corresponding to patients who had a previous repair of a AAA and that underwent TEVAR for a thoracic or a thoracoabdominal aortic aneurysm (TAAA) in a separate time period (the second aneurysm was not identified at the time of the initial surgery).
The primary endpoint of this study was the occurrence of SCI which was defined as paraparesis or paraplegia according to the Tarlov scale.9
Paraplegia or paraparesis observed immediately or upon awakening were defined as immediate neurologic deficits. Those occurring after a period of normal neurologic function were classified as delayed deficits. The deficits could also be permanent or temporary.
As secondary endpoints we analyzed the stroke rate, acute kidney injury and death.
Data was obtained from our institution's database and we collected the following factors for both groups: age, sex, classification of the patient's physical status according to the ASA scale (American Society of Anesthesiologists), prior chronic kidney disease (creatinine level >1.5mg/dL), diameter of the aneurysm, number of stent-grafts used in the TEVAR procedure, extent of coverage of the thoracic aorta, location of the proximal landing zone in accordance to the classification of the aortic arch proposed by Ishimaru9, if a debranching procedure of the supra-aortic trunk was needed, time interval between the initial intervention and the second one and the occurrence of intraoperative hypotension (systolic blood pressure less than 80mmHg for longer than 10min).
The applicability of endovascular techniques and the planning of the procedure was performed with a preoperative CT Scan evaluation using the software Osirix.
The stent-grafts used in the TEVAR procedures were: GORE-TAG (WL Gore and Assoc, Flagstaff, Ariz) in one case; Cook-Zenith TX 2 (William Cook Europe Aps, Bjaeverskov, Denmark) in 8 cases and Medtronic Valiant (AVE/Medtronic Inc, Santa Rosa, Calif) in 2 cases.
All procedures were undertaken under general anesthesia and followed the institution's protocol for the prevention of spinal cord dysfunction:
- -
CSF drain was inserted before the induction of anesthesia (usually the afternoon before the procedure). Passive CSF drainage was kept below 10mmHg for 48–72h in the absence of SCI symptoms.
- -
While under CSF drainage all patients were kept in the ICU with regular evaluation of their neurological status.
- -
Mean arterial pressure was maintained above 90mmHg.
- -
Whenever possible we assured the patency of the left subclavian artery (with a revascularization procedure if necessary) and of, at least, one of the hypogastric arteries.
Of the 58 cases of patients undergoing TEVAR 18.9% had a synchronous or metachronous aneurysms.
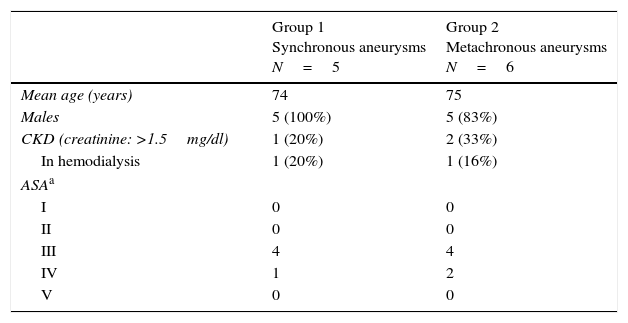
In Group 1 five male patients were included, with a mean age of 74 years (Table 1).
Patients’ characteristics.
| Group 1 Synchronous aneurysms N=5 | Group 2 Metachronous aneurysms N=6 | |
|---|---|---|
| Mean age (years) | 74 | 75 |
| Males | 5 (100%) | 5 (83%) |
| CKD (creatinine: >1.5mg/dl) | 1 (20%) | 2 (33%) |
| In hemodialysis | 1 (20%) | 1 (16%) |
| ASAa | ||
| I | 0 | 0 |
| II | 0 | 0 |
| III | 4 | 4 |
| IV | 1 | 2 |
| V | 0 | 0 |
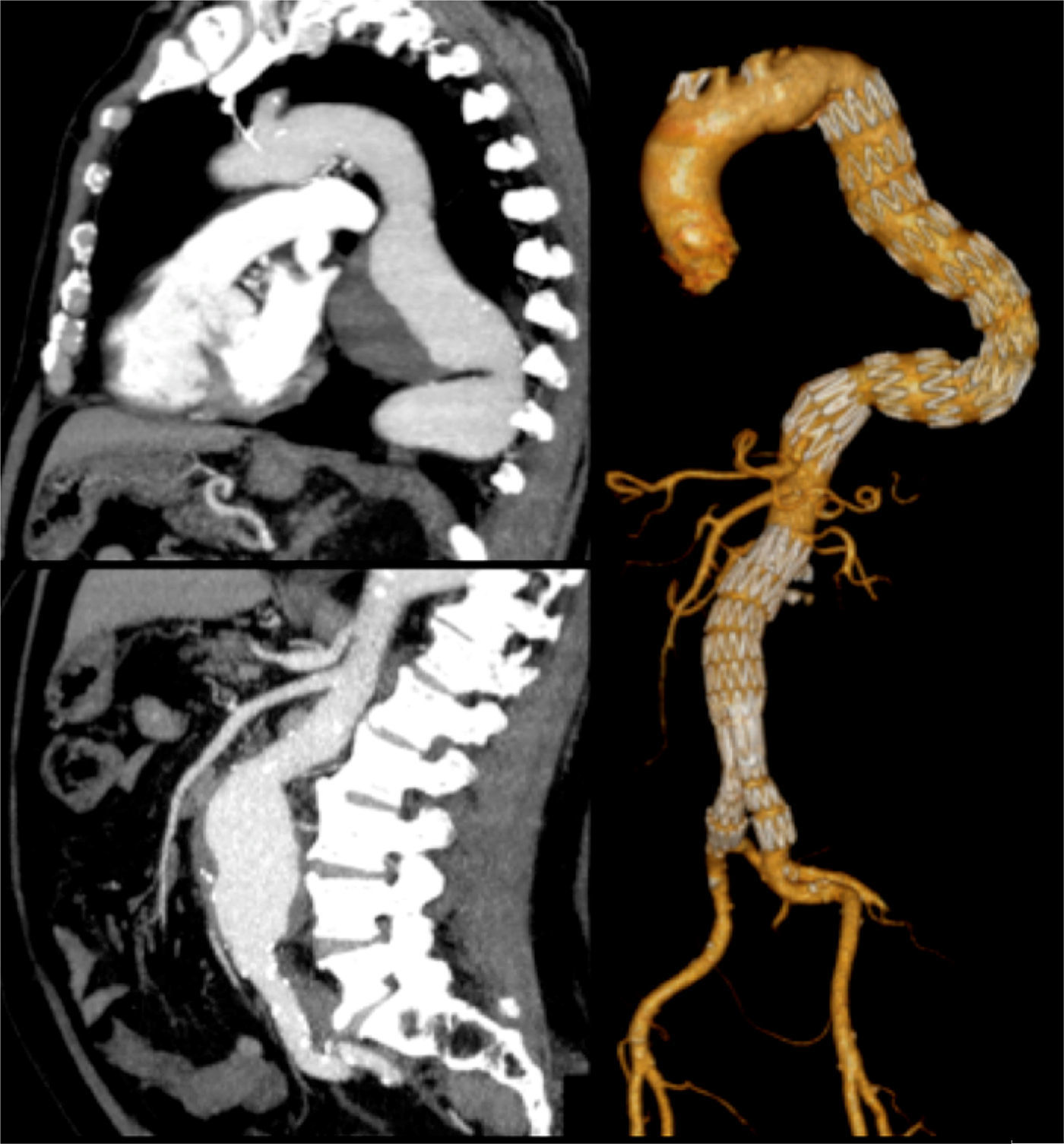
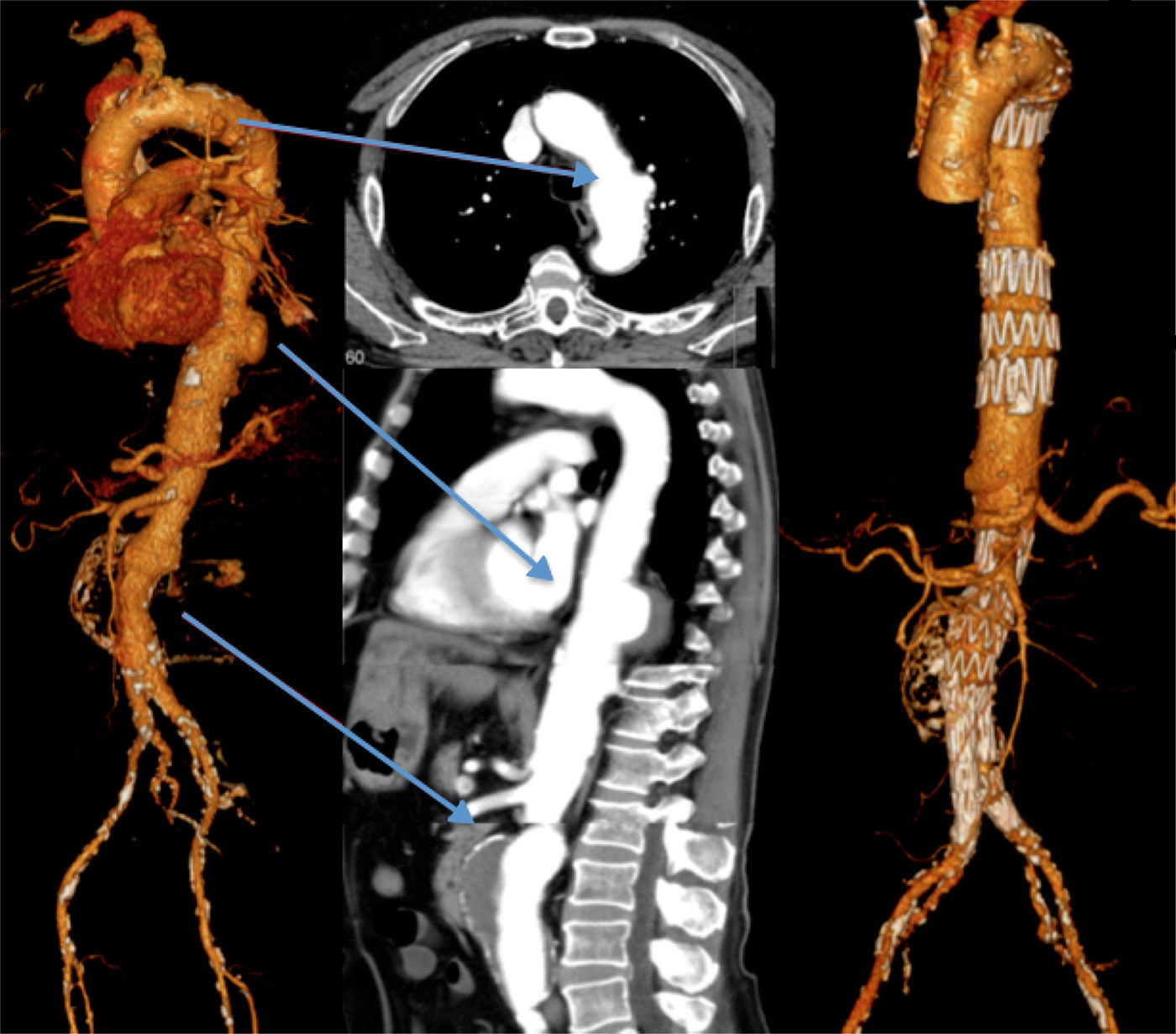
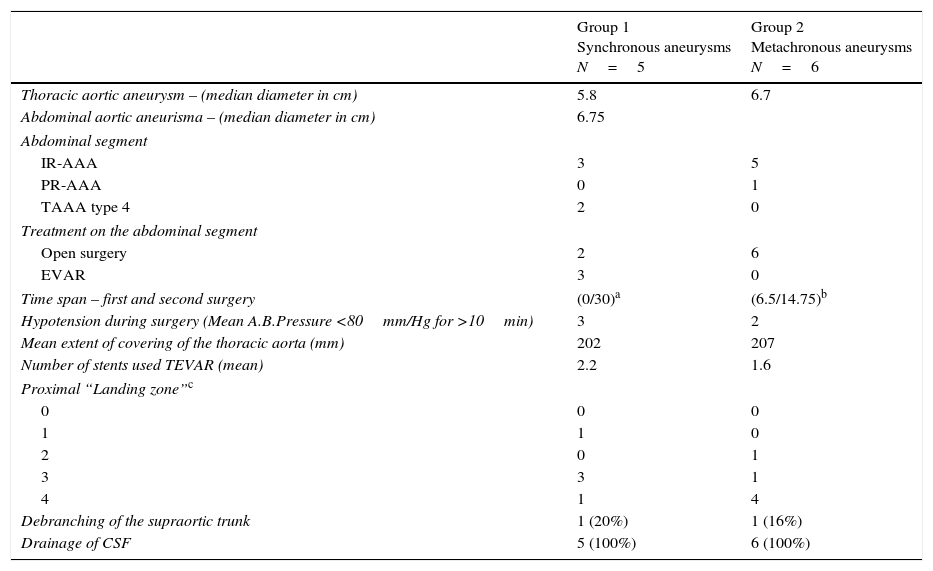
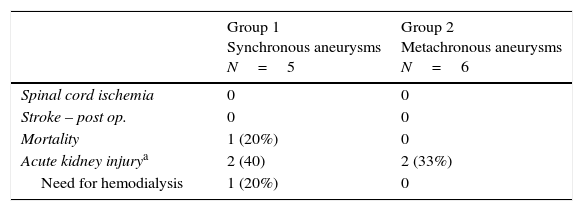
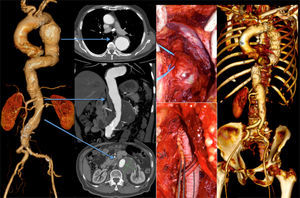
Three patients underwent TEVAR+EVAR in the same surgical procedure for aneurysms of the descending thoracic aorta and of the infrarenal aorta (Fig. 1) and one was also treated for a penetrating ulcer of the proximal thoracic aorta which was addressed with the deployment of a third separate endoprosthesis (Fig. 2).
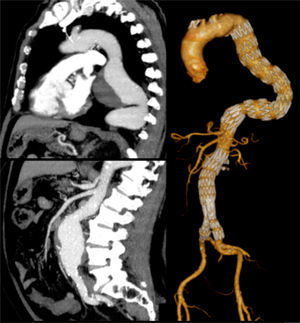
One other patient presented with a 7cm type 4 thoracoabdominal aneurysm synchronous to an 6cm aneurysm of the descending thoracic aorta. According to the aneurysm diameter the TAAA was treated first by OR (Crawford technique), followed by TEVAR for the TAA (during the same hospital stay) (Fig. 3).
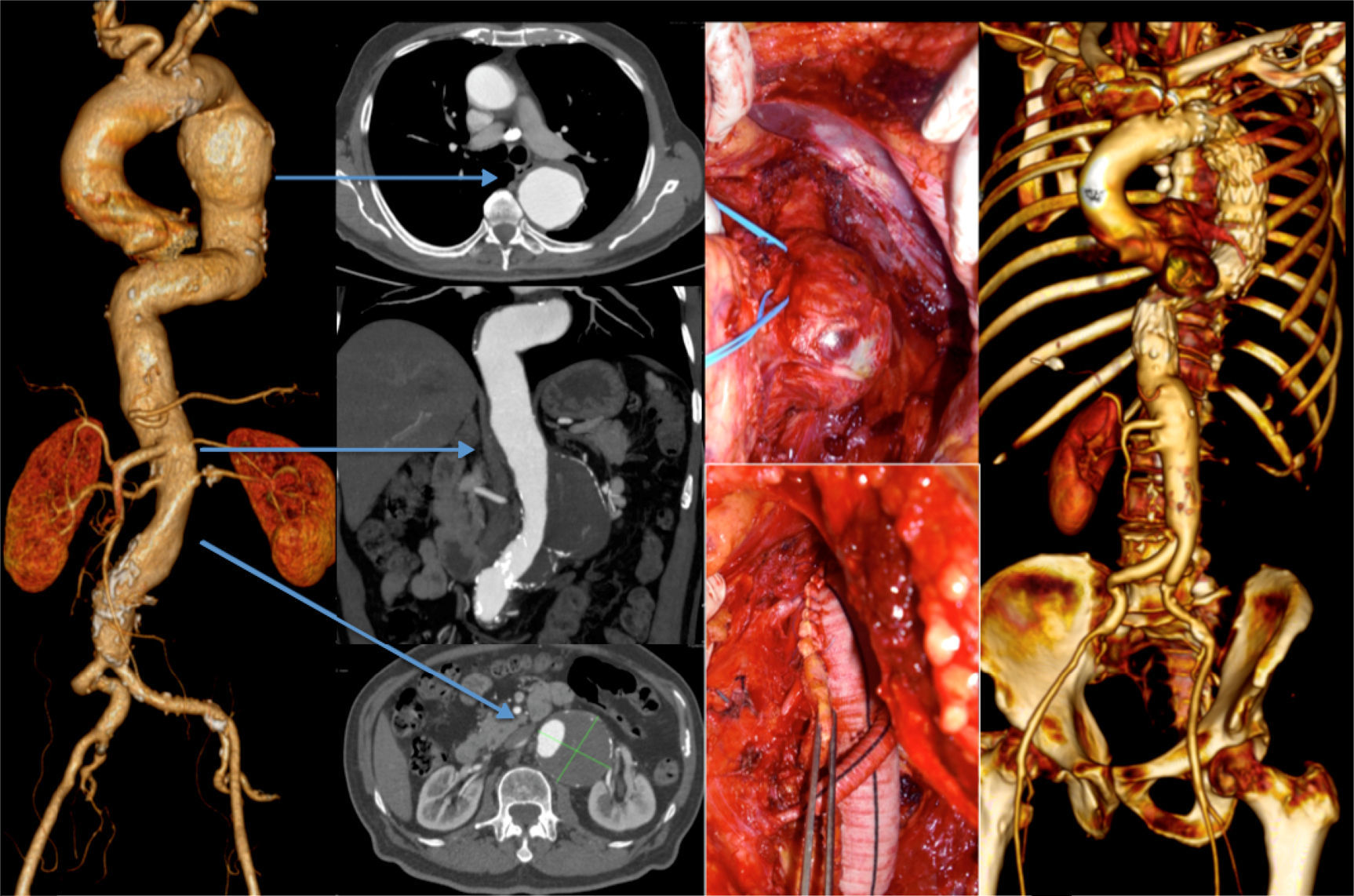
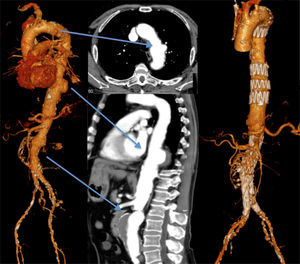
Finally, the fifth patient was admitted due to a contained rupture of an aneurysm of the left aortic hemi-arch and proximal descending thoracic aorta. He had also a type 4 TAAA that showed no signs of acute complication. The patient was submitted to a zone 1 debranching procedure of the supra-aortic trunks before the endovascular exclusion of the TAA. The intervention was completed by coil embolization of the left subclavian artery. There were no immediate complications after the first intervention and the patient was discharged home for recovery, being the second procedure schedulded for 2 months after. However, while waiting for the second intervention, the TAAA ruptured and was repaired in emergency setting by OR using the Crawford's technique. The patient survived the operation, which was uneventful, but died 5 weeks later in the ICU due to resistant respiratory tract infection that led to multiorgan dysfunction (Table 3).
In this group there were no complications of SCI or stroke. One patient required dialysis due to multiorgan dysfunction (the aforementioned fifth patient) and one other had a transient worsening of the renal function (Table 3).
Group 2 (metachronous aneurysms) included six patients (5 males and 1 female) with a mean age of 75 years (Table 1). All had previous abdominal aortic open surgery, 5 due to infrarenal AAA and one due to a pararenal AAA which was treated by an abdominal debranching procedure followed by EVAR.
The median time between the first and the second interventions was 6.5 years (Table 2).
Patient data.
| Group 1 Synchronous aneurysms N=5 | Group 2 Metachronous aneurysms N=6 | |
|---|---|---|
| Thoracic aortic aneurysm – (median diameter in cm) | 5.8 | 6.7 |
| Abdominal aortic aneurisma – (median diameter in cm) | 6.75 | |
| Abdominal segment | ||
| IR-AAA | 3 | 5 |
| PR-AAA | 0 | 1 |
| TAAA type 4 | 2 | 0 |
| Treatment on the abdominal segment | ||
| Open surgery | 2 | 6 |
| EVAR | 3 | 0 |
| Time span – first and second surgery | (0/30)a | (6.5/14.75)b |
| Hypotension during surgery (Mean A.B.Pressure <80mm/Hg for >10min) | 3 | 2 |
| Mean extent of covering of the thoracic aorta (mm) | 202 | 207 |
| Number of stents used TEVAR (mean) | 2.2 | 1.6 |
| Proximal “Landing zone”c | ||
| 0 | 0 | 0 |
| 1 | 1 | 0 |
| 2 | 0 | 1 |
| 3 | 3 | 1 |
| 4 | 1 | 4 |
| Debranching of the supraortic trunk | 1 (20%) | 1 (16%) |
| Drainage of CSF | 5 (100%) | 6 (100%) |
Four cases underwent a TEVAR due to a TAA.
One patient had a type 5 TAAA and chronic occlusion of the coeliac trunk and was already on dyalisis. He was submitted to a TEVAR with a chimney graft to the superior mesenteric artery.
The sixth patient had a TEVAR after zone 2 debranching procedure of the left subclavian artery.
In Group 2 there were also no signs of SCI. Two patients showed worsening of the renal function with complete recovery at discharge. There was no mortality or cerebrovascular events (Table 3).
Endpoints.
| Group 1 Synchronous aneurysms N=5 | Group 2 Metachronous aneurysms N=6 | |
|---|---|---|
| Spinal cord ischemia | 0 | 0 |
| Stroke – post op. | 0 | 0 |
| Mortality | 1 (20%) | 0 |
| Acute kidney injurya | 2 (40) | 2 (33%) |
| Need for hemodialysis | 1 (20%) | 0 |
On the one month CT scan controls we only found one case of type 1 endoleak in the patient from group 2 that underwent TEVAR with chimney graft to the superior mesenteric artery and that is currently under surveillance.
DiscussionSpinal cord ischemia is a devastating complication of proximal aortic surgery. It is associated with higher operative mortality and has serious long-term repercussions on quality of life and socio-economic status.
Retrospective studies have shown that the major predictor of spinal cord dysfunction is the aortic extension involved in open surgery or endovascular treatment.10
SCI after OR of thoracoabdominal aneurysms has been investigated and its prevalence ranges from 3.8%11 to 13.2%12 in centers with extensive experience. Nevertheless, it can reach higher rates if we evaluate the results of centers with less experience, in the so called “real world”.10,13
In general, the risk is substantially higher in more extensive aneurysms (type 1 and 2 TAAA).
If we consider aneurysms of the descending thoracic aorta, the risk of SCI after open surgical procedures is also high and was reported around 14%.14
The endovascular approach on descending thoracic aortic aneurysms was initially considered a game changer in regard to SCI with reports of 2–3%.4–6 When we compare endovascular to open surgery there are some conceptual differences: the endoluminal approach does not require aortic clamping and perioperative hipotension is much less frequent and prolonged than in OR. However, it also excludes intercostal arteries from circulation and those below T8 seem to play a critical role on spinal cord perfusion.
Postoperative SCI appears to be multifactorial and it is not yet fully understood.9
The spinal cord perfusion pressure is defined as the pressure difference between the systemic blood pressure and the cerebrospinal fluid pressure. During open procedures, aortic clamping causes a sudden drop in blood perfusion pressure which may cause a shift in spinal cord perfusion pressure. The drainage of CSF is advised and aims to decrease CSF pressure in order to reduce the gradient and improve perfusion.
During endovascular procedures there is no need for aortic clamping and the hemodinamic insult is generally lower, but other mechanisms that inflict SCI may be present, beyond the simple exclusion of critical aortic branches. Distal embolization to small end arteries in the spinal cord may occur due to catheter or graft manipulation in the aorta15 and Griepp et al.16 have suggested that blood may drain away from the spinal cord via retrograde flow from segmental vessels into the excluded aneurysm sac. Such steal may further increase the vulnerability of the spinal cord in patients undergoing endovascular procedures. However, the main mechanism seems to be the complete and sudden occlusion of several segmental vessels after the deployment of the grafts.
In recent years, it was emphasized the importance of a network of blood vessels responsible for the spinal cord perfusion and directly located around it. This network is directly perfused by the anterior spinal artery (artery of Adamkiewicz), which supplies the anterior portion of the spinal cord, and is also feeded by the vertebral, the hypogastric and other anterior radiculomedullary arteries.17
It is interesting to note that the occlusion of the Adamkiewicz's artery does not, necessarily, lead to SCI due to the maintenance of blood supply from the collateral network. This multilevel spinal cord blood supply stresses the relevance of maintaining perfusion in arteries like the median sacral, the internal iliacs and the subclavians in order to keep perfusion in the spinal cord collateral network. This was clearly described by Strauch et al.18 after clamping the median sacral artery in pigs and observing that the spinal cord became dramatically more prone to injury. Khoynezhad et al.19 also showed that covering the hypogastric artery is a significant risk factor for SCI.
Considering extensive branched/fenestrated endovascular thoracoabdominal grafting for TAAA, recent publications showed that the SCI risk is higher than what was previously thought stressing the importance of long aortic segmentar branch occlusions.13,20,21
Strategies like early intra-operative hypogastric and limb reperfusion, staged procedures, late endograft branch occlusion (keeping transient aneurysm perfusion) or embolization of collateral vessels to the spinal cord in order to develop neovascularization are under investigation.22,23
According to the previous arguments it is not a surprise that patients with simultaneously thoracic and abdominal aneurysms, or previously submitted to abdominal aortic aneurysm repair and undergoing TEVAR, may have an increased risk of post-procedural SCI and this was reported by some authors.15,24,25 However, most of these studies included a small number of patients which limit its validity.
Schlosser et al. noted that in 75 patients with previous abdominal surgery, the incidence of SCI after TEVAR was 12.5% and this was significantly higher in comparison to patients without prior abdominal replacement (12.5% versus 1.7% p<0.0001).8
The European Collaborators on Stent/Graft Techniques for Aortic Aneurysm Repair (EUROSTAR) evaluated the risk factors for SCI after TEVAR and determined that concomitant open abdominal surgery was statistically and independently associated with an increased risk of SCI (OR 5.52; p=0.0371).7 This has led some authors to suggest that whenever possible the procedures should be staged in order to allow the development of the collateral network and thus decrease the incidence of SCI.5,9
This topic remains controverse regarding the best endovascular approach and some authors question whether there is an increased risk of SCI in combined endovascular treatments of the thoracic and abdominal aorta and in comparison to conventional surgery as small series have been published on TEVAR+EVAR or concomitant OR that showed no increase in risk.26–28 According to Lucas et al. in a population of 49 patients (18 cases undergoing EVAR+TEVAR; 21 cases TEVAR+OR; 10 cases TEVAR+debranching), there was no SCI increase. They showed, however, fewer complications and lower mortality than in simultaneous or staged OR of thoracic and abdominal aneurysms.2
Some consensus exists regarding the preventive measures to be implemented in order to reduce the SCI risk and the avoidance of hypotension (its severity and duration) and the drainage of SCI appear as the most valued measures in the literature.
The importance of keeping the blood pressure at normal levels in the intra- and postoperative period and the avoidance of hypotension, is acknowledged and permissive hypertension is even suggested by several authors to increase the spinal cord perfusion pressure, whilst giving time for the collateral network to adapt to the surgical aggression.29,30
Despite being less well documented in the literature, the importance of CSF drainage in patients undergoing endovascular treatments is supported by most authors in cases that requires extensive exclusion of aortic segments.5,9
The occlusion of the left subclavian artery and consequently of the vertebral artery and the origin of the anterior spinal artery while treating TAA, relates to a 4-fold increase in the risk of paraplegia8 and should be avoided by the liberal use of revascularization procedures.7
The present study reports our experience in a group of patients with multilevel aortic aneurysms (synchronous and metachronous), with the thoracic component treated by endovascular exclusion under a SCI prevention protocol, and we found no increase in the risk of SCI.
Ethical responsibilitiesProtection of people and animalsThe authors declare that the proceedings followed abide by the ethical legislation from the ethical committee on responsible human experimentation, the World Health Organization, and the Declaration of Helsinki.
Confidentiality of dataThe authors declare that they have followed their center protocols on the publishing of data from patients.
Right to privacy and informed consentThe authors have obtained the written informed consent from patients and/or subjects referred to in this paper. This document belongs to the corresponding author.
Conflicts of interestThe authors have no conflicts of interest to declare.